Abstract
We have previously shown that separation of the amnion from choriodecidua occurs as an integral part of the fetal membranes (FM) rupture process. We have also reported that spontaneous separation of FM is nearly universal with term vaginal delivery. The etiology of this spontaneous FM separation is unknown. If biochemical degradation at the amnion-choriodecidua interface is a factor, decreased adhesive force between the FM components prior to their complete separation would be expected. The purpose of this project was to develop and validate machinery and procedures to measure the adhesive force between amnion and choriodecidua. Commercial tensile testing equipment was adapted to perform a standard T-peel test, per the American Society for Testing and Materials (ASTM) guidelines. FM test strip dimensions, peel speed, and peel force data measurements from force versus displacement curves were optimized for reproducibility. Test system validation was performed using Shurtape CP 60 (slow release painter's masking tape) as the standard. Equipment and procedures for a standard T-peel test on FM were developed. Shurtape CP 60 of decreasing widths showed reproducible, linear changes in the adhesive force range for FM (r2 = 0.96). The adhesive force between FM components ranged from 0.017–0.262 N/cm. Reproducibility was optimal with FM test strips of 4x6 cm and a peel speed of 25.4 cm/min. FM showed greater adhesive force adjacent to the placental disc than distal from the disc (p<0.05). We have developed equipment and procedures to accurately and reproducibly measure adhesive force between the FM amnion and choriodecidua.
Introduction
Fetal membranes (FM) weaken primarily as a result of a programmed, biochemical, process prior to onset of labor, and FM rupture frequently occurs after contractions begin.1 We have previously shown that separation of the FM components, amnion from choriodecidua, occurs as an integral part of the process of FM rupture and that energy may be expended in separating the FM components.2 This separation may effectively contribute to weakening of the membranes. We have also reported that some spontaneous separation of FM at delivery is nearly universal and that FM separation is associated with increased gestational age at delivery, spontaneous rupture of FM (SROM), shorter duration of contractions, and vaginal delivery with term labor.3 Etiology of this spontaneous FM separation is unknown. If biochemical degradation at the amnion-choriodecidua interface is a factor, then decreased adhesive forces between the FM components prior to complete separation would be expected.
Currently there is no method to measure adherence of the amnion to choriodecidua, that is, the force holding the components of the FM together. Although peel testing has often been used by industry to test adhesives and tapes, biomaterials have been tested to a very limited degree.4–7 The purpose of this project was to develop and validate machinery and procedures to measure the adhesive force between the FM amnion and choriodecidua.
Methods
Biological samples
Thirty FM [20 SROM vaginal deliveries and 10 elective Cesarean Section (C/S)] were obtained with Institutional Review Board (IRB) waiver for anonymous discarded specimens and were utilized to determine optimal peeling speed, optimal test sample dimensions, and refine methods of data analysis. With IRB approval and written consent, 10 additional FM were then collected from patients undergoing uncomplicated elective scheduled C/S (n=5) or vaginal delivery with SROM (n=5) for preliminary studies of adherence variability over the FM surface. For the C/S patients, the FM weak zone overlying the cervix was visually identified and marked using our previously reported methodology.8
Membrane sampling procedure
FM were processed within 20 minutes of delivery. Membranes were gently washed with ice cold saline and all clots were removed. Membrane pieces were kept moist with cold saline throughout the peel testing. The entire FM was cut in such a manner that location and orientation of each tested piece, was maintained relative to each other, to the placental disc and to the area of FM previously overlying the cervix (for C/S FM specimens).8 After laying the placental membranes flat, the area previously overlying the cervix was identified and a series of cuts were made starting from this region and extending to the placental disc. This allowed the membranes to lay completely flat. Serial secondary cuts were made along a line extending from marked area / tear line (vaginal deliveries) extending to the placental disc to obtain tissue test pieces (4 × 6 cm). Additional membrane sections, parallel and adjacent to this group, were cut maintaining similar orientation. This allowed an average of 3 pieces in series (with each series extending from placental disk to rupture line) and a total of 3–5 series providing up to 15 (9–15) test pieces per placenta. The FM test strips were used to determine the strength of FM adherence by relative location of membrane piece (paracervical area versus near the placental disc versus central locations).
To minimize the shear stress along the cut margins, a 4 cm microtome blade was used by pressing it perpendicularly over the membrane firmly. A 4 × 6 cm template made with cork sheet was placed gently over the membranes to guide the microtome blade and obtain uniform sized membrane pieces (Figure 1A).
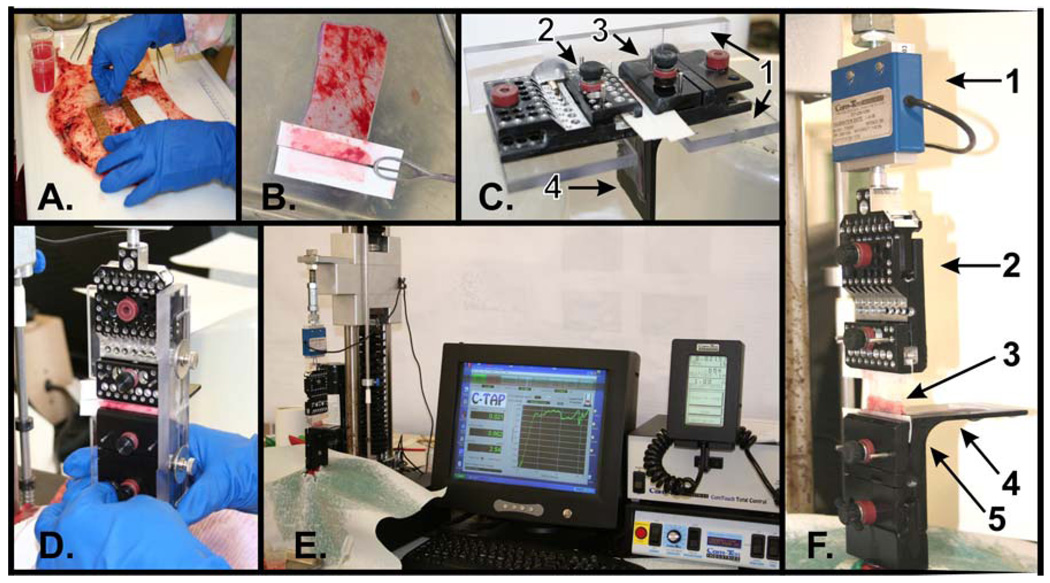
Figure 1. Peel testing procedures.
Tissue samples are cut out from FM kept moist and layed flat. (A). Stiff filter paper stints assist in holding and mounting the partially manually peeled amnion and choriodecidua by maintaining shape (B). Membrane piece mounted on custom clamps which are maintained in fixed position by a plexiglass mount support to assist sample loading. The clamps-plexiglass mount support unit is placed horizontal to assist with sample loading (1=plexiglass mount support, 2=upper clamp holding 1 cm peeled amnion with filter paper, 3=lower clamp holding 1 cm peeled choriodecidua with filter paper, 4=horizontal platform of the lower clamp supporting intact membrane piece) (C). Sample loaded clamps with supporting Plexiglas mount as a single unit attached to the peel testing device (D). Testing in progress; the peel tester with the FM piece loaded and the plexiglass mount support removed (E). Amnion and choriodecidua being peeled apart between the clamp jaws (1=load cell, 2=upper clamp pulling amnion vertically upwards, 3=amnion peeling from choriodecidua, 4=horizontal platform of the lower clamp supporting unpeeled FM floating over cold saline, 5=lower clamp holding choriodecidua) (F).
T-peel testing equipment
Commercial industrial tensile testing equipment (701 Universal Test System, Com-Ten Industries, St. Petersburg, Fl) was adapted to perform a standard engineering T-peel test (ASTM D1876)9 to measure FM adhesive force (Figure 1A–F). The equipment has wide speed range (2 to 101 cm/min). It is computer controlled with custom software (CTAP) which continuously acquires, displays and stores data. A 0.227 kg S-Block Load Cell (sensitivity of 0.00005 kg) was incorporated into the design and the system was adjusted so that the load cell operated in its mid-sensitivity range.
Tissue clamp assembly and mounting of the FM tissue samples
Along one 4 cm width end of a sample (4 × 6 cm), approximately 1 cm of the amnion and choriodecidua were gently separated from one another. The 1 cm peeled ends were mounted on stiff filter paper (1 × 6 cm) strips (Figure 1B). This enabled the filter paper adherent amnion and choriodecidua to be loaded into the clamps without wrinkles, and facilitated sample alignment with respect to each other and to the clamps (Figure 1C).
Ends of each FM component were secured in a custom made 5 cm wide tissue clamp assembly consisting of an upper clamp that holds the amnion, a lower clamp that holds the choriodecidua and a Plexiglas mounting bracket that temporarily holds the clamps a fixed distance apart while mounting the membrane-clamp assembly in the testing machine. The sample was secured with this clamp assembly positioned horizontal prior to attaching to peel tester (Figure 1 C). This system was designed and manufactured at Case School of Medicine Design and Fabrication Center (drawings available upon request). The lower clamp has a platform to support the tissue which has not yet been separated in order to prevent the FM from dragging against the fixture. The platform was covered with cold saline so that the sample is floating to minimize friction.
After securing the sample (Figure 1C), the Plexiglas mounting bracket with the clamps and membrane is mounted to the peel tester as a single unit (Figure 1D). The Plexiglas mounting bracket is then removed and peel testing begins (Figure 1E). The lower clamp is held stationary while the upper clamp attached to the load cell is raised vertically by the peel tester, peeling the amnion from the choriodecidua (Figure 1F).
Validation of the T-Peel Test system and methodology
Shurtape CP 60 (Shurtape Technologies, Hickory, NC, USA), an acrylic, uniform, but low adhesive based 60 day painter’s masking tape, conforming to ASTM standards, was used to validate the device over the range of adhesion force of interest. This particular tape was chosen because of its high quality and adhesive force similar to that of FM (verbal communication by John Johnston, technical advisor to Pressure Sensitive Tape Council). Adhesive sides of two strips of Shurtape were uniformly applied to each other and the peel force required to separate them was measured.
Further optimization of the test system to identify optimal peel speed, the optimal test sample size and to refine methods of data analysis was all done with human FM.
Data analysis
Peel force and displacement data were imported into Microsoft Office Excel. The data for each sample was organized as a Scatter Plot Graph with distance (displacement – the total amount of peeling) as the independent variable and force (N) as the dependent variable. Average peel force for each sample was computed for a consistently defined displacement region. Average peel force for each placenta was determined by dividing the sum of average forces of all samples by the number of all samples. Similarly, average peel forces between different regions (adjacent to the placental disc, tear line / marked paracervical region and central) were determined and the comparisons were made using a three way ANOVA with post-hoc correction. Differences in peel strength between two groups were analyzed using the Student t-test, two tail, with P<0.05 considered statistically significant.
Results and Discussion
Reproducibility of the measured peel force
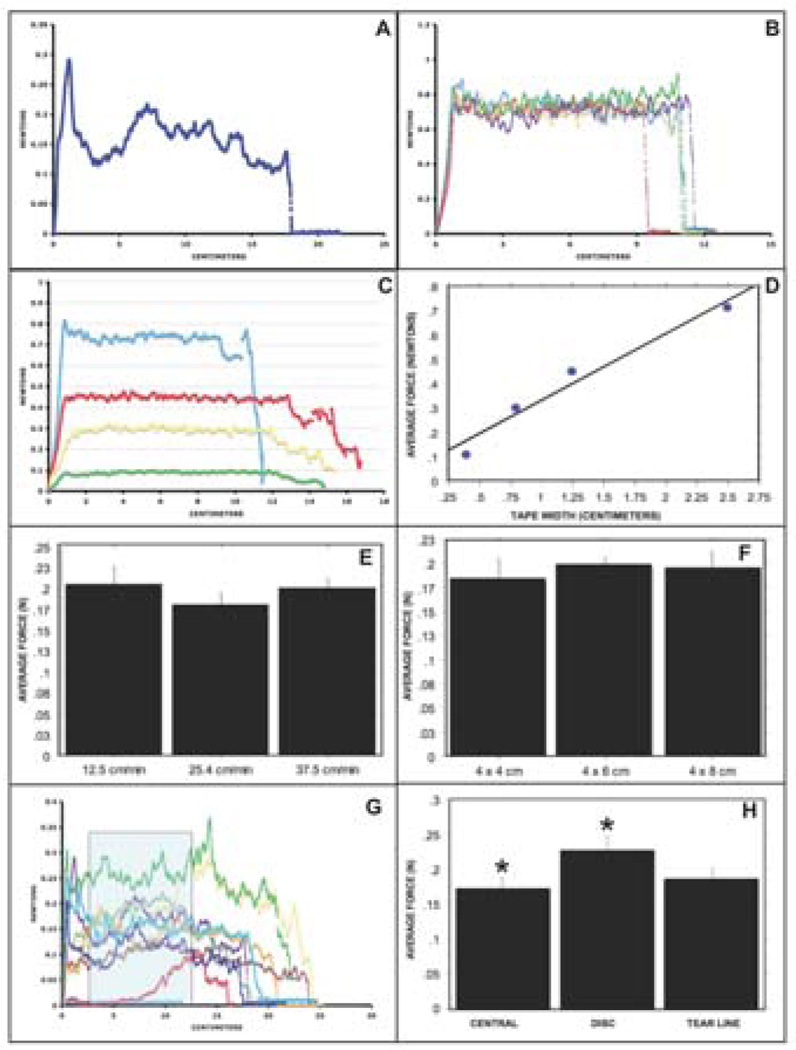
Five individual peel tests using 2.5 cm wide Shurtape demonstrated reproducible average peel force (Figure 2B). Force (0.712–0.106 N) required to peel Shurtape decreased proportionately with decreasing width (2.5, 1.25, 0.8, 0.4; cm) with remarkable correlation (r2 =0.96) (Figure 2C and 2D). The measured peel forces with Shurtape overlapped the range of FM peeling (0.017–0.262 N/cm). The equipment thus provides an accurate, reproducible measurement of peeling force over the range of FM adherence. There was little peel force variability during the steady state peeling using Shurtape CP 60 (Figure 2B). This is expected given that it conforms to industry standards of less than 20% variability in adhesive force over the entire roll of tape.
Figure 2. Peel test validation and optimization.
A typical force (N; Y axis) versus displacement (cm; X axis) curve for FM peel test (A). The peel tester is capable of remarkably reproducible results as demonstrated with Shurtape CP 60 tape tests (B). Average forces required to peel same length but decreasing (2.5 cm blue; 1.25 cm red; 0.8 cm yellow; 0.4 cm green) width Shurtape decreased with remarkable correlation (r2 =0.96) (C & D). Average peel force variability at speeds of 12.5, 25.4 and 37.5 cm/min were similar (E). The average peel force for the 3 tested sample sizes was similar with 4x6 cm pieces demonstrating the least standard deviation (F). For optimal measurement of peel force, the initial 2.5 cm of the peel force displacement curve was ignored and the force was measured for the subsequent 10.1 cm of the curve (G). The FM pieces from near the placental Disc were more adherent than from other areas (Disc versus Central, p=0.046; Disc versus Tear line, p=0.17) (H).
Typical FM peeling force displacement curve
When peeling began, after initial slack, the force increased rapidly to a peak value that was often the maximal force for that sample (Figure 2A). Thereafter, the force dropped coinciding with steady state peeling and representing true measurement of adhesion force, which unlike the Shurtape, is variable along the strip.
Optimization of peel testing speed
FM tissue samples were tested at three different speeds. Average peel force variability at 12.5 cm/min (24 samples from 5 FM), 25.4 cm/min (32 samples from 5 FM) and 37.5 cm/min (25 samples from 5 FM) was similar (Figure 2E). We chose to use a peel test speed of 25.4 cm/min which is the ASTM D1876 industry standard and was used in a recent biomaterials methodology report.4,9
Optimizing the tissue sample size
Multiple tissue samples of 4 × 4 cm (19 samples from 2 FM), 4 × 6 cm (39 samples from 5 FM) and 4 × 8 cm (33 samples from 5 FM) were tested at a peel test speed of 25.4 cm/min. The average peel force measured for the three sizes respectively was similar (0.184±0.099, 0.198±0.063, and 0.198±0.099; N) (2F). Notably the 4x6 cm group showed the smallest standard deviation. This size also enabled us to obtain an adequate number (10–15) of samples per placenta.
Optimizing peel force measurement
In contrast to Shurtape CP 60 which showed minimal peel strength variability, all of the 38 (4x6cm) FM tissue samples demonstrated inter and intra sample variability. This FM peel strength variability is a real phenomenon, unrelated to technique, and likely represents the strength of adherence between the amnion and choriodecidua within different regions of the same tissue sample piece and between different tissue samples from the same region. Conforming to the ASTM peel testing guidelines, we ignored the initial 2.5 cm of the force versus peel displacement curve (containing the starting peak) and determined the average peel force for the subsequent 10.1 cm curve (2G).9,10
Variability in FM adherence with sample location
Our previous data show that FM strength, as measured by rupture force, varies with membrane sample location relative to the paracervical area where the membrane is significantly weaker.8,11 To determine whether similar differences in regional FM adherence occur, pooled samples from both 5 vaginal and 5 C/S deliveries were peel tested. Average peel force for Disc group (pooled samples pieces from adjacent to the placental disc, n=10 pieces), Tear line group (pooled sample pieces from spontaneous tear line in vaginal deliveries or paracervical marked FM area in C/S, n=8) and Center group (n=10) samples demonstrated regional differences. The FM pieces from the Disc group were more adherent than those from the Center group (0.224±0.070 versus 0.171±0.051, N; p=0.046) and the Tear line group (0.224±0.070 versus 0.186±0.038, N; p=0.17) (2H).
Summary
We have developed equipment and a standardized, highly reproducible, procedure for T-peel testing to measure strength of adherence between the FM components; amnion and choriodecidua. The adhesive force between FM membrane components ranged from 0.017–0.262 N/cm. Reproducibility was optimal with FM test strips of 4 cm wide by 6 cm length peel tested at a speed of 25.4 cm/min. FM showed significantly greater adhesive force adjacent to the placental disc than remote from the disc. We expect this equipment and procedure will allow us to gain insight into the mechanisms of separation of the FM and the role of separation in FM weakening and rupture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Location where study was performed: MetroHealth Medical Center, Cleveland, Ohio
References
- 1.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties Placenta. 2006;27:1037–1051. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Arikat S, Novince RW, Mercer BM, Kumar D, Fox JM, Mansour JM, Moore JJ. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194:211–217. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Shaniuk P, Smith J, Bryant K, Moore RM, Novak J, Mercer BM, Moore JJ. San Diego, California: Abstracts of the Society for Gynecological Investigation (Reproductive Sciences, Vol. 15, Supplement); 2008. Duration of rupture of fetal membranes and labor are both associated with spontaneous separation of fetal membrane (Amnion and Choriodecidua) components; p. 260A. [Google Scholar]
- 4.Bundy K, Schlegel U, Rahn B, Geret S, Perren S. An improved peel test method for measurement of adhesion to biomaterials. J Mat Sc: Mat Med. 2000;11:517–521. doi: 10.1023/a:1008965926086. [DOI] [PubMed] [Google Scholar]
- 5.Englert C, Greiner G, Berner A, Hammer J. T-peel test for the analysis of articular cartilage integration. Stud Health Technol Inform. 2008;133:95–102. [PubMed] [Google Scholar]
- 6.Karwoski AC, Plaut RH. Experiments on peeling adhesive tapes from human forearms. Skin Res Technol. 2004;10:271–277. doi: 10.1111/j.1600-0846.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto K, Masuda K, Inoue N, Okuma M, Muehleman C. An HS. Anti-adhesion properties of a thrombin-based hemostatic gelatin in a canine laminectomy model: a biomechanical, biochemical, and histologic study. Spine. 2006 Feb 15;31(4):E91–E97. doi: 10.1097/01.brs.0000199902.80607.ce. [DOI] [PubMed] [Google Scholar]
- 8.El Khwad M, Stetzer B, Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72:720–726. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 9.ASTM Standard D1876-01. Standard test method for peel resistance of adhesives (T-Peel Test) West Conshohocken, PA: ASTM International; 2001. [Google Scholar]
- 10.ASTM Standard D 6862-04. Standard test method for 90 degree peel resistance of adhesives. West Conshohocken, PA: ASTM International; 2004. [Google Scholar]
- 11.El Khwad M, Pandey V, Stetzer B, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13:191–195. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]