Abstract
Glycosylation of a synthetic aglycone using precursor-directed biosynthesis is facilitated by a chemical ketosynthase knockdown’ of the apoptolidin producer Nocardiopsis sp. This synthetic approach facilitated the preparation of an unnatural disaccharide derivative of apoptolidin D that substantially restores cytotoxicity against H292 cells and deconvolutes the role of the decorating sugars in apoptolidin bioactivity.
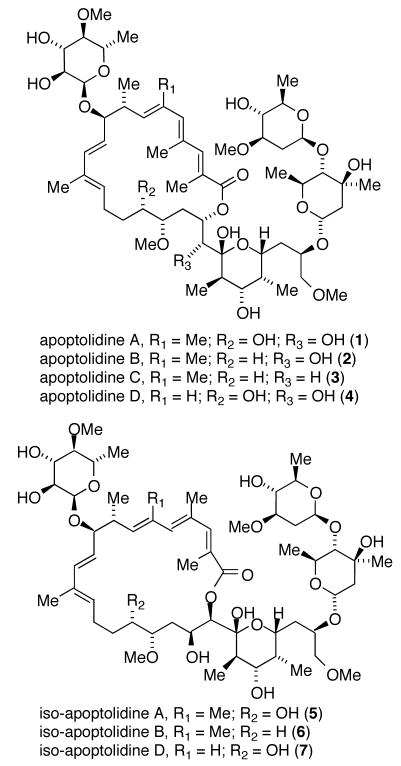
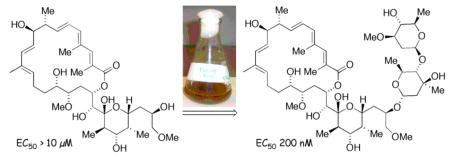
Actinomycetes have long been a source of important bioactive natural products possessing a high level of molecular complexity.1 For example the macrolide apoptolidin A (1), produced by an actinomycete of the genus Nocardiopsis (FERM BP-5871), selectively induces apoptosis in rat glia transformed cells and exhibits low cytotoxicity against nontransformed cell lines.2,3 The gross structural features of apoptolidins A-D (1–4)4 and related iso-apoptolidins (5–7)5 are a central aglycone conjugated to three sugar units (Figure 1). Apoptolidin A is reported to inhibit growth of H292 cancer cells (lung carcinoma) in the nanomolar range (EC50 = 30 nM)6a and removal of the sugar units results in complete loss of cytotoxicity (apoptolidinones A and D, EC50 >10 μM) emphasizing the significance of the sugar fragments in relation to apoptolidin bioactivity.6
Figure 1.
Structures of apoptoidins and iso-apoptolidins.
From the perspective of total synthesis, the challenge of assembling the complete molecular matrix of apoptolidin, a complex aglycone conjugated to three deoxy sugar units, remains significant.7 In particular the introduction of sugar units by chemical methods poses multiple problems in selectivity requiring complex protecting group schemes and methods of stereocontrol in the key glycosylation step. One approach to circumventing these difficulties is to employ in vitro enzymatic glycosylation methods using purified enzymes.8 A second option, mutasynthesis, is an extension of precursor-directed biosynthesis whereby a organism, engineered to eliminate the aglycone encoding polyketide synthase, allows glycosylation of a synthetic unnatural aglycone without competitive interference from the endogenous aglycone in the producing organism.9 However, in vitro expression of glycosyltransferases or mutasynthesis requires appropriately active recombinant enzymes and mutasynthesis presumes knowledge of the biosynthetic gene cluster and well developed methods for genetic manipulation of the producing organism.9 A third alternative, requiring no knowledge of sequence or genetic method, is to employ a selective chemical ‘knockdown’ of the interfering aglycone polyketide synthase. In this way, polyketide synthase (PKS) inhibitors may facilitate the introduction of a foreign or unnatural aglycone and its subsequent enzymatic glycosylation by the PKS cured system. This strategy was successfully demonstrated by Omura in the early 1980’s in which the ketosynthase inhibitor cerulenin was used to silence the spiromycin pathway, inhibiting production of the endogenous aglycone and allowing for glycosylation of a exogenously introduced aglycone protylonolide.10 Herein we report the precursor directed glycosylation of completely synthetic aglycones of apoptolidin using a chemical knockdown methodology.
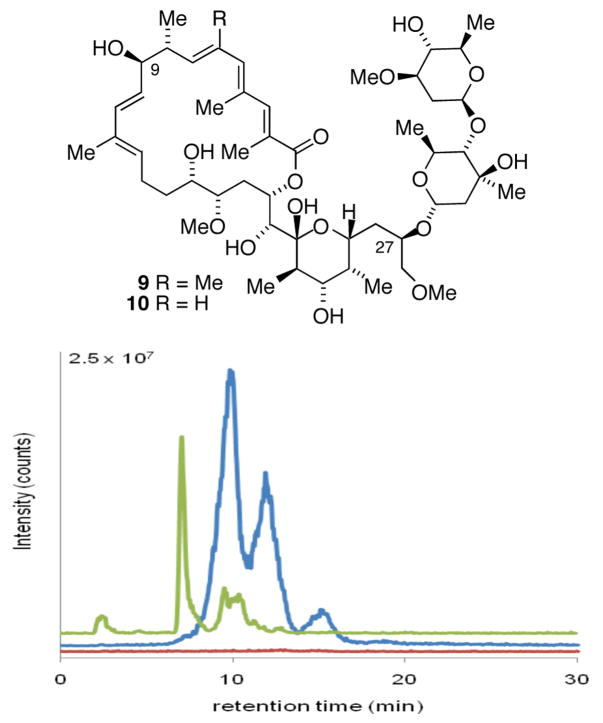
The aglycones of apoptolidins A and D (Figure 2) were prepared by chemical synthesis following our previously published synthetic routes.6a In order to introduce the sugar units we elected to examine glycosylation of apoptolidinones A and D using precursor-directed biosynthesis employing a whole cell culture of the apoptolidin producer (actinomycete Nocardiopsis). Our approach was to employ the promiscuous ketosynthase inhibitor cerulenin11 allowing the unnatural aglycone to effectively compete as a substrate for the extant glycosyl transferase enzymes. Due to the cross reactivity of cerulenin in essential fatty acid biosynthesis, concentrations were titrated to identify a concentration range that inhibited apoptolidin production, as measured by HPLC/MS, without substantially inhibiting cell growth, as measured by pelleted mycelial mass. Optimized conditions comprising pulsed feeding of 0.2 mM of cerulenin/day under apoptolidin fermentation conditions in Nocardiopsis reduced apoptolidin production below 5% relative to control cultures without effecting cell growth (< 5 %). When the addition of cerulenin was accompanied by pulsed addition of synthetic apoptolidinone A, only trace amounts of apoptolidin A were detected, however for the first time apoptolidin A disaccharide (9) was evident by LC-MS (Figure 3). Notably, indication of disaccharide conjugate 9 by LC-MS is not observed in the control fermentation12 suggesting the C9 glucose sugar may be introduced at the seco acid stage and the C27 disacharride following macrolactonization.13,14
Figure 2.
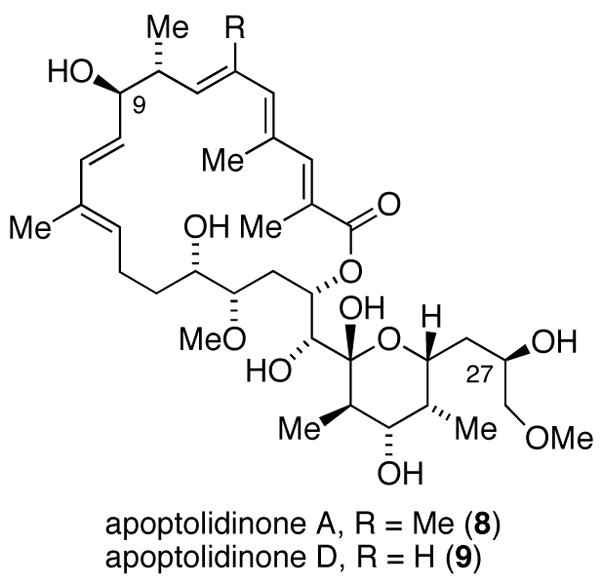
Structure of apoptolidinone A and D.
Figure 3.
HPLC/MS detection of apoptolidins in fermentation extracts of the apoptolidin producer innoculated with 0.2 mM cerulenin/day: no aglycone supplmentation (red); supplemented with synthetic apoptolidinone A (blue ES+ M+NH4/z = 986.6); supplemented with apoptolidinone D (green ES+ M+NH4/z = 972.6).
The addition of apoptolidinone D to the compromised culture led to the isolation of apoptolidine D disaccharide (10), once again unique to the aglycone supplemented culture (Figure 3). Analysis of the crude extract of this culture showed the primary product to be apoptolidin D disaccharide (10) accompanied by minor amounts of apoptolidin and iso-apoptolidin (see supplemental for HPLC analysis). Apoptolidin D disaccharide (9) was isolated by preparative HPLC and fully characterized by NMR analysis.15
Upon evaluation against H292 lung carcinoma cells apoptolidin D disaccharide inhibited cell growth in the sub-micromolar range (EC50 = 100–300 nM), showing less then one order loss of activity relative to apoptolidin A. Notably, the observed significant recovery of cytotoxicity on incorporation of the C27 disaccharide is in accord with earlier indications of the biological significance of this sugar residue. 6b,16
In conclusion we have demonstrated the glycosylation of apoptolidinone D (6-normethylapoptolidinone A) by employing whole cells of the natural apoptolidin producer actinomycete Nocardiopsis in combination with cerulenin as a ketosynthase inhibitor. Such chemical knockdowns of polyketide biosynthesis provide rapid and organism nonspecific methods to access the glycosylation apparatus of a wide range of biosynthetic systems.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (CA 059515) and the Vanderbilt Institute of Chemical Biology.
Footnotes
Supporting Information Available Experimental procedures and characterization data and NMR spectra of apoptolidin D disaccharide (9). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]; (b) Berdy J. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hayakawa Y, Kim JW, Adachi H, Shinya K, Fujita K, Seto H. J Am Chem Soc. 1998;120:3524–3525. [Google Scholar]; (b) Kim JW, Adachi H, ShinYa K, Hayakawa Y, Seto H. J Antibiot. 1997;50:628–630. doi: 10.7164/antibiotics.50.628. [DOI] [PubMed] [Google Scholar]
- 3.Review on chemistry and biology of apoptolidin: Daniel PT, Koert U, Schuppan J. Angew Chem Int Ed. 2006;45:872–893. doi: 10.1002/anie.200502698.
- 4.(a) Wender PA, Longcore KE. Org Lett. 2007;9:691–694. doi: 10.1021/ol0630245. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, Sukopp M, Longcore K. Org Lett. 2005;7:3025–3028. doi: 10.1021/ol051074o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Wender PA, Gulledge AV, Jankowski OD, Seto H. Org Lett. 2002;4:3819–3822. doi: 10.1021/ol0266222. [DOI] [PubMed] [Google Scholar]; (b) Pennington JD, Williams HJ, Salomon AR, Sulikowski GA. 2002;4:3823–3825. doi: 10.1021/ol026829v. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ghidu VP, Wang JQ, Wu B, Liu QS, Jacobs A, Marnett LJ, Sulikowski GA. J Org Chem. 2008;73:4949–4955. doi: 10.1021/jo800545r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schuppan J, Wehlan H, Keiper S, Koert U. Chem Eur J. 2006;12:7364–7377. doi: 10.1002/chem.200600461. [DOI] [PubMed] [Google Scholar]
- 7.Total synthesis of apoptolidin A: Nicolaou KC, Fylaktakidou KC, Monenschein H, Li YW, Weyershausen B, Mitchell HJ, Wei HX, Guntupalli P, Hepworth D, Sugita K. J Am Chem Soc. 2003;125:15433–15442. doi: 10.1021/ja0304953.Wehlan H, Dauber M, Fernaud MTM, Schuppan J, Keiper S, Mahrwald R, Garcia MEJ, Koert U. Chem Eur J. 2006;12:7378–7397. doi: 10.1002/chem.200600462.Crimmins MT, Christie HS, Long A, Chaudhary K. Org Lett. 2009;11:831–834. doi: 10.1021/ol802829n.
- 8.(a) Thibodeaux CJ, Melancon CE, Liu HW. Angew Chem Int Ed. 2008;47:9814–9859. doi: 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mendez C, Luzhetskyy A, Bechthold A, Salas JA. Curr Top Med Chem. 2008;8:710–724. doi: 10.2174/156802608784221532. [DOI] [PubMed] [Google Scholar]; (c) Walsh C, Meyers CLF, Losey HC. J Med Chem. 2003;46:3425–3436. doi: 10.1021/jm030257i. [DOI] [PubMed] [Google Scholar]; (d) Thorson JS, Hosted TJ, Jiang JQ, Biggins JB, Ahlert J. Curr Org Chem. 2001;5:139–167. [Google Scholar]
- 9.(a) Kennedy J Nat Prod Rep. 2008;25:25–34. doi: 10.1039/b707678a. [DOI] [PubMed] [Google Scholar]; (b) Venkatraman L, Salomon CE, Sherman DH, Fecik RA. J Org Chem. 2006;71:9853–9856. doi: 10.1021/jo062047u. [DOI] [PubMed] [Google Scholar]; (c) Ashley GW, Burlingame M, Desai R, Fu H, Leaf T, Licari PJ, Tran C, Abbanat D, Bush K, Macielag M. J Antibiot. 2006;59:392–401. doi: 10.1038/ja.2006.56. [DOI] [PubMed] [Google Scholar]
- 10.(a) Nakagawa A, Omura S. J Antibiot. 1996;49:717–741. doi: 10.7164/antibiotics.49.717. [DOI] [PubMed] [Google Scholar]; (b) Omura S, Sadakane N, Tanaka Y, Matsubara H. J Antibiot. 1983;36:927–930. doi: 10.7164/antibiotics.36.927. [DOI] [PubMed] [Google Scholar]
- 11.(a) Nakagawa A, Omura S. J Antibiot. 1996;49:717–741. doi: 10.7164/antibiotics.49.717. [DOI] [PubMed] [Google Scholar]; (b) Omura S, Sadakane N, Tanaka Y, Matsubara H. J Antibiot. 1983;36:927–930. doi: 10.7164/antibiotics.36.927. [DOI] [PubMed] [Google Scholar]
- 12.We typically isolate a combined mass of 150–200 mg per liter of apoptolidin and iso-apoptolidin. Under our fermentation conditions we have not observed by LC-MS apoptolidins B-D. To date, no detection of partially glycosylated apoptolidin metabolites by LC-MS has been observed in control fermentations.
- 13.Studies of linear and cyclic aglycones as glycosyltransferase substrates: Kao CL, Borisova SA, Kim HJ, Liu HW. J Am Chem Soc. 2006;128:5606–5607. doi: 10.1021/ja058433v.Borisova SA, Kim HJ, Pu XT, Liu HW. Chembiochem. 2008;9:1554–1558. doi: 10.1002/cbic.200800155.
- 14.Although not proven we assume the second major peak corresponding in mass to apoptolidin A disaccharide is the 21-membered macrolactone or iso-apoptolidin A disaccharide (cf. Figure 1).
- 15.Addition of 15 mg of apoptolidinone D to a cured culture afforded 3.7 mg (ca. 18% isolated yield) of apoptolidin D disaccharide (9) following HPLC purification. Our attention was focused on apoptolidinone D as a substrate for precursor-directed biosynthesis as at the time we assumed this to be an unnatural aglycone. It was only during the course of our investigations that Wender’s group discovered apoptolidin D (ref. 4a).
- 16.(a) Salomon AR, Zhang Y, Seto H, Khosla C. Org Lett. 2001;3:5–59. doi: 10.1021/ol006767d. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, Jankowski OD, Tabet EA, Seto H. Org Lett. 2003;5:2299–2302. doi: 10.1021/ol0346335. [DOI] [PubMed] [Google Scholar]; (c) Wender PA, Jankowski OD, Longcore K, Tabet EA, Seto H, Tomikawa T. Org Lett. 2006;8:589–592. doi: 10.1021/ol052800q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nicolaou KC, Li Y, Sugita K, Monenschein H, Guntupalli P, Mitchell HJ, Fylaktakidou KC, Vourloumis D, Giannakakou P, O’Brate A. J Am Chem Soc. 2003;125:15443–15454. doi: 10.1021/ja030496v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.