Abstract
Purpose
To study the time spent with radiation-induced dermatitis during a course of radiation therapy for breast cancer in women treated with conventional or intensity-modulated radiation therapy (IMRT).
Materials and methods
The study population consisted of 804 consecutive women with early-stage breast cancer treated with breast-conserving surgery and radiation from 2001 – 2006. All patients were treated with whole-breast radiation followed by a boost to the tumor bed. Whole-breast radiation consisted of conventional wedged photon tangents (n=405) earlier in the study period and mostly of photon IMRT (n=399) in later years. All patients had acute dermatitis graded each week of treatment.
Results
The breakdown of the cases of maximum acute dermatitis by grade was as follows: 3%, grade 0; 34%, grade 1; 61%, grade 2; and 2%, grade 3. The breakdown of cases of maximum toxicity by technique was as follows: 48%, grade 0/1, and 52%, grade 2/3, for IMRT, and 25%, grade 0/1, and 75%, grade 2/3, for conventional radiation therapy (p<0.0001). IMRT patients spent 82% of weeks during treatment with grade 0/1 dermatitis and 18% with grade 2/3 dermatitis, compared with 29% and 71% of patients, respectively, treated with conventional radiation (p<0.0001). Further, the time spent with grade 2/3 toxicity was decreased in IMRT patients with small (p=0.0015), medium (p<0.0001), and large (p<0.0001) breasts.
Conclusions
Breast IMRT is associated with both a significant decrease in the time spent during treatment with grade 2/3 dermatitis and in the maximum severity of dermatitis compared with conventional radiation regardless of breast size.
Keywords: Breast cancer, radiation therapy, IMRT
Introduction
Breast-conserving surgery and radiation therapy are the standard alternatives to mastectomy for eligible patients with Stage 0, I, or II invasive breast cancer 1, 2. Postoperative whole-breast radiation therapy is associated with long-term local control on the order of 85–95% with survival outcomes equivalent to those seen in women who undergo mastectomy 3–5. Postoperative radiation therapy for invasive breast cancer has also been associated with improved local control 4, 6 and overall survival 7 compared to breast-conserving surgery alone.
Despite this success, there is room for improvement in conventional tangential breast radiation therapy, which is often associated with a greater than 10% dose inhomogeneity across the target volume, which may in turn increase the incidence of acute side effects and negatively impact the long-term cosmesis that is a particular benefit of treatment 8–11. In particular, the increase in dose inhomogeneity is associated with acute skin toxicity that increases in severity with increasing breast size 9, 12. Acute dermatitis, including moist desquamation of the skin during or within 6 weeks of radiation, is seen in 30–50% of women treated with conventional radiation therapy 13, 14. Moist desquamation is associated with decreased quality of life during radiation treatment 14. The dose inhomogeneity associated with conventional radiation, in which the target receives significantly greater doses than the doses prescribed, could also result in an increased incidence of long-term complications. For example, a worse long-term cosmetic outcome and a greater complication rate have been associated with doses above 50 Gy or a daily dose per fraction of 2.5 Gy or higher delivered using conventional radiation therapy 15–17.
Intensity-modulated radiation therapy (IMRT) involves the use of optimized non-uniform radiation beam intensities to create more conformal dose distributions around the targets of irradiation. IMRT involves many aspects already established as a part of 3D conformal radiation: patient immobilization, the definition of target volumes and organs and normal structures in three dimensions by drawing contours on cross-sectional images using CT simulation, the optimization of treatment plans based upon isodose coverage of target structures, and multi-leaf collimation. However, IMRT also requires more advanced radiation therapy treatment planning that depends on computer algorithms for inverse dose planning. IMRT also has special physics requirements, including the requirement for new protocols for acceptance testing, commissioning, and rigorous quality assurance. Initial experiences with IMRT for breast cancer have shown it is associated with improved dose distributions in the treated breast, lower doses delivered to normal heart or lung tissue, a low incidence of acute toxicity, and a reduced incidence of subacute complications such as breast edema and of undesirable cosmetic changes compared with standard techniques 18–25.
All of these previous prospective and retrospective studies of breast IMRT have looked at the incidence of acute dermatitis only in terms of maximum grade occurring during a course of radiation therapy. Given that a typical course of adjuvant radiation therapy for breast cancer lasts 6 weeks, the reporting of only the maximally occurring grade of acute dermatitis could obscure large differences in the patient experience with that toxicity. For example, although 2 patients may have grade 2 dermatitis, in 1 patient this may occur only in week 5 of treatment and in the other patient in weeks 3 through 6 of treatment. We hypothesized that this difference in the onset and duration of acute toxicity is a more important determinant of quality of life during treatment than whether a patient experiences grade 2 toxicity at all.
This study is the first to our knowledge to analyze the incidence of acute dermatitis separately during each week of a course of radiation therapy for breast cancer. We also examined the respective effects of IMRT and wedged tangential irradiation on both the incidence of radiation dermatitis and the time spent with grade 2 or higher radiation dermatitis.
Methods and Materials
In this retrospective study, the study population consisted of 804 consecutive women with early-stage breast cancer treated with breast-conserving surgery and radiation therapy from 2001 – 2006. Inclusion criteria were that the patient have American Joint Committee on Cancer stages 0, I, or II breast cancer 26; received radiation therapy at the Fox Chase Cancer Center, and completed radiation therapy. Exclusion criteria included male breast cancer, T3-T4 disease, stage IV disease, mastectomy, or the patient’s treatment did not include radiation. Patient demographics, tumor characteristics, and treatment-related information were entered prospectively into a database that was maintained and updated by a single data manager. The collection, storage, and retrieval of data were all done in compliance with the hospital’s Institutional Review Board and the Health Insurance Privacy and Portability Act.
All patients were treated with whole-breast radiation (46–50 Gy), with or without regional nodal radiation, and a boost to the tumor bed (10–18 Gy). The total dose was generally determined by the extent of surgery and the final margin status and ranged from a median of 60 Gy for a negative margin to 64 Gy for a close margin to 66 Gy for a positive final margin. Whole-breast radiation therapy consisted of conventional wedged photon tangents (n=405) earlier in the study period, while the majority of patients in later years received photon IMRT (n=399). Details of the conventional radiation treatment policy during this study period have been previously described 27. In summary, all conventionally treated patients were treated with breast tangents to a median dose of 46 Gy. A 6 MV linear accelerator was used in most patients. The primary tumor bed was boosted in 99% of patients, and this boost was delivered with electrons in almost all patients.
The IMRT technique consisted of a combination of open and segmented tangential fields using volume-based inverse dose planning and step-and-shoot beam delivery. Patients underwent simulation with a dedicated CT scanner and conventional fluoroscopy to define the clinical target volume (CTV) of the breast tissue and normal structures. Patients were placed in an alpha-cradle cast on a 10–20% wedged breast board for set-up reproducibility. The physician defined the CTV as the palpable breast tissue anterior to the chest wall to within 5 mm of the skin, and with a margin of 2 cm in the superior, inferior, and lateral directions. To facilitate the transition to IMRT and to make as valid a comparison as possible, the definition of the CTV by the physician, patient positioning, tangential beam orientation, and field sizes were kept the same as possible as those used for the previous conventional tangential photon technique used at our institution. Patients have been prescribed a standard fractionation dose of 2 Gy per day to a total of 46–50 Gy over 4 ½-5 weeks with the IMRT. This is followed by a sequential electron boost to 14 – 20 Gy to the tumor bed and scar. Treatment energy depended on patient chest wall separation.
Because of the differences in the years of treatment between IMRT and conventional radiation treatment, there were some differences in the use of systemic therapies as well (Table 2). Specifically, more patients receiving IMRT than conventional radiation therapy had chemotherapy, but sequencing was done prior to radiation therapy in most cases in both groups. For conventional radiation patients, chemotherapy consisted of adriamycin/cytoxan in 58 patients, adriamycin/cytoxan/taxane in 45 patients, other adriamycin-based regimens in 8 patients, and other regimens in 5 patients. For IMRT patients, these chemotherapy types were given in 60, 76, 16, and 7 patients, respectively. Another difference in treatment practice over time was that most IMRT patients began tamoxifen after radiation, while half of the conventional treatment patients started tamoxifen concurrently with radiation.
Table 2.
Patient characteristics. (numbers in table represent number of patients)
| Conventional | IMRT | p value | |
|---|---|---|---|
| Number of Patients | 405 | 399 | |
| Bra Size | < 0.0001 | ||
| Small (32; 34A,B; 36A) | 56 | 43 | |
| Medium (34C; 36B,C; 38A,B,C) | 151 | 161 | |
| Large (any D or size 40+) | 105 | 145 | |
| Unknown | 93 | 50 | |
| Chest wall separation | 0.002 | ||
| Mean | 21.4 | 22.2 | |
| Range | 13.2 – 32.6 | 15.6 – 32.5 | |
| Tumor stage | 0.02 | ||
| Tis | 66 | 65 | |
| T1 | 289 | 256 | |
| T2 | 50 | 78 | |
| Nodal stage | NS | ||
| N0 | 228 | 250 | |
| N1-3 | 90 | 77 | |
| N4+ | 19 | 11 | |
| NX | 68 | 61 | |
| Breast dose (Gy) | NS | ||
| Median | 46 | 46 | |
| Range | 40 – 50.4 | 34 – 60 | |
| Total dose (Gy) | NS | ||
| Median | 60 | 60 | |
| Range | 50 – 66 | 34 – 66 | |
| Energy (MV) | <0.0001 | ||
| 6 | 219 | 165 | |
| 10 | 131 | 194 | |
| 18 | 55 | 38 | |
| Chemotherapy | 0.005 | ||
| No | 274 | 228 | |
| Yes – before radiation | 116 | 159 | |
| Yes – concurrent with radiation | 4 | 1 | |
| Yes – after radiation | 11 | 11 | |
| Tamoxifen | 0.006 | ||
| No | 161 | 143 | |
| Yes – before and/or concurrent | 22 | 7 | |
| Yes – after radiation | 222 | 249 | |
NS = not statistically significant at the 0.05 level
The study endpoint was acute skin toxicity. The maximum toxicity was scored for each patient during and within 6 weeks of radiation using the common terminology criteria for adverse events (CTC), version 3, for acute radiation dermatitis (Table 1) 28. A physician and nurse prospectively evaluated skin toxicity weekly during radiation therapy at standard on-treatment patient visits. A nursing flow sheet was used for all patients that recorded maximum skin toxicity each week, including scores for skin color and integrity. Breast size was grouped according to bra size as small (34A,B; 36A), medium (34C; 36B,C; 38A,B,C), or large (any D or size 40+) according to a definition used in the RTOG 97–13 trial 14. Chi-square test, Wilcoxon test, and generalized estimating equations were used for univariate and multivariate analyses.
Table 1.
Common Terminology Criteria for Adverse Events for Acute Radiation Dermatitis Grade Acute Dermatitis Scoring
| Grade | Acute Dermatitis Scoring |
|---|---|
| 0 | No change |
| 1 | Faint erythema or dry desquamation |
| 2 | Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema |
| 3 | Moist desquamation other than skin folds and creases; bleeding induced by minor trauma or abrasion |
| 4 | Skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site |
| 5 | Death |
Results
Patient characteristics are shown in Table 2. There were 405 patients treated with conventional radiation and 399 patients treated with IMRT. Significant differences were seen between groups in terms of breast size, chest wall separation, tumor stage, treatment energy, use of chemotherapy prior to radiation, and use and timing of tamoxifen. There were no significant differences in nodal stage, whole-breast dose, or total dose, which consisted of the whole-breast plus boost dose.
In terms of the maximum acute dermatitis seen during treatment in all patients, 3% showed grade 0, 34% grade 1, 61% grade 2, and 2% grade 3 dermatitis. The maximum toxicity by technique was as follows: grade 0/1 in 48% of patients and grade 2/3 in 52% of patients for IMRT, and grade 0/1 in 25% of patients and grade 2/3 in 75% of patients for conventional radiation (Table 3). The maximum dermatitis by breast size subgroup and by technique is also shown in Table 3. Subgroup analysis showed that the time spent with grade 2/3 toxicity was decreased in IMRT patients with small (p=0.0015), medium (p<0.0001), and large (p<0.0001) breasts.
Table 3.
Maximum acute dermatitis by radiation technique and breast size. Numbers in parentheses are actual number of patients.
| Breast Size | Technique | # | Grade 0/1 | Grade 2/3 | p value |
|---|---|---|---|---|---|
| Small | 0.004 | ||||
| Conventional | 56 | 40% (22) | 60% (34) | ||
| IMRT | 43 | 70% (30) | 30% (13) | ||
| Medium | <0.0001 | ||||
| Conventional | 151 | 30% (45) | 70% (106) | ||
| IMRT | 161 | 59% (95) | 41% (66) | ||
| Large | <0.0001 | ||||
| Conventional | 105 | 10% (10) | 90% (95) | ||
| IMRT | 145 | 32% (47) | 68% (98) | ||
| All Patients | <0.0001 | ||||
| Conventional | 405 | 25% (101) | 75% (304) | ||
| IMRT | 399 | 48% (192) | 52% (207) |
The odds ratio was 2 for grade 2/3 toxicity for conventional radiation compared with IMRT, which was significant on multivariate analysis correcting for the other significant variables (Table 4). Other variables shown to be significant on multivariate analysis were large or unknown breast size compared with small breast size, increasing week of treatment, increasing chest wall separation, and use of chemotherapy or tamoxifen.
Table 4.
Multivariate analysis for significant predictors of grade 2 or higher radiation dermatitis.
| Variable | Comparison | Odds Ratio | 95% range | p value |
|---|---|---|---|---|
| Technique | Conventional vs. IMRT | 2.03 | 1.50–2.75 | < 0.001 |
| BRA size | Medium vs. small | 1.50 | 0.94–2.40 | 0.093 |
| BRA | Large vs. small | 2.73 | 1.63–4.60 | < 0.001 |
| BRA | Unknown vs. small | 2.07 | 1.22–3.52 | 0.007 |
| Treatment week | Continuous variable | 2.36 | 2.21–2.51 | < 0.001 |
| Energy (MV) | 10 vs. 6 | 0.91 | 0.62–1.32 | 0.615 |
| Energy (MV) | 18 vs. 6 | 1.21 | 0.69–2.12 | 0.496 |
| Chestwall size | Continuous variable | 1.06 | 1.00–1.12 | 0.059 |
| Chemotherapy* | Yes vs. No | 1.86 | 1.11–3.12 | 0.018 |
| Tamoxifen* | No vs. Yes | 1.40 | 1.05–1.86 | 0.021 |
Yes = chemotherapy or tamoxifen prior to or during radiation. No = none or only after radiation was completed.
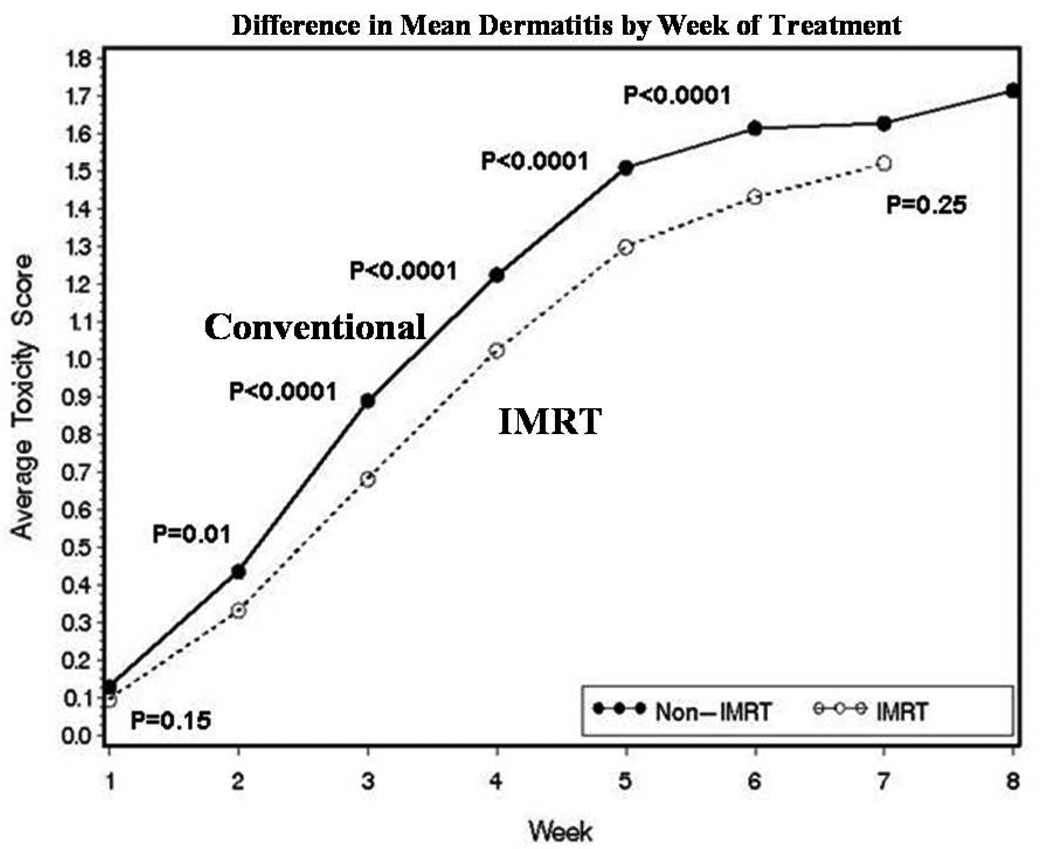
The mean grade of dermatitis was reduced during all weeks of treatment in IMRT patients compared to conventional radiation patients, and the difference was statistically significant for weeks 2–6 (Fig. 1). The time spent per week of radiation with grade 2/3 dermatitis was also lower in the IMRT patients. Further, 82% of weeks in IMRT patients were spent with grade 0/1 and 18% with grade 2/3 dermatitis, compared with 29% and 71%, respectively, in conventional radiation patients (p<0.0001).
Figure 1.
Mean frequency of dermatitis by week of treatment during radiation therapy for conventional (n=405) and IMRT (n=399) treated patients.
The frequency of grade 2/3 toxicity when chemotherapy preceded radiation was 27% without chemotherapy vs. 19% with chemotherapy. However, this may be confounded due to a large imbalance in the number of women with large breasts treated with chemotherapy (n = 73) as opposed to no chemotherapy (n = 177). In a two-by-two comparison, toxicity grade was consistently greatest each week in patients treated with conventional radiation and no chemotherapy and lowest in patients treated with IMRT and chemotherapy, with the toxicity grade in the other two groups falling in between these two extremes.
Discussion
This study of over 800 patients treated for breast cancer is unique in assessing the degree of skin toxicity each week during a course of radiation therapy as opposed to only the maximum degree of toxicity. We observed that IMRT reduces the incidence of grade ≥ 2 dermatitis in women of all breast sizes, which prior studies have also shown. However, we also found that IMRT reduced the absolute number of weeks spent with this degree of acute dermatitis. In particular, women treated with IMRT experienced grade 2 toxicity approximately 1 to 2 weeks later during their course of therapy, or not at all, than did women treated with conventional tangential radiation. In addition, IMRT patients spent the majority of their weeks of treatment with grade 0–1 toxicity, as opposed to conventionally treated patients, who spent the majority of their weeks with grade 2 dermatitis. In this way, IMRT may be associated with an overall better quality of life throughout a course of treatment, a difference that could be obscured if the maximal toxicity is compared only in the last week of radiation. Other determinants of acute dermatitis in this study were increasing breast size and increasing radiation dose each week of treatment. While patients treated with chemotherapy had a lower overall incidence of dermatitis, this may be more an effect of the confounding preponderance of large-breasted women in the non-chemotherapy–treated group; thus, we cannot conclude that the lower incidence was due to the use of IMRT itself in these women.
Initial experiences with IMRT for breast cancer have shown improved dose distributions in the treated breast, lower doses delivered to the normal heart or lung tissue, a lower incidence of acute toxicity, and reduced subacute complications such as breast edema or unfavorable cosmetic changes compared with the findings in women treated with standard techniques 18–24. In our initial assessment of IMRT for breast cancer, we examined 73 women with early-stage breast cancer treated with IMRT and compared them to a matched control group of 60 women treated with conventional radiation therapy on the basis of breast size and chest wall separation 24. The earlier study showed that the degree of acute desquamation was greater in conventionally treated patients than in IMRT-treated patients, but subgroup analysis found this to be a significant factor only in women with small and large but not medium-sized breasts. In women with large breasts, the incidence of grade 2 moist desquamation was 48% in IMRT patients compared with 79% in conventionally treated patients. Limitations of this study were our small number of patients and the inability to match all IMRT patients to an appropriate control.
There have now been reports of two prospective randomized trials of breast IMRT compared to conventional tangential radiation therapy that have shown improvement in both acute dermatitis 18 and breast cosmesis at 5 years 25. In the first study, a randomized trial from Canada with a patient population of 358 patients, Pignol et al compared standard wedge compensated conventional radiation therapy to IMRT and found that IMRT was associated with improved dose homogeneity and a reduced incidence of moist desquamation (31% vs. 48%, p=0.0019) 18. This was observed in all breast quadrants, but a particularly large difference was noted for the inframammary fold, whereas a 26% incidence of moist desquamation was seen in IMRT patients as opposed to a 43% incidence in patients treated with standard techniques (p=0.0012). In the second study, a randomized trial from the United Kingdom reported by Donovan et al, standard radiotherapy was compared to IMRT in early-stage breast cancer 25. There were 240 evaluable patients, out of 306 patients, for whom there were photographs that could be reviewed to assess changes in breast appearance. There was a negative change in breast appearance in 58% of women randomized to receive 2D conventional treatment compared with a negative change in breast appearance in only 40% of women randomized to receive IMRT. 2D radiotherapy in this trial was therefore 1.7 times more likely than IMRT to cause a negative change in breast appearance (p=0.008). Fewer patients treated with IMRT were also found to develop palpable induration on objective examination.
Despite the results of these two prospective randomized trials, the utilization of IMRT for breast cancer has been met with difficulties, including skepticism regarding its merits and reimbursement issues. In contrast, IMRT for prostate cancer is uniformly accepted without such evidence-based trial research. Our study provides additional proof that there are observable benefits to moving beyond conventional radiation therapy for breast cancer. Our study did not address the controversy over whether IMRT is required in all cases. It also did not assess whether techniques to improve dose homogeneity in the treated breast, such as 3D conformal dose compensation, could reduce the radiation dose delivered by standard therapy to normal organs such as the lung and heart, thereby achieving results similar to those of IMRT 29. And there is also a need for research and consensus on what should constitute IMRT for breast cancer – the technique used in this study was effective in reducing the duration and frequency of acute dermatitis, but definitions of breast IMRT vary across studies and these variations need to be considered. Future research may determine the relative advantages and disadvantages of the IMRT used in this study compared with other possible IMRT methods.
Acknowledgment
The authors thank Cindy Rosser for her collection and management of the data for the study population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abstract presented at the scientific session of the 49th annual meeting of the American Society for Therapeutic Radiology and Oncology in Los Angeles, CA October 28-Novermber 1, 2007.
Conflict of Interest: none.
References
- 1.National Comprehensive Cancer Network. NCCN practice guidelines for breast cancer. 2008. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 2.NIH Consensus Conference. Treatment of early-stage breast cancer. J.A.M.A. 1991;265:391–395. [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J. Clin. Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17. Intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz TA, Gurgoze E, Bice WS, et al. Dosimetric analysis of intact breast irradiation in off-axis planes. Int. J. Radiat. Oncol. Biol. Phys. 1997;39:261–267. doi: 10.1016/s0360-3016(97)00292-7. [DOI] [PubMed] [Google Scholar]
- 9.Das IJ, Cheng CW, Fein DA, et al. Patterns of dose variability in radiation prescription of breast cancer. Radiother. Oncol. 1997;44:83–89. doi: 10.1016/s0167-8140(97)00054-6. [DOI] [PubMed] [Google Scholar]
- 10.Chin LM, Cheng CW, Siddon RL, et al. Three-dimensional photon dose distributions with and without lung corrections for tangential breast intact treatments. Int. J. Radiat. Oncol. Biol. Phys. 1989;17:1327–1335. doi: 10.1016/0360-3016(89)90545-2. [DOI] [PubMed] [Google Scholar]
- 11.Solin LJ, Chu JCH, Sontag MR, et al. Three-dimensional photon treatment planning of the intact breast. Int. J. Radiat. Oncol. Biol. Phys. 1991;21:193–203. doi: 10.1016/0360-3016(91)90178-7. [DOI] [PubMed] [Google Scholar]
- 12.Neal AJ, Torr M, Helyer S, et al. Correlation of breast dose heterogeneity with breast size using 3D CT planning and dose-volume histograms. Radiother. Oncol. 1995;34:210–218. doi: 10.1016/0167-8140(95)01521-h. [DOI] [PubMed] [Google Scholar]
- 13.Back M, Guerrieri M, Wratten C, et al. Impact of radiation therapy on acute toxicity in breast conservation therapy for early breast cancer. Clinical Oncology. 2004;16:12–16. doi: 10.1016/j.clon.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97–13. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1307–1310. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 15.Taylor ME, Perez CA, Halverson KJ, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:753–764. doi: 10.1016/0360-3016(94)00480-3. [DOI] [PubMed] [Google Scholar]
- 16.Gorodetsky R, Lotan C, Piggot K, et al. Late effects of dose fractionation on the mechanical properties of breast skin following post-lumpectomy radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:893–900. doi: 10.1016/s0360-3016(99)00257-6. [DOI] [PubMed] [Google Scholar]
- 17.Johansson S, Svensson H, Denekamp J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2002;52:1207–1219. doi: 10.1016/s0360-3016(01)02743-2. [DOI] [PubMed] [Google Scholar]
- 18.Pignol J, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 19.Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:1336–1344. doi: 10.1016/s0360-3016(02)03746-x. [DOI] [PubMed] [Google Scholar]
- 20.Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: Initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 21.Hong L, Hunt M, Chui C, et al. Intensity-modulated tangential beam irradiation of the intact breast. Int. J. Radiat. Oncol. Biol. Phys. 1999;44:1155–1164. doi: 10.1016/s0360-3016(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 22.Chui C, Hong L, Hunt M, et al. A simplified intensity modulated radiation therapy technique for the breast. Medical Physics. 2002;29:522–529. doi: 10.1118/1.1460875. [DOI] [PubMed] [Google Scholar]
- 23.Harsolia A, Kestin L, Grills I, et al. Intensity-Modulated Radiotherapy Results in Significant Decrease in Clinical Toxicities Compared With Conventional Wedge-Based Breast Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1375–1380. doi: 10.1016/j.ijrobp.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Freedman GM, Anderson PR, Li J, et al. Intensity modulated radiation therapy (IMRT) decreases the acute skin toxicity for women receiving radiation therapy for breast cancer. Am. J. Clin. Oncol. 2006;29:66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 25.Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother. Oncol. 2007;82:254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 26.American Joint Committee on Cancer. 5th ed. Philadelphia: Lippincott-Raven; 1997. AJCC cancer staging manual. [Google Scholar]
- 27.Fowble B, Freedman G. Cancer of the Breast. In: Wang CC, editor. Clinical Radiation Oncology: indications, techniques and results. second ed. Wiley-Liss, inc; 2000. [Google Scholar]
- 28.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events: National Cancer Institute. http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 29.Haffty BG, Buchholz TA, McCormick B. Should intensity-modulated radiation therapy be the standard of care in the conservatively managed breast cancer patient? J. Clin. Oncol. 2008;13:2072–2074. doi: 10.1200/JCO.2007.15.9442. [DOI] [PubMed] [Google Scholar]