Abstract
Transfer of antigen-specific T cells into antigen-expressing lymphopenic recipients leads to the sequential generation of Th1 and Th17 effector and protective CD25+FoxP3+ regulatory cells in the periphery with surprisingly different kinetics. Such an experimental model is potentially valuable for defining the stimuli that regulate lineage decision and plasticity of various T cell effectors and peripheral regulatory T cells. Our studies have shown that IL-17 production occurs rapidly and declines within the first week with the appearance of IFN-γ producing T cells. Regulatory T cells appear during the recovery phase of the disease. The factors that mediate this complex differentiation originating from a starting naïve T cell population remain to be defined.
Keywords: Interferon-gamma, IL-17, FoxP3, Th17 cells, Th1 cells, Regulatory T cells
1. Introduction
It has been known for over twenty years that following antigen stimulation, T helper (Th) cells may differentiate into at least two major subtypes with distinct cytokine profiles and functions in the immune system. Th1 cells typically produce interferon (IFN)-γ and TNF-α, are involved in macrophage activation and play a role in autoimmune disease and tissue damage associated with chronic infections. Th2 cells on the other hand produce Interleukin (IL)-4, IL-5 and IL-13, and play a role in allergic diseases and defense against helminthic parasites. The third and more recently discovered subset is called the Th17 subset because its signature cytokine is IL-17. The role of IL-17 producing T cells in autoimmunity was discovered by the finding that loss of IFN-γ surprisingly resulted in worsened autoimmunity in mouse models of collagen-induced arthritis and EAE [1, 2]. Th17 cells are now considered a distinct lineage separate from Th1 and Th2 CD4+ T cells [3–7].
IL-17 refers to a cytokine family comprising IL17 A, B C, D, E (IL-25) and F [8, 9]. IL-17 is a potent proinflammatory cytokine that aids in coordinating tissue inflammation by inducing chemokines and other cytokines and matrix metalloproteases, and stimulating proliferation and migration of neutrophils, reviewed in [10]. IL-17 expression can be detected in sera and tissues in various autoimmune diseases – RA, MS, SLE – and other diseases of immune dysregulation like asthma [11–14]. Furthermore, IL-17 has been shown to be directly involved in mouse models of joint inflammation of both cartilage and bone [15, 16] and in the development of EAE [17, 18].
Regulatory T cells, identified by surface expression of CD4 and CD25 and by expression of the functionally essential transcription factor FoxP3, have been investigated extensively in animal models and humans. They have been shown to play a role in the induction and maintenance of tolerance in a variety of different models. They may originate from the thymus as well as from the periphery [19, 20] . The latter is particularly interesting because it raises the possibility that the nature of an immune response may dictate whether the same naïve CD4+ T cells develops into a Th1, Th2, Th17 or Treg cell. Elucidating the exact mechanisms that determine along which pathway a CD4+ T cell differentiates has obvious implications for the physiologic regulation as well as the manipulation of immune responses involved in pathogen defense, autoimmunity and tolerance. In this article, we will review some of the regulatory mechanisms that influence differentiation of CD4+ T helper cells into Th1, Th17 or regulatory T cells. We will also highlight how these CD4+ T cell subpopulations interact with each other and thus might influence the differentiation pathways.
2. Sequential development of Th17, Th1 and regulatory T cells
We have established a transgenic mouse model in which a soluble model antigen (chicken ovalbumin, OVA) is expressed ubiquitously in the mouse, subsequently referred to as soluble Ovalbumin transgenic mice (sOVA Tg) [21]. If OVA-specific CD4+ T cells from DO11.10 T cell receptor (TCR) transgenic mice (DO11) are transferred into these sOVA Tg recipients, they undergo a brief initial expansion and abortive activation followed by cell-intrinsic anergy, and eventually, most of the antigen specific T cells are deleted [21]. Tolerance induction in this system is not associated with any pathology and is extremely robust. In contrast, if the sOVA Tg recipients are crossed onto a lymphocyte-deficient background, i.e. Rag−/−, T cells transferred into these “empty” recipients expand, and cause a severe systemic disease characterized by weight loss and skin inflammation with many similarities to graft versus host disease (GvHD) [22–24]. By isolating the antigen specific T cells after transfer, we were able to determine their surface marker expression, cytokine profile and other aspects of their functional capacity at various time points of the immune response. One of the strengths of this model is the fact that we have eliminated several variables that may influence Th lineage decisions. By limiting the T cell population to T cells expressing a single specificity, differences in TCR fine specificity and affinity cannot account for differences in T cell differentiation. Furthermore, in most of these experiments we use DO11 mice that were crossed onto a Rag−/− background, and thus all of the transferred T cells are homogenous naïve cells.
Following the transferred DO11 T cells over time and in various tissues, including lymphoid organs and peripheral sites, and determining their functional properties allows us to follow differentiation of the T cells into the distinctive lineages and discern the intrinsic and environmental mechanisms that influence the decision along which Th pathway the T helper cells differentiate. In addition, by using antibody blockade or cytokine knock-out (KO) mice as donors for adoptive transfer, we are also able to delineate how the Th subtypes influence each other. Using these methods, we established that the disease that develops after the DO11 cells recognize their antigen in the absence of endogenous polyclonal T cells is characterized by an early burst of interleukin-17 secretion by the transferred T cells [25]. Subsequently, this IL-17 response wanes, and the response is replaced by high numbers of IFN-γ producing cells. We observed this cytokine profile in lymphoid organs as well as in the skin, which is one of the principal target organs in this disease model [25]. Similar to our model, Korn et al showed that EAE was associated with an early burst of IL-17 and subsequently high numbers of IFN-γ producing cells isolated from the central nervous system at various time points following immunization with MOG 35–55/CFA. In the EAE model, the IL-17 response was almost completely abrogated on an IL-6 knock-out background whereas the interferon-gamma response was preserved [26]. These data argue that in the context of a strong inflammatory stimulus, e.g. mediated by IL-6, there are 2 subsequent phases of the immune response, the first one being dominated by IL-17, which is subsequently replaced by IFN-γ. We postulate that in lymphopenic recipients, the lack of competition with other endogenous lymphocytes makes the transferred antigen-specific T cells responsive to the antigen even in the absence of external inflammatory stimuli. However, once the response is initiated, it recapitulates the same basic sequence of differentiation pathways, dominated by the early Th17 response.
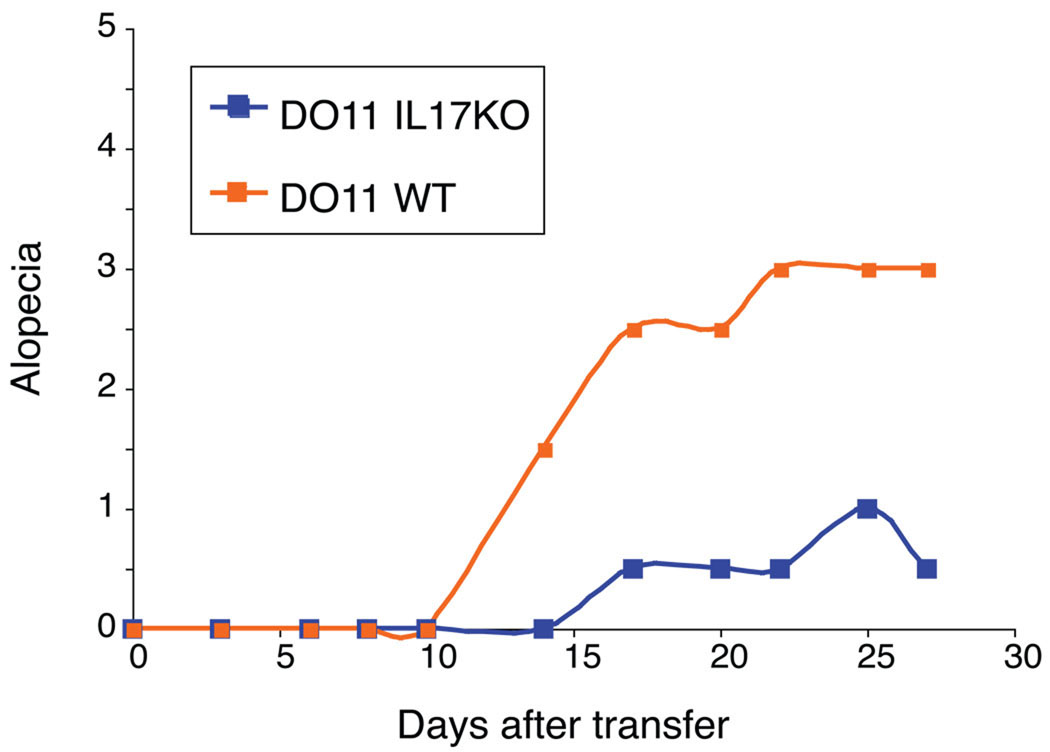
In our model, antibody blockade or transfer of IL-17 deficient DO11 cells results in significant reduction of skin disease Figure 1, [25]. These data demonstrate that IL-17 plays an essential role in skin inflammation. However, we did not see any differences in weight loss or survival of the IL-17 KO T cell recipients, suggesting that IL-17 may control only the inflammation in peripheral tissues and not systemic manifestations. We established that even though we obtained a very strong Th1 response in our model system, IFN-γ does not play a role in any aspect of the pathology – neither systemic disease as measured by weight loss nor tissue inflammation. In contrast, lack of IFN-γ or Tbet exacerbated the skin disease by increasing the interleukin-17 response [25]. Hirota et al used a mouse model of autoimmune arthritis (the SKG mutant) resembling rheumatoid arthritis. Similar to our data, transfer of IL-17 wild-type CD4+ T cells from SKG mice into Rag−/− animals caused arthritis, but the transfer of IL-17 KO CD4+ T cells did not, indicating a pathogenetic role for IL-17 in the tissue inflammation. IL-6 in this scenario was necessary for induction of arthritis and was produced mostly by the antigen-presenting cells. Spontaneous arthritis developed only in SKG animals on an IFN-γ KO background and CD4+ T cells from IFN-γ KO SKG mice that were transferred into Rag−/− recipients produced larger amounts of IL-17 in an IL-6 dependent manner [27]. Using another in vivo model in which adoptive transfer of naïve CD4+ T cells into Rag−/− mice causes a T cell dependent colitis, Noguchi et al showed that colitis can be inhibited by blocking IL-6 receptor. However, transfer of naïve CD4+ T cells from IL17 KO mice did not inhibit the disease, suggesting that IL-17 is just one of the effector mechanisms that is triggered by IL-6 [28].
Figure 1. DO11 IL-17 KO T cells cause less alopecia.
1 × 106 sorted CD4+CD25− DO11 T cells from either WT or IL-17 KO mice were transferred into sOVA Tg Rag−/− recipients and alopecia was scored over time (alopecia < 1 cm = 1, 1 – 2 cm = 2, and > 2 cm = 3 points, respectively).
These data stress several important points about the regulation of T cell differentiation in vivo. First, Th1 and Th17 cells may arise with different kinetics. In our model, Th17 cells are seen first and subsequently give way to a developing Th1 response. Second, whether or not Th1 or Th17 cells are harmful not only depends on the specific disease model but also on the target tissue. Third, different aspects of disease may be affected by different subtypes of differentiating T cells. In our disease model, Th17 cells have an important role in the development of the skin disease but do not influence systemic disease as measured by weight loss and wasting. While the initial immune response in our disease model is characterized by Th17 and Th1 cells, there is a third, late phase in our disease model that is dominated by development of Treg cells and recovery from disease, which we will discuss in detail below.
3. Role of IL-2, transforming growth factor (TGF)-beta and Treg on Th17 T cell differentiation
3.1. Development of peripheral Treg
It is well established that CD4+ T cells that recognize self-antigens in the thymus and are not deleted develop into CD4+CD25+ regulatory T cells that express FoxP3. In addition to these centrally generated regulatory T cells, Treg cells may also be generated in the periphery. Thorstenson et al. used a model of DO11 T cell transfer into mice which had been immunized with OVA peptide and observed that the absolute number of CD25+ DO11 cells increased when using a lower antigen dose and a weaker immunogenic stimulus [29]. Apostolou et al. demonstrated that CD25+FoxP3+ Treg could be generated in thymectomized TCR Tg Rag−/− animals in which the cognate antigen was secreted by an osmotic pump [20]. Similar observations were made when antigen was delivered coupled to DC-specific monoclonal anti-DEC205 antibody and Treg, presumably generated in the periphery, were generated with and without inflammatory stimuli, but with different kinetics and after a different number of cell divisions [30]. Treg have also been shown to be accumulating in the context of an immune response to a pathogen such as leishmania major [31].
In our model, the early phase of the reaction, with strong effector function and early IL-17 and subsequent IFN-γ production and severe clinical phenotype, is followed by clinical recovery and the establishment of tolerance. This later phase coincides with the appearance of CD4+ CD25+FoxP3+ Treg about two weeks after T cell transfer. Similar to the studies outlined above, these cells are generated in the periphery since the transferred DO11 cells are on a Rag−/−background and do not contain any FoxP3+ cells prior to transfer and the experiments were repeated in thymectomized animals [19].
It is unknown which signals drive peripheral Treg development. One might speculate that the microenvironment in which a T cell undergoes activation determines which differentiation route a cell takes. This could be through cytokine secretion, innate immune stimuli indirect via TLR signaling to dendritic cells, and also through interaction with other T cells within that environment.
It has been described in vitro that regulatory T cells can promote interleukin-17 production by means of TGF-β secretion [5, 6]. Using T cells from mice that carry a TGF-β dominant negative receptor we were able to show that IL-17 production is reduced if TGF-β signals are blocked (unpublished results). It is, however, unlikely that peripherally generated Treg in this model could be the source of TGF-β driving IL-17 production, because IL-17 production has tapered off by the time a significant Treg population is present.
3.2. Protective and potentially pro-inflammatory roles of Treg
Bettelli et al showed that TGF-β, although extensively shown to have protective properties in various models of autoimmunity, can have deleterious effects in EAE mediated by interleukin-17. In an EAE model where TGF-β transgenic mice were immunized with MOG 35–55 peptide the TGF-β transgene exacerbated the disease and this was associated with increased production of IL-17 and decreased numbers of FoxP3+ cells [6]. While one could hypothesize based on these data that regulatory T cells may actually exacerbate the skin disease depending on the timing of their appearance, in our model we have failed to demonstrate any such effect by co-transferring Treg at various time points. In our model Treg are found when the Th17 response has already declined. In order to evaluate whether Treg, either by TGF-β or other mechanisms, have the capacity to increase the Th17 response we co-transferred thymically generated Treg together with naive DO11 Rag−/− cells. These experiments showed that Treg may indeed increase the ratio of IL-17 producing cells, however, their effect on limiting the initial proliferation of naïve T cells is so profound that the net effect is a severe reduction of absolute Th17 cell numbers.
We have also found that the co-transferred Treg substantially inhibited Th1 cytokines including IL-2 and IFN-γ. While IFN-γ does not play a pathogenic role in our model but rather a protective one by limiting IL-17 production, IL-2 is crucially important for pathogenesis, and in the absence of IL-2, mortality and systemic disease is almost completely abrogated. By co-transferring Treg at different time points after the effector cells have already accumulated we have attempted to define a time point at which the Treg mediated inhibition of cell proliferation is irrelevant and the Treg mediated shift towards Th17 producing cells may predominate. However, at any time point and cell dose that we studied, the protective effect of Treg, either by limiting proliferation and accumulation of effector cells or by limiting certain types of effector cytokines, always outweighed their potential to increase inflammation and pathogenicity by promoting Th17 cells. It is, therefore, unclear if there are any circumstances under which the effect of promoting Th17 cells by Treg dominates over their protective function and thus makes them pathogenic. It is possible that Treg influence other proinflammatory targets in a similar way as they promote IL-17. However, given that there are many studies demonstrating the protective effect of Treg in a variety of different models, under most circumstances their protective effect appears to outweigh their inflammatory potential.
3. 3. Role of IL-2 in controlling and stimulating pathologic T cell responses
We addressed the role of IL-2 in the development of disease in our model system by using donor T cells and recipients on an IL-2 KO background. Since IL-2 has been described as a crucial cytokine for both Th1 development and Treg generation, we hypothesized that disease would be exacerbated since both the Th1 response as well as the regulatory T cell development confer a protective effect. Indeed, we observed significant worsening of the skin disease associated with larger numbers of antigen-specific Th17 cells that migrated to the skin in the absence of IL-2. Transferred IL-2 KO T cells produce IL-17 to a much larger extent than WT T cells. Interestingly, only the skin disease was more severe, while systemic disease as measured by weight loss and wasting was significantly milder [19, 25]. This is different from published data on in vitro induction of IL-17 production where there was no difference in IL-17 production in the presence or absence of IL-2. However, other in vivo studies also show that IL-17 production is increased in the absence of IL-2 [32], confirming that IL-2 does play a role in limiting IL-17 production in vivo.
These and data from other groups demonstrate that a particular cytokine cannot strictly be perceived as either protective or pathogenic but the exact effects that a cytokine has depend on multiple parameters.
Finally, these results raise an important question of how stable or plastic the various T cell lineages are, an issue that is currently under active investigation in many labs. Recent data from Xu et al have shown that in vitro stimulation of CD4+FoxP3+ T cells from FoxP3-reporter mice in the presence of IL-6 led to differentiation of FoxP3+ cells into IL-17 producing cells. Interestingly, the FoxP3+ cells did not differentiate into IFN-γ producing cells in vitro if stimulated under Th1 differentiation conditions [33]. Thus, some plasticity between FoxP3+ cells and Th17 cells appears to exist, whereas the dichotomy between FoxP3+ cells and Th1 appears to be more rigid, at least in vitro.
4. Conclusions
In summary, many variables contribute to regulation and effector functions of Th1, Th17 and regulatory T cells. Most of the available data suggest that strong inflammation mediated by IL-6 or other cytokines results in early Th17 differentiation, which gives way to subsequent Th1 development and inhibits Treg development. Inhibition of Th1 development promotes Th17 differentiation and increases the pathology that is mediated by IL-17. IL-2 has multiple roles in this sequence of events. It is a major factor for Th1 development but also for Treg differentiation and limits IL-17 production. In our model, lack of IL-2 leads to both higher numbers of Th17 cells in the peripheral organ and lymphatic system, demonstrating that strong induction of pathogenic Th17 cells can occur in the absence of Treg and their ability to produce TGF-β is completely dispensable. IL-17 has been shown to have pathogenic effects in models of EAE and arthritis and affects skin disease in our model. These results provide insight into the complex relations between various CD4+ T cell subsets. The potential relationship between Th1, Th17 and regulatory T cells is demonstrated in Figure 2.
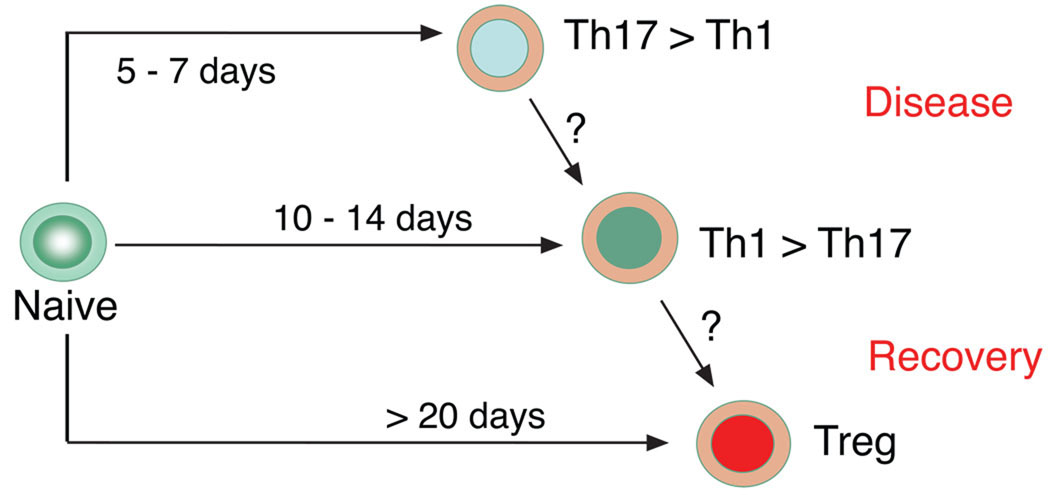
Figure 2. Model of plasticity and lineage relationship of Th1, Th17 effector cells and regulatory T cells.
Transfer of naïve CD4 T cells into lymphopenic antigen-expressing recipients leads to initial Th17 development that is followed by predominance of IFN-γ producing cells. Peripherally generated regulatory T cells appear during the recovery phase of the disease.
It has also become clear that the development of these CD4+ helper cell subtypes is crucially dependent on multidimensional variables. These include 1) the specific disease model, which may be defined by the kind and the distribution of the antigen that elicits the immune response, 2) the timing of cytokine secretion by the T cells themselves, by the stimulating APC, or other bystander cell populations, 3) the organ site involved, and 4) other stimulatory events, which may differ on the specific type of APC and TLR profile involved. Furthermore, lymphopenia might play a role in exacerbating T cell activation and leading to breakdown of T cell tolerance. Potential scenarios include prolonged or increased effector differentiation, imbalances in the differentiation of effector and regulatory cells and prevention or slower kinetics of anergy induction. Interestingly certain autoimmune diseases such as systemic lupus erythematosus and type 1 diabetes may be associated with some degree of lymphopenia. The effect of lymphopenia on T cell activation, however, will likely depend on multifactorial changes within the host, which will be difficult to tease out.
This multidimensional scenario may be complicated even more by possible plasticity and de-differentiation between these subpopulations. While in vitro studies are the indispensable first step to define basic mechanisms, more in vivo studies are needed to further define the significance of each of these regulatory pathways in vivo in mice and ultimately in man.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 2.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 9.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 10.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 12.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol. 2005;137 Suppl 1:51–54. doi: 10.1159/000085432. [DOI] [PubMed] [Google Scholar]
- 14.Linden A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15:973–977. doi: 10.1034/j.1399-3003.2000.15e28.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 16.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 17.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 18.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoechel B, Lohr J, Zhu S, Wong L, Hu D, Ausubel L, Abbas AK. Functional and molecular comparison of anergic and regulatory T lymphocytes. J Immunol. 2006;176:6473–6483. doi: 10.4049/jimmunol.176.11.6473. [DOI] [PubMed] [Google Scholar]
- 22.Deeg HJ, Storb R. Graft-versus-host disease: pathophysiological and clinical aspects. Annu Rev Med. 1984;35:11–24. doi: 10.1146/annurev.me.35.020184.000303. [DOI] [PubMed] [Google Scholar]
- 23.Klingebiel T, Schlegel PG. GVHD: overview on pathophysiology,incidence, clinical and biological features. Bone Marrow Transplant. 1998;21 Suppl 2:S45–S49. [PubMed] [Google Scholar]
- 24.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 25.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi D, Wakita D, Tajima M, Ashino S, Iwakura Y, Zhang Y, Chamoto K, Kitamura H, Nishimura T. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a Th17-independent manner. Int. Immunol. 2007;19:1431–1440. doi: 10.1093/intimm/dxm114. [DOI] [PubMed] [Google Scholar]
- 29.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 30.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 31.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]