Abstract
Small molecule-regulated transcription has broad utility and would benefit from an easily delivered self-contained regulatory cassette capable of robust, tightly controlled target gene expression. We describe the delivery of a modified dimerizer-regulated gene expression system to cells on a single retrovirus. A transcription factor cassette responsive to the natural product dimerizer rapamycin was optimized for retroviral delivery by fusing a highly potent chimeric activation domain to the rapamycin-binding domain of FKBP-rapamycin-associated protein (FRAP). This improvement led to an increase in both the potency and maximal levels of gene expression induced by rapamycin, or nonimmunosuppressive rapamycin analogs. The modified transcription factor cassette was incorporated along with a target gene into a single rapamycin-responsive retrovirus. Cell pools stably transduced with the single virus system displayed negligible basal expression and gave induction ratios of at least three orders of magnitude in the presence of rapamycin or a nonimmunosuppressive rapamycin analog. Levels of induced gene expression were comparable to those obtained with the constitutive retroviral long terminal repeat and the single virus system performed well in four different mammalian cell lines. Regulation with the dimerizer-responsive retrovirus was tight enough to allow the generation of cell lines displaying inducible expression of the highly toxic diphtheria toxin A chain gene. The ability to deliver the tightly inducible rapamycin system in a single retrovirus should facilitate its use in the study of gene function in a broad range of cell types.
Systems for regulating gene expression by means of cell-permeant inducing agents have many applications in the study of gene function. The ability to vary expression levels of a gene product at will and to monitor effects on cells or whole animals can give powerful insights into its biological role. Regulation also facilitates the study of genes encoding products whose constitutive expression would be detrimental or lethal to cells. Finally, systems for the small molecule control of gene expression are applicable to gene therapies that require intermittent therapeutic protein delivery or delivery at levels that fall within a narrow therapeutic window.
Several methods for the small molecule control of gene expression have been described including those induced by tetracycline, antiprogestins, and ecdysone (1). We have described a transcriptional switch based on rapamycin, a small molecule natural product that mediates the formation of heterodimers between the immunophilin FK506-binding protein (FKBP) and the lipid kinase homolog FRAP (2–5). By fusing FKBP domains to a DNA-binding domain called ZFHD1 and the rapamycin-binding domain of FRAP to a transcriptional activation domain from the p65 subunit of human NF-κB, reconstitution of a functional transcription factor and expression of a target gene can be made dependent on the presence of rapamycin (4). This system has been shown to regulate target gene transcription effectively in cell culture and in animals and is characterized by low basal expression levels and high dose-dependent inducibility with the addition of rapamycin (4, 6, 7).
To date, the target gene and transcription factor components of the system have been delivered on separate plasmids in vitro (4) or on separate adeno-associated virus or adenoviral vectors in vivo (6, 7). In cell culture, the generation of responsive cell lines requires the sequential stable integration of plasmids containing the transcription factors and target gene. This process can be laborious and time consuming and is not feasible in cell lines that are difficult to transfect. The ability to deliver all components in a single vector would facilitate the generation of inducible cell pools and clones in vitro and expand the range of applications for this gene regulation technology in vivo. To this end, we have incorporated the transcription factor and target gene components of the system into a single vector. Retroviral vectors were chosen for this purpose, because they combine the requisite insert capacity with an ability to deliver genes rapidly, efficiently, and stably to a broad range of cell types. Here we describe the development of an improved dimerizer-responsive transcription factor cassette and its incorporation, along with a regulatable target gene into a single retrovirus.
Materials and Methods
Plasmid Construction.
Full plasmid details are available on request. All retroviral vectors were constructed in pLXSN2, a derivative of pLXSN (ref. 8; CLONTECH) in which the pBR322 replication origin is replaced by a pUC replication origin. Transcription factor cassettes were inserted downstream of the 5′ long terminal repeat (LTR) in pLXSN2 and are constructed as described (4), except for the following. The minimal FRB domain of human FRAP contains a threonine-to-leucine mutation at amino acid 2,098 (6); the FRAP*-S3H fusion contains a chimeric activation domain comprising amino acids 281–551 of the p65 subunit of human NF-κB fused to amino acids 406–529 of the human heat shock factor 1 (HSF1) transcription factor (9, 10); and the FRAP*-AD fusions lack an epitope tag and contain an amino-terminal nuclear localization sequence derived from the human c-myc protein (amino acids 364–374; ref. 11). To reduce the likelihood of recombination during the retroviral life cycle, DNA-binding domain fusions (ZFn) containing multiple copies of FKBP were constructed by using nonidentical FKBP coding sequences bearing silent mutations.
L4+S3H-ZF1-S and L4-S3H-ZF1/2-S retroviral plasmids were constructed by inserting a secreted alkaline phosphatase (SEAP) reporter gene under control of a minimal IL-2 gene promoter and 12 ZFHD1 binding sites (Z12-IL-2-SEAP; ref. 4) between the neo gene and 3′ LTR of the appropriate transcription factor vector in the forward or reverse orientation, respectively. The Z12-IL-2-SEAP gene in L4-S3H-ZF1/2-S also includes the simian virus 40 polyadenylation sequence. L4-S3H-ZF2-DT-A was constructed by replacing the SEAP coding sequence in pL4-S3H-ZF2-S with amino acids 1–189 of the diphtheria toxin A chain (DT-A) coding sequence (isolated by the PCR from pDT201; ref. 12). The SEAP coding sequence from Z12-IL-2-SEAP (without the Z12-IL-2 promoter), or the enhanced green fluorescent protein (EGFP) coding sequence from pEGFP (CLONTECH) was inserted downstream from the LTR in pLXSN2 to construct pLS and pLE respectively.
Retrovirus Production and Infection.
Phoenix-Ampho packaging cells (obtained from Garry Nolan, Stanford University) were seeded at a density of 2× 106 cells per 60-mm dish and transfected by using FuGene reagent (Roche Molecular Biochemicals), according to the manufacturer's protocol. Retroviral supernatants were harvested 48 h after transfection and filtered through a 0.45-μM filter before infection. Cells were plated for infection at a density of 1.7× 105 cells per well in 6-well plates (1× 105 cells per well for Ba/F3). Retroviral supernatant (1 ml) was added to 3 ml of medium containing polybrene at a final concentration of 4 μg/ml. Cells were centrifuged at room temperature at 650 × g for 90 min before overnight incubation at 37oC. At 48 h after infection, cells were placed under selection in medium containing G418 at 500 μg/ml (HT1080) or 800 μg/ml (Ba/F3, NIH 3T3, and C2C12). Retroviral titers (in colony-forming unit/ml) were estimated based on G418-resistant colony formation in infected HT1080 cells and were found to be as follows: FRAP*-AD/ZFn transcription factor retroviruses, 1× 104; LE, 1× 105; L4+S3H-ZF1-S, 1× 104; L4-S3H-ZF1-S, L4-S3H-ZF2-S, and L4-S3H-ZF2-DT-A, 1× 103.
Cell Lines and Culture.
HT1080 (CCL1211), NIH 3T3 (CRL1658), and C2C12 (CRL1772) cells were obtained from the American Type Culture Collection. Ba/F3 cells were a gift from Bernard Mathy-Prevot (Harvard Medical School, Boston, MA). The HT1080L cell line contains a retrovirally integrated Z12-IL-2-SEAP reporter gene, as described (4). HT1080 cells were grown as described (4). NIH 3T3 and C2C12 cells were maintained in DMEM/10% (vol/vol) FBS, and Ba/F3 cells were cultured in RPMI medium 1640/10% (vol/vol) FBS in the presence of 1 ng/ml recombinant murine IL-3 (R & D Systems).
Measurement of Transcriptional Activation.
Addition of drugs and SEAP assays in Figs. 1B, 2B, and 3 were performed as described (4, 13). For the experiment shown in Fig. 4, cells were incubated in medium with or without 100 nM AP22565 for 24 h before washing and the addition of fresh medium (plus or minus AP22565). SEAP assays were performed after an additional 24 h as described, except that SEAP fluorescence units were converted to milliunits/ml by assaying samples alongside known concentrations of human placenta alkaline phosphatase (Calbiochem).
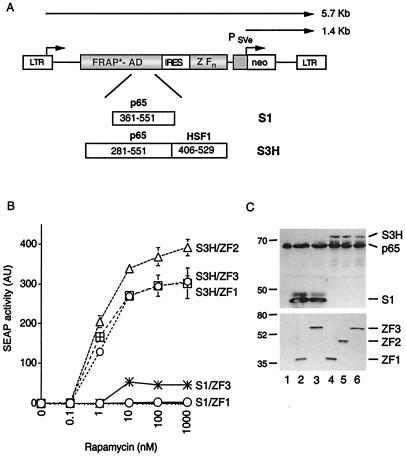
Figure 1.
Structure and activity of rapamycin-responsive transcription factor retroviruses. (A) Schematic representation of retroviral transcription factor expression vectors. The LTR drives expression of a bicistronic gene in which the first cistron encodes amino acids 2,021–2,113 of human FRAP fused to the S1 or S3H activation domain (FRAP*-AD, * indicates that the FRAP sequence contains a T2098L mutation). The second cistron, translated from an internal ribosome entry site (IRES), encodes ZFHD1 fused to one, two, or three copies of FKBP (ZFn). Retroviral vectors also contain a neo gene (neo) under the control of the simian virus 40 early promoter (PSVe). Viral transcripts and start sites of transcription are indicated by arrows (transcript sizes are for the S3H/ZF1 retrovirus). (B) Rapamycin-regulated gene expression in retrovirally infected cells. HT1080L cell pools stably transduced with transcription factor retrovirus were incubated with the indicated concentration of rapamycin for 24 h, and SEAP activity secreted into the growth medium was measured. Assays were performed in triplicate, and mean values (in arbitrary units, AU) ± SD were plotted. (C) Levels of transcription factor fusions in retrovirally infected pools. Activation domain (S1, S3H) and DNA-binding domain fusions (ZF1, ZF2, ZF3) were detected by immunoblotting extracts from retrovirally infected pools with anti-p65 antibody (Upper) or with anti-influenza hemagglutinin epitope HA.11 (Lower), respectively. p65 indicates endogenous p65 protein. Lane 1, uninfected HT1080 cells; lane 2, S1/ZF1 pool; lane 3, S1/ZF3 pool; lane 4, S3H/ZF1 pool; lane 5, S3H/ZF2 pool; and lane 6, S3H/ZF3 pool. Numbers to the left indicate molecular mass (in kDa).
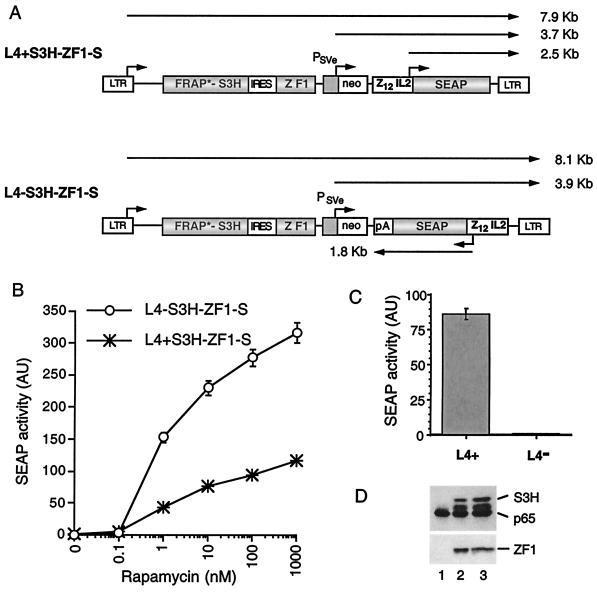
Figure 2.
Effect of target gene orientation on inducibility in the rapamycin-regulatable single virus system. (A) The structure of retroviruses containing the complete rapamycin-responsive gene expression system. Retroviral vectors L4+S3H-ZF1-S and L4-S3H-ZF1-S are similar to those described in Fig. 1, except that a regulatable SEAP target gene has been placed between the neo gene and the 3′ LTR. The SEAP gene is under the control of a minimal IL-2 gene promoter flanked by 12 copies of the ZFHD1 binding site (Z12IL2). The SEAP gene in L4-S3H-ZF1-S is followed by the polyadenylation signal of the simian virus 40 late gene (pA). IRES, internal ribosome entry site. (B) Rapamycin-regulated gene expression in cells infected with retroviruses containing the complete rapamycin system. Retrovirally infected cell pools were assayed for rapamycin-responsive SEAP gene expression as described in the legend to Fig. 1B. AU, arbitrary units. (C) SEAP gene expression in L4+S3H-ZF1-S and L4-S3H-ZF1-S infected pools in the absence of rapamycin. Cells were incubated in the absence of rapamycin for 24 h, and SEAP activity was assayed as before except that the duration of the SEAP reaction was extended to 18 h. (D) Levels of transcription factor fusions in L4+S3H-ZF1-S and L4-S3H-ZF1-S pools. FRAP*S3H and ZF1 expression levels were measured by immunoblotting as described in the legend to Fig. 1C. Lane 1, uninfected HT1080 cells; lane 2, L4-S3H-ZF1-S pool; lane 3, L4+S3H-ZF1-S pool.
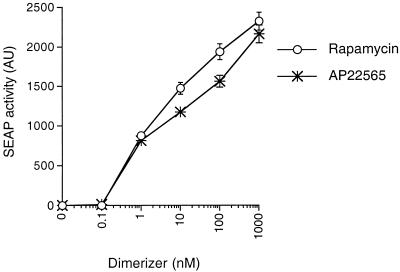
Figure 3.
Dose-responsive activation of gene expression by rapamycin and a rapamycin analog in L4-S3H-ZF1-S-infected HT1080 cells. SEAP activity secreted into the growth medium was measured after a 24-h incubation of cells with the indicated concentration of rapamycin or AP22565. SEAP gene expression was assayed as described in the legend to Fig. 1B, except that the growth medium was diluted 10-fold before incubation with SEAP reaction buffer. Assays were performed in triplicate, and mean values (in arbitrary units, AU) ± SD were plotted.
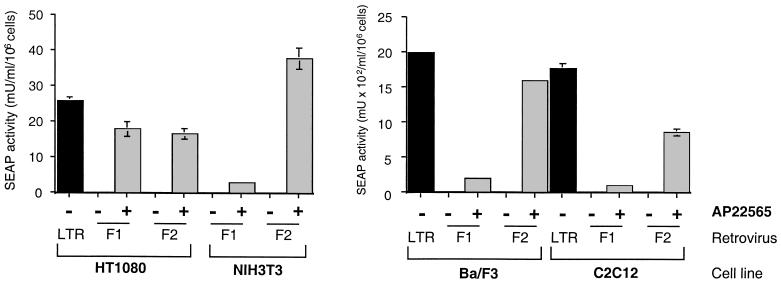
Figure 4.
Comparison of LTR-driven and AP22565-activated gene expression in different retrovirally infected cell lines. HT1080, NIH 3T3, Ba/F3, and C2C12 cells were infected with L4-S3H-ZF1-S (F1) or L4-S3H-ZF2-S (F2) retrovirus. HT1080, Ba/F3, and C2C12 cells were also infected with the constitutive LS (LTR) retrovirus. SEAP activity secreted into the growth medium was measured after a 48-h incubation in the presence (+) or absence (−) of 100 nM AP22565. SEAP assays were performed in triplicate, as described in the legend to Fig. 2B. To facilitate comparison between experiments performed at different times with different cell types, arbitrary units were converted to milliunits (mU)/ml by using a SEAP standard curve and expressed as milliunits/ml per 106 cells.
Immunoblot Analysis of Transcription Factors.
Whole cell extracts from infected cells were prepared, and equal amounts of protein were fractionated by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-NF-κB p65 rabbit polyclonal antibody (Santa Cruz Biotechnology) at 0.5 μg/ml or with anti-influenza hemagglutinin epitope HA.11 (Babco, Richmond, CA) at 1 μg/ml. Blots were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) diluted 1:10,000 and visualized by chemiluminescence (Renaissance, Dupont/NEN).
Crystal Violet Staining.
Medium was removed from the dishes, and cells were stained in a solution containing 0.1% (wt/vol) crystal violet and 20% (vol/vol) methanol for 15 min.
Results
Construction and Retroviral Delivery of Improved RapamycinRegulated Transcription Factors.
For retroviral delivery of rapamycin-responsive transcription factors, we assembled a series of LXSN-derived constructs, the basic structure of which is shown in Fig. 1A. Transcription factors are expressed from an LTR-driven transcription unit containing an internal ribosome entry site that permits expression of both transcription factor fusions from a single bicistronic message. The vector also contains an internal neomycin phosphotransferase expression cassette that allows stably transduced cells to be isolated by using G418 selection. To test whether cells stably transduced with retroviral transcription factor expression vectors can regulate gene expression in response to rapamycin, infectious retroviral particles were generated by transient packaging in Phoenix-Ampho cells. The resulting retroviral supernatants were used to infect the HT1080L cell line, which contains a retrovirally integrated ZFHD1 responsive SEAP reporter gene (4). After retroviral transduction, cells were placed under G418 selection, and the resulting G418-resistant pools were assayed for rapamycin-dependent SEAP expression.
In the previously described rapamycin system, FRAP is joined to amino acids 361–551 of human p65 (FRAP-S1), and ZFHD1 is linked to three copies of FKBP (ZF3; ref. 4). Cells expressing these transcription factor fusions induced SEAP expression after a 24-h incubation with rapamycin at concentrations of 10 nM or greater (S1/ZF3 in Fig. 1B). However, cells stably transduced with a retrovirus expressing FRAP-S1 in conjunction with ZFHD1 fused to a single FKBP (ZF1) failed to respond to rapamycin at any concentration (S1/ZF1 in Fig. 1B). We have recently generated a particularly strong hybrid activation domain, termed S3H, by joining the activation domain of human HSF1 (amino acids 406–529) to a longer portion of p65 (amino acids 281–551; S.N., unpublished results). To determine whether this improved activation domain could enhance the performance of the retrovirally delivered system, we constructed vectors containing FRAP-S3H activation domain fusions. Cells were stably transduced with transcription factor retroviruses expressing FRAP-S3H in conjunction with ZF1, ZF2, or ZF3. All FRAP-S3H-expressing pools gave maximal rapamycin-induced SEAP levels that were at least 6-fold greater than the level of the FRAP-S1/ZF3-expressing pool (S3H/ZF1, S3H/ZF2, and S3H/ZF3 in Fig. 1B). The improved cassette also enhanced potency, such that half-maximal levels of SEAP expression were obtained at a rapamycin concentration of about 1 nM. Furthermore, in contrast to the original system, removal of one or more FKBP domains from the DNA-binding domain fusion did not reduce rapamycin-responsiveness significantly in HT1080 cells. In fact, the S3H/ZF2-expressing pool showed slightly better rapamycin-responsiveness than either the S3H/ZF1 or S3H/ZF3 pool. Expression levels of the DNA-binding domain fusions in all pools were equivalent (Fig. 1C Lower); however, expression levels of fusions containing the strong S3H activation domain were lower than those containing the S1 domain (Fig. 1C Upper; compare lanes 4, 5, and 6 with lanes 2 and 3). This observation is consistent with previous reports demonstrating an inverse correlation between activator potency and steady-state expression levels (14, 15).
Construction and Characterization of Retroviral Vectors Incorporating Both the Transcription Factors and Target Gene.
We next wanted to incorporate the improved transcription factor cassette into vectors that also contained a regulatable target gene. We chose to use the ZF1 DNA-binding domain in conjunction with FRAP-S3H, because it allowed us to reduce the size of the transcription factor cassette without significantly impairing performance in HT1080 cells. The FRAP-S3H/ZF1 transcription factor retrovirus (Fig. 1A) was modified to allow the insertion of the regulatable SEAP target gene between the neo gene and the 3′ LTR (see Materials and Methods). In retroviruses bearing multiple genes, the inducibility and basal expression levels of the regulatable target gene may be affected by interactions with other transcription units within the provirus. We reasoned that effects caused by transcriptional read-through or proximity to other transcriptional control elements would differ according to the orientation of the regulatable target gene. We therefore decided to test the target gene in each orientation (relative to viral transcription) by constructing the two vectors shown in Fig. 2A. L4+S3H-ZF1-S contains a ZFHD1-responsive SEAP reporter gene inserted so that SEAP transcription occurs in the same direction as viral transcription. L4-S3H-ZF1-S contains the SEAP reporter gene in the antisense orientation relative to viral transcription. This vector also incorporates a polyadenylation signal in the SEAP transcription unit. Transiently packaged retroviral supernatant was prepared for each vector and used to infect HT1080 cells under conditions in which only a small percentage (10% or less) of cells was transduced. This approach ensured that the majority of infected cells in the pool contained a single integrated provirus. The titer of the L4-S3H-F1-S virus was found to be 10-fold lower than that of the L4+S3H-F1-S virus and 100-fold lower than that of LE, a simple LXSN-derived virus containing an LTR-driven enhanced green fluorescent protein gene (see Materials and Methods). This result suggests that the orientation of transcription units, as well as their size, can affect viral titer. After selection of G418-resistant pools, stably transduced cells were incubated for 24 h with increasing concentrations of rapamycin before SEAP assay. Both the L4+S3H-ZF1-S- and L4-S3H-ZF1-S-infected pools displayed a dose-dependent increase of SEAP expression in response to rapamycin (Fig. 2B). The L4+S3H-ZF1 pool gave lower induced SEAP expression levels than the L4-S3H-ZF1-S pool and also gave rise to detectable SEAP expression in the absence of rapamycin. This basal expression is clearly evident in Fig. 2C, which shows SEAP activity in reactions that were allowed to incubate overnight. Overall, this resulted in a relatively low 30-fold induction for the L4+S3H-ZF1 pool. In contrast, the L4-S3H-ZF1 pool induced SEAP expression to high levels in the presence of rapamycin with little or no basal expression (Fig. 2 B and C). The L4-S3H-ZF1 pool therefore displayed much tighter regulation with an induction of at least 300-fold. Both pools expressed similar amounts of the transcription factor fusions (Fig. 2D). Based on these results, the L4-S3H-ZF1-S retrovirus seemed to be the optimally configured vector and was analyzed further.
Induction Characteristics of an L4-S3H-ZF1-S-Transduced Pool in Response to Rapamycin and a Nonimmunosuppressive Rapamycin Derivative.
We next sought to examine the induction characteristics of the L4-S3H-ZF1-transduced pool in greater detail; in particular, we wanted to confirm the lack of detectable SEAP expression in the absence of rapamycin. Rapamycin treatment and SEAP assays were performed as before, except that samples from cells incubated with rapamycin were diluted 10-fold relative to samples from cells incubated without rapamycin. We also increased the duration of the SEAP assay 8-fold. These modifications effectively amplify any basal SEAP expression that may occur in the absence of inducer. Under these conditions, basal SEAP expression remained undetectable, with 2,000 arbitrary fluorescence units being produced at 100 nM rapamycin (Fig. 3), leading to an induction ratio of at least three orders of magnitude. SEAP activity was detectable in the culture medium 8 h after rapamycin addition, and induction kinetics were similar to those described for the related FK1012-inducible gene expression system (ref. 5; data not shown). Analysis of 10 individual G418-resistant clones revealed that 5 of them induced SEAP expression to levels equal to or greater than the parental pool and displayed little or no basal expression in the absence of inducer (data not shown).
We have recently synthesized rapamycin derivatives that cannot interact with endogenous FRAP and are therefore free of the immunosuppressive and cell-cycle inhibitory effects of rapamycin itself (L. W. Rozamus, W. Yang, T.C., and D. A. Holt, unpublished results; details available on request). The FRAP coding sequence of all constructs described in this study incorporates a mutation that alters the rapamycin-binding interface to allow binding to these derivatives (see legend to Fig. 1A and Materials and Methods). Cells transduced with these constructs are therefore able to induce gene expression in response to the nonimmunosuppressive rapamycin derivatives as well as rapamycin.
Fig. 3 shows that one such nonimmunosuppressive rapamycin derivative, AP22565, is able to induce SEAP expression in the L4-S3H-ZF1-S transduced pool almost as well as rapamycin. All further experiments were performed therefore by using AP22565 as the inducer.
Comparison of Inducible and LTR-Driven Expression Levels in Different Cell Types.
We wanted to assess the performance of the single retrovirus rapamycin system in a range of cell types and to compare levels of induced gene expression with those obtained from a constitutive viral enhancer. The L4-S3H-ZF1-S retrovirus was used to infect the following cell lines: NIH 3T3 (murine fibroblasts), Ba/F3 (murine pre-B lymphocytes), and C2C12 (murine myoblasts). HT1080, Ba/F3, and C2C12 cells were transduced in parallel with LS, an LXSN-derived retroviral vector containing a SEAP gene under the control of the constitutive MoMLV LTR. L4-S3H-ZF1-S pools undergo a lag time between addition of inducer and secretion of SEAP into the medium, during which transcriptional initiation, mRNA synthesis, and protein translation occur. Therefore, to provide an appropriate comparison with constitutively expressing LS pools, cells were incubated (in the presence or absence of AP22565) for 24 h before washing and replacing with fresh medium (plus or minus AP22565) for an additional 24 h before assaying for SEAP.
In HT1080 cells stably transduced with L4-S3H-ZF1-S, the level of SEAP expression in the presence of 100 nM AP22565 was comparable to constitutive LTR-driven SEAP expression (see Fig. 4, compare HT1080 LTR with HT1080 F1 + AP22565). In contrast, when the L4-S3H-ZF1-S retrovirus was used to infect NIH 3T3, Ba/F3, or C2C12 cells, induced levels of SEAP expression were very low (Fig. 4, NIH 3T3, Ba/F3, C2C12, lanes F1 + AP22565). In the original rapamycin system, there is a strict requirement for multiple FKBPs on the DNA-binding domain fusion (ref. 4; Fig. 1B). Therefore, although ZF1 suffices for efficient activation in HT1080 cells, we reasoned that adding an additional FKBP to the DNA-binding domain fusion might improve the performance of the system in the refractory cell types. We constructed a second retrovirus incorporating a DNA-binding domain fusion with two copies of FKBP (L4-S3H-ZF2-S). This virus was used to infect each cell line. In HT1080 cells, pools made by using the L4-S3H-ZF1-S and L4-S3H-ZF2-S retroviruses performed similarly in response to AP22565 (Fig. 4, compare HT1080 F1 + and F2 +). This observation is consistent with results obtained with retroviruses bearing transcription factors alone in this cell type (see Fig. 1). In contrast, use of the L4-S3H-ZF2-S retrovirus dramatically improved the performance of the system in NIH 3T3, Ba/F3, and C2C12 cells. In each L4-S3H-ZF2-S-infected cell type, basal expression was undetectable, and SEAP levels in the presence of 100 nM AP22565 were comparable to those obtained with a constitutive LTR-driven reporter (Fig. 4, compare LTR with F2 + AP22565 in NIH 3T3, Ba/F3, and C2C12). These results show that the single retrovirus rapamycin system supports robust, tightly regulated transcription in several different cell types.
Inducible Expression of the DT-A Gene.
To demonstrate the usefulness and stringency of the dimerizer-responsive retroviral system, we set out to establish stable cell lines capable of inducible expression of the highly toxic DT-A protein (16). To generate the L4-S3H-ZF2-DT-A retroviral plasmid, the SEAP gene in L4-S3H-ZF2-S was replaced with DT-A. Transiently packaged L4-S3H-ZF2-DT-A retroviral supernatant was used to infect HT1080 cells. A parallel infection was performed with a retrovirus bearing a nontoxic (enhanced green fluorescent protein) target gene. Both infections gave rise to several hundred G418-resistant clones. The similarity of the L4-S3H-ZF2-DT-A titer to the nontoxic control suggests that basal DT-A expression did not occur in the majority of L4-S3H-ZF2-DT-A transduced cells. Infected L4-S3H-ZF2-DT-A clones (n = 26) were expanded and tested for inducible DT-A expression by plating each clone in the presence or absence of 10 nM AP22565 and monitoring cell growth over a period of 5 days. A dramatic response was given by 11 clones (80–100% cell death) after a 5-day incubation in the presence of AP22565, indicating that these clones supported inducible DT-A expression. The effect of AP22565 on two of the highly responsive clones is shown in Fig. 5. A 5-day incubation with AP22565 had no effect on growth of a cell line transduced with a transcription factor retrovirus lacking the DT-A target gene (Fig. 5). These results demonstrate that regulation with the rapamycin-responsive retrovirus is stringent enough to allow inducible expression of extremely cytotoxic proteins.
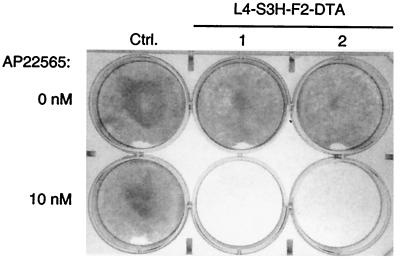
Figure 5.
Inducible DT-A chain expression in L4-S3H-ZF2-DT-A transduced clones. Two L4-S3H-ZF2-DT-A transduced clones and a control (Ctrl.) clone bearing transcription factors but lacking the inducible DT-A gene were plated in 6-well dishes (4× 104 cells per well) and grown for 5 days in the presence or absence of 10 nM AP22565 before staining with crystal violet.
Discussion
We have improved the transcription factor components of the rapamycin-regulated gene expression system and combined them with a regulated target gene in a single dimerizer-responsive retrovirus. The single virus system displayed high induction ratios in response to rapamycin, functioned well in multiple cell types, and has several advantages over the previously described two-vector system. First, all components of the regulatory system are delivered to cells in a single step, obviating the need for sequential transfection steps to introduce each of two separate plasmids stably. Second, retroviral infection of cells is more efficient than stable plasmid transfection; cell pools consisting of hundreds or thousands of independent transduction events can be generated rapidly. Third, delivery by means of retroviral infection extends the range of target cells in which gene function can be studied beyond those cell types that can be efficiently transfected. In addition, the vectors described here incorporate a modified FRAP sequence that has allowed us to induce gene expression with nonimmunosuppressive rapamycin analogs.
A key improvement in the system described here is the use of a highly potent activation domain, which increased both the dose-responsiveness and maximal levels of induced gene expression. Such increases were found to be particularly advantageous in a retroviral context, where low copy number generally results in lower expression levels than those achieved with plasmid vectors (data not shown). The S3H domain was constructed from sequences derived from the activation domains of the human p65 and HSF1 transcription factors. These subdomains seemed to act synergistically (perhaps by contacting separate components of the transcriptional initiation machinery; ref. 17), because tandem duplications of either the p65 or HSF1 sequence did not give the same level of activation as S3H (data not shown).
We found that the single virus system worked best when the target gene was placed in the antisense orientation relative to viral transcription. In this configuration, target gene expression could be induced to levels comparable to those obtained with a constitutive LTR, with little or no basal expression. In fact, basal levels were low enough to allow the generation of cell lines bearing an inducibly expressed DT-A gene, a protein reported to be lethal at concentrations as low as one molecule per cell (16). This lack of “leakiness” was observed in cell pools representing hundreds of independent integration events and occurred despite the proximity of the regulated IL-2 promoter to the 3′ proviral LTR. These findings suggest that the minimal IL-2 promoter lacks regulatory elements required for the LTR or nearby cellular enhancers to activate transcription. It is also possible that any basal expression of the SEAP gene in the absence of rapamycin is subject to antisense inhibition by constitutively expressed genomic viral transcripts. This explanation has been invoked to explain lowered basal-gene expression in a similarly configured tetracycline-responsive retrovirus (18). If such antisense inhibition does occur, however, it does not seem to interfere with high level target-gene expression on rapamycin addition. We obtained induction ratios of at least three orders of magnitude with the L4-S3H-ZF1-S retrovirus with the addition of rapamycin or AP22565. This result compares favorably with reported tetracycline-responsive single retrovirus systems (18–23).
Although the ZF1-DNA binding domain functioned as well as ZF2 when assayed in HT1080 cells, this was not true for the other cell types examined. In NIH 3T3, Ba/F3, and C2C12 myoblasts, the ZF2 DNA-binding domain gave much more efficient rapamycin-dependent gene expression, suggesting that L4-S3H-ZF2-based vectors will be of more general use. The reasons for this difference are not clear but may be linked to lower levels of FRAP-S3H expression in at least one of the refractory cell types (C2C12; data not shown). One explanation may be that under conditions of limiting activation domain fusion, an avidity effect provided by fusing multiple copies of FKBP to the DNA-binding domain becomes important for efficient transcription factor reconstitution and subsequent gene activation.
The dimerizer-inducible retrovirus should prove useful in studies of gene function, particularly those encoding proteins that promote cell death, block the cell cycle, or are otherwise toxic. In addition, similarly configured vectors may ultimately find a role in gene therapy applications involving retroviral and perhaps lentiviral delivery of therapeutic genes that can be controlled by small molecules.
Acknowledgments
We are grateful to Dale Talbot for synthesis of nonhomologous FKBP coding sequences, Len Rozamus and Wu Yang for providing AP22565, and Bob Kingston for providing a plasmid containing the HSF1 coding sequence. G. R. Crabtree is a consultant to, and has equity in, ARIAD Pharmaceuticals Inc. Reagents described in this study, as well as a polylinker version of L4-S3H-ZF2, are being distributed to the research community and are available on request through our web site at www.ariad.com.
Abbreviations
- DT-A
diphtheria toxin A chain
- LTR
long terminal repeat
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230446297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230446297
References
- 1.Clackson T. Curr Opin Chem Biol. 1997;1:210–218. doi: 10.1016/s1367-5931(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 2.Standaert R F, Galat A, Verdine G L, Schreiber S L. Nature (London) 1990;346:671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- 3.Brown E J, Albers M W, Shin T B, Ichikawa K, Keith C T, Lane W S, Schreiber S L. Nature (London) 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 4.Rivera V M, Clackson T, Natesan S, Pollock R, Amara J F, Keenan T, Magari S R, Phillips T, Courage N L, Cerasoli F, Jr, et al. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 5.Ho S N, Biggar S R, Spencer D M, Schreiber S L, Crabtree G R. Nature (London) 1996;382:822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 6.Rivera V M, Ye X, Courage N L, Sachar J, Cerasoli F, Jr, Wilson J M, Gilman M. Proc Natl Acad Sci USA. 1999;96:8657–8662. doi: 10.1073/pnas.96.15.8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Rivera V M, Zoltick P, Cerasoli F, Jr, Schnell M A, Gao G, Hughes J V, Gilman M, Wilson J M. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 8.Miller A D, Rosman G J. BioTechniques. 1989;7:980–982. , 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz M L, Baeuerle P A. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabindran S K, Giorgi G, Clos J, Wu C. Proc Natl Acad Sci USA. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang C V, Lee W M. Mol Cell Biol. 1988;8:4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong D, Coleman K D, Murphy J R. Science. 1983;220:515–517. doi: 10.1126/science.6403984. [DOI] [PubMed] [Google Scholar]
- 13.Amara J F, Clackson T, Rivera V M, Guo T, Keenan T, Natesan S, Pollock R, Yang W, Courage N L, Holt D A, et al. Proc Natl Acad Sci USA. 1997;94:10618–10623. doi: 10.1073/pnas.94.20.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molinari E, Gilman M, Natesan S. EMBO J. 1999;18:6439–6447. doi: 10.1093/emboj/18.22.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salghetti S E, Muratani M, Wijnen H, Futcher B, Tansey W P. Proc Natl Acad Sci USA. 2000;97:3118–3123. doi: 10.1073/pnas.050007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaizumi M, Mekada E, Uchida T, Okada Y. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 17.Ptashne M, Gann A. Nature (London) 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 18.Paulus W, Baur I, Boyce F M, Breakefield X O, Reeves S A. J Virol. 1996;70:62–67. doi: 10.1128/jvi.70.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann A, Nolan G P, Blau H M. Proc Natl Acad Sci USA. 1996;93:5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshimaru M, Ray J, Sah D W, Gage F H. Proc Natl Acad Sci USA. 1996;93:1518–1523. doi: 10.1073/pnas.93.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang J J, Scuric Z, Anderson W F. J Virol. 1996;70:8138–8141. doi: 10.1128/jvi.70.11.8138-8141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida A, Chen S T, Friedmann T, Yee J K. J Virol. 1996;70:6054–6059. doi: 10.1128/jvi.70.9.6054-6059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindemann D, Patriquin E, Feng S, Mulligan R C. Mol Med. 1997;3:466–476. [PMC free article] [PubMed] [Google Scholar]