Abstract
Background and Aims
Species may occur over a wide geographical range within which populations can display large variation in reproductive success and genetic diversity. Neotinea maculata is a rare orchid of conservation concern at the edge of its range in Ireland, where it occurs in small populations. However, it is relatively common throughout the Mediterranean region. Here, factors that affect rarity of N. maculata in Ireland are investigated by comparing Irish populations with those found in Italy, where it is more common.
Methods
Vegetation communities, breeding system and genetic diversity were compared using three amplified fragment length polymorphism (AFLP) primer pairs in populations in Ireland and Italy. Vegetation was quantified using quadrats taken along transects in study populations, and hand pollination experiments were performed to assess reliance of N. maculata on pollinators in both Irish and Italian populations.
Key Results
Neotinea maculata occupies different vegetation communities in Italian and Irish populations. Breeding system experiments show that N. maculata is 100 % autogamous, and there are no differences in fruit and seed production in selfed, outcrossed and unmanipulated plants. AFLP markers revealed that Irish and Italian populations have similar genetic diversity and are distinct from each other.
Conclusions
Neotinea maculata does not suffer any negative effects of autogamous reproduction; it self-pollinates and sets seed readily in the absence of pollinators. It occupies a variety of habitats in both Ireland and Italy; however, Irish populations are small and rare and should be conserved. This could be due to climatic factors and the absence of suitable soil mycorrhizas to allow recruitment from seed.
Key words: Neotinea maculata, AFLP, autogamy, conservation, genetic diversity, Lusitanian species, pollination
INTRODUCTION
Species that occur over a large geographical area often have populations that occupy a variety of habitats (Caughley et al., 1988; Hall et al., 1992). However, not all habitats provide populations with equal ecological advantages. Differences in both biotic (e.g. competition, genetic structure and mutualists) and abiotic (e.g. light, moisture and temperature) factors may affect abundance of populations and individuals within populations in a given region (Griggs, 1914; Caughley et al., 1988; Lawton, 1993; Carey et al., 1995). Most species occur at high frequency at only a few sites within their geographical range (Lawton, 1993; Blackburn et al., 1999). One instance in which a species may occur at a lower frequency, in terms of both the number of populations and the number of individuals within populations, is at the edge of its geographical range (Hanski, 1982; Lawton, 1993). This may be, at least in part, due to optimal biotic and abiotic conditions for such species occurring at the core of its distribution, whereas at the edge of the range the species may suffer from a suboptimal environment (Carey et al., 1995; Channell and Lomolino, 2000). Consequently, this has led to the assumption of an ‘abundant centre’ distribution in ecology (e.g. Sagarin and Gaines, 2002).
Edge populations may suffer from reduced reproduction and recruitment (Busch, 2005; Moeller and Geber, 2005). This can be due to a decrease in animal-mediated outcrossing, as pollinators may not be present at the edge of the range (Herlihy and Eckert, 2002), or there may be a lack of specialist pollinators that occur in the centre of the species range. Populations of self-compatible species may autonomously self-pollinate in order to provide reproductive assurance at the edge of their range (Fausto et al., 2001; Herlihy and Eckert, 2002; Busch, 2005). Theoretically, selfing should be favoured when there is low pollinator attention, whereas outcrossing should be favoured when there is low vigour of selfed progeny (inbreeding depression) (Barrett and Harder, 1996; Takebayashi and Morrell, 2001). In addition to lower pollinator abundance, other factors such as soil and vegetation communities may not be suitable for individuals to establish and survive owing to competition or a lack of nutrients (Brown, 1984). As a result, populations may be fewer and smaller at the edge of a species range due to habitat constraints (Gaston and Kunin, 1997).
Populations of species that occur at the edge of the range have been highlighted for conservation concern because of their low abundance and unique ecological regimes (Lesica and Allendorf, 1995). Such populations may experience different selection pressures owing to different environmental conditions (e.g. selection for autogamy in times of low pollinator abundance; Kalisz and Vogler, 2003). Also, the expected heterozygosity, number of alleles and proportion of polymorphic loci can increase with increasing population size and be lower in small populations (Leimu et al., 2006). They may be highly inbred and suffer from low genetic diversity and/or problems with reproductive traits (Ellstrand and Elam, 1993; Vucetich and Waite, 2003), such as impaired stigma receptivity and low pollen viability (e.g. Nelson Hayes et al., 2005). In addition to occurring at the edge of their range, species may be naturally rare in a community or geographical region. Rare species occur in all natural communities, with most communities containing many species with few individuals (Rabinowitz et al., 1986; Gaston and Kunin, 1997). Human-mediated fragmentation of natural habitats has caused many previously common species to become rare (Rathcke and Jules, 1993; Young et al., 1996). An understanding of the differences between natural and human-induced rarity is imperative for appropriate conservation action. Therefore, good knowledge of habitat differences and genetic diversity is necessary for adequate conservation of a species (de Lange and Norton, 2004; Pillon et al., 2007).
Certain plant groups, such as orchids, are adapted for insect-mediated outcrossing (Nilsson, 1992; Tremblay et al., 2005) and have specific habitat requirements (e.g. soil mycorrhizal associations for successful establishment from seed; Rasmussen, 2002). In addition, some species of orchid occur across large geographical ranges, and many are self-compatible (Darwin, 1862; Dressler, 1981; Neiland and Wilcock, 1998). This makes them good model species to test theories related to fitness of populations at the edge of their range. However, little work has been done in this area using orchid species. The dense-flowered orchid, Neotinea maculata, occurs throughout the Mediterranean region in Europe and reaches its western geographic extreme in western Ireland where it has a relatively restricted distribution. In Ireland, N. maculata is considered rare, occurring in the west of the country in only thirteen 10 × 10 km squares (Preston et al., 2002). It forms small, scattered populations in Ireland with few flowering individuals (1–20 flowering individuals; K J. Duffy, pers. obs.). However, it is common in the Mediterranean region and can form large populations (typically >100 individuals). Little is known of its reproductive ecology, although it is thought to be self-compatible and reproduce autogamously (>80 % of flowers per inflorescence mature fruit; van der Cingel, 1995). Neotinea maculata has small flowers (at full anthesis: approx. 2 mm corolla diameter), is scented and may contain a nectar reward, which can help attract potential pollinators (van der Cingel, 1995). Putative pollinators are flies and wasps, though pollinators have never been recorded (van der Cingel, 1995), and pollinators may be present in Italy but not in Ireland. The other members of Neotinea are nectarless and depend on pollinators for outcrossing (N. ustulata, N. tridentata, N. lactea; van der Cingel, 1995; Tali et al., 2004). Neotinea maculata has a staggered flowering period throughout Europe, commencing in March–April in the south Mediterranean, whereas more northerly populations begin flowering in April–May, allowing comparison of populations within one field season.
Here, factors that may influence the limited distribution of N. maculata in Ireland are investigated. Ecology and population genetics of N. maculata were investigated in populations in both Ireland (edge populations) and Italy (centre populations). Specifically, it is hypothesized that at the edge of its range compared with the centre, N. maculata populations: (a) are restricted to a smaller number of vegetation communities; (b) experience reduced fruit and seed production because of a lack of mutualists and increased reliance on self-pollination; and (c) have lower genetic diversity, higher differentiation and higher levels of inbreeding.
MATERIALS AND METHODS
Vegetation analysis
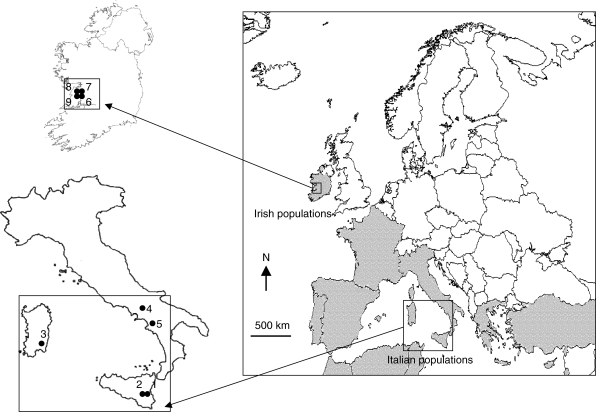
In the spring and early summer of 2006, the vegetation that co-occurs with flowering N. maculata was quantified using 1 × 1 m quadrats taken at regular intervals (5 m intervals in Irish populations; 10 m intervals in Italian populations). These were taken along a 100 m transect in three Italian populations (two in mainland Italy and one in Sardinia) and along a 50 m transect in four Irish populations (see Table 1 and Fig. 1). The length of transect reflected the approximate occurrence of flowering populations of N. maculata. The Vesuvio population was much larger, although all flowering individuals occurred in a homogeneous habitat, which was represented in the quadrats sampled (K. J. Duffy, pers. obs.). Due to logistical constraints, Sicilian populations (Buccheri and Monti Rossi) were not surveyed. All plant species that occurred in each quadrat were recorded, and the percentage abundance was estimated for each species. Composite soil samples were taken from the 0–5 cm layer in at least three patches surrounding N. maculata (which is within the range of the entire root length of N. maculata; K. J. Duffy, pers. obs.) from each population. These were analysed for (a) pH; (b) loss on ignition (LOI,%); (c) available phosphorus (mg kg−1); (d) total C (%); and (e) total N (%).
Table 1.
Soil and vegetation diversity values of populations of N. maculata
| Population | Location | ANP | pH | P (mg/kg) | C ( %) | N ( %) | Organic matter (% LOI) | Mean Shannon diversity index H (± s.d.) |
|---|---|---|---|---|---|---|---|---|
| Buccheri | Sicily | ? | 6·89 | 32·8 | 3·71 | 0·303 | 27·00 | – |
| Monti Rossi | Sicily | ? | 6·75 | 46·4 | 3·91 | 0·208 | 19·00 | – |
| Roccamonfina | Italy | ∼100 | 5·76 | 39·2 | 2·52 | 0·195 | 20·20 | 2·149 (0·190) |
| Vesuvio | Italy | ∼100 | 5·84 | 44 | 30·07 | 1·204 | 71·43 | 0·356 (0·176) |
| San Gregorio | Sardinia | >1000 | 6·51 | 38·4 | 8·91 | 0·519 | 16·38 | 0·664 (0·340) |
| Loch Bunny | Ireland | 30 | 7·73 | 6·4 | 16·18 | 1·393 | 30·35 | 2·072 (0·194) |
| Loch Gealáin | Ireland | 20 | 7·10 | 34·4 | 26·23 | 2·056 | 64·95 | 1·979 (0·225) |
| Mullach Mór | Ireland | 30 | 7·55 | 7·6 | 17·80 | 1·470 | 38·83 | 1·141 (0·216) |
| Oughtmama | Ireland | 20 | 7·84 | 5·2 | 11·04 | 0·679 | 13·28 | 1·893 (0·217) |
P, available phosphorus; C, total carbon; N, total nitrogen; LOI, loss on ignition; ANP, approximate number of plants.
For Italian populations, ANP is the number of flowering plants only. For Irish populations, it is the approximate number of individuals that produced rosettes.
Fig. 1.
Locations of Neotinea maculata study populations. The shaded area represents the approximate distribution of N. maculata. Population number codes: 1 = Monti Rossi; 2 = Buccheri; 3 = San Gregorio; 4 = Roccamonfina; 5 = Vesuvio; 6 = Loch Bunny; 7 = Loch Gealáin; 8 = Oughtmama; 9 = Mullach Mór.
Non-metric multidimensional scaling (NMDS) was used to evaluate vegetation composition and abundance data. Vegetation community analyses were performed in PC-Ord 4 (McCune and Mefford, 1999). The autopilot mode of NMDS in PC-Ord 4 was run at the slow and thorough setting and incorporated a maximum of 400 iterations, a stability criterion of 0·00005 with 20 iterations to evaluate stability, six starting axes, 40 runs with real data, and 50 runs with randomized data. Sorensen's distance and a random starting configuration were used (McCune and Mefford, 1999). A multiresponse permutation procedure (MRPP) was used to test for vegetation differences between all populations and between Ireland and Italy. This procedure calculates a distance measure in ordination space within each group (Sorensen's distance in this case), and tests whether the difference between the observed and expected distances is due to chance. The test statistic (T) describes the separation between the groups; the more negative T is, the stronger the separation between groups. In addition, another statistic (A) describes the within-group homogeneity, compared with random expectation (McCune and Grace, 2002). Vegetation diversity within each population was measured using the Shannon diversity index, as calculated in PC-Ord 4 (McCune and Mefford, 1999).
Breeding system and pollination
In order to assess the extent to which N. maculata relies on pollinators, hand pollination treatments were performed. Twenty inflorescences were bagged prior to anthesis, and the following treatments were each performed on five flowers from five inflorescences when flowers opened: (a) pollen removal (emasculation) to test for spontaneous seed production (agamospermy); (b) within-flower pollen transfer (autogamy); (c) pollen added from an individual >10 m away (xenogamy); and (d) no manipulation (control). These treatments were performed on individuals in two Italian populations (Roccamonfina and Vesuvio) and two Irish populations (Loch Bunny and Loch Gealáin). As N. maculata has small flowers, a 12× head lens with a headlight was used to observe flowers and a fine-tipped wooden stick was used to transfer pollen. In addition, fruits were collected from unbagged individuals in each population. Mature capsules were collected and put in Eppendorf® or Falcon tubes with some silica gel and stored at 4 °C, and were counted within 1 year of collection. The total number of fruit set was recorded, and seed per fruit from treated individuals was estimated. As orchid seeds are minute, the seeds were liberated from the capsule, spread evenly and the remaining seeds stuck in the capsule were washed with 70 % ethanol. Unlike water, ethanol does not have surface tension, which stops seeds from clumping and allows for a more even spread on the Petri dish. Seeds were counted immediately as it was noticed that the alcohol dehydrates seeds if left immersed for >2 h. As orchid capsules contain thousands of seeds, to estimate the number of seeds per fruit, the number of seeds from 10 × 10 mm squares were sub-sampled using a 9 cm diameter circle of graph paper (to fit the underside of a Petri dish). All the seeds within 10 randomly sampled squares were counted and they were multiplied by the total number of squares occupied by seeds.
Differences in seed set were tested for among (a) pollination treatment (agamospermy, autogamy, xenogamy and unmanipulated); and (b) population, using a two-factor analysis of variance (ANOVA). For this analysis, R 2·6·1 was used (R Core Development Team, 2007).
To test whether N. maculata offers a reward to flower visitors, an attempt was made to extract nectar using 0·5 µL microcapillary tubes. However, because N. maculata has minute flowers it was not possible to extract nectar using microcapillary tubes or other methods (e.g. folded filter paper; Dafni, 1992). Instead, tests were made for the presence/absence of a sugar reward by adding 1 µL of distilled water to the base of the labellum with a microcapillary tube and the resulting solution was tested for sugar using a standard diabetic test kit (Dafni, 1992).
To test for both pollen viability and stigma receptivity, one flower from ten separate inflorescences was examined in each of two populations in Italy and two populations in Ireland. Pollen was stained with Alexander's stain (Alexander, 1980) to test for viability. Stigmas were tested for receptivity using Peroxtesmo Ko test paper (Dafni and Maues, 1998).
Molecular methods
No more than one-half of a leaf from two- or more-leaved individuals was taken. All samples were collected and stored in zip-lock plastic bags containing a mean of 14·2 g (s.e. = 1·3 g; n = 10) of silica gel (Chase and Hills, 1991) for between 1 and 8 months until total DNA was extracted. Leaf material was sampled from five Italian populations (Buccheri, Monti Rossi, San Gregorio, Roccamonfina and Vesuvio) and three Irish populations (Loch Bunny, Loch Gealáin and Mullach Mór). Sampling of Irish N. maculata was undertaken during December 2006 when fresh leaves had emerged, and Italian samples were collected in the field during other experimental work in spring 2006 from individuals in full anthesis.
A DNA fingerprinting method was employed using amplified fragment length polymorphisms (AFLPs; Vos et al., 1995), which has been successfully used across a wide range of orchid taxa (e.g. Smith et al., 2004; Flanagan et al., 2006; Tali et al., 2006; Pillon et al., 2007). AFLPs were chosen for this study because they require no prior knowledge of the DNA sequence and provide large amounts of data with reproducible results. In addition, AFLPs were recently successfully used on the congeneric N. ustulata (Tali et al., 2006). DNA was extracted from approx. 0·05–0·1 g of dried material using a modified 2× CTAB (cetryltrimethyl ammonium bromide) procedure (Doyle and Doyle, 1987), purified on a QIAquick column (Qiagen Ltd, Crawley, UK) according to the manufacturer's protocol and quantified using a spectrophotometer. AFLP analysis was performed according to the AFLP Plant Mapping protocol of Applied Biosystems Inc. (Foster City, CA, USA). Sampled DNA was restricted with the endonucleases EcoRI and MseI, and ligated to appropriate double-stranded adaptors according to the manufacturer's protocols. Two steps of amplification followed: a pre-selective amplification using primer pairs with one selective base was followed by a selective amplification with additional selective bases to reduce the number of fragments further. Because genome size can have a marked effect on the quality of AFLP traces (Fay et al., 2005) and the congeneric N. ustulata has a large genome (Tali et al., 2006), the Applied Biosystems protocol, which uses three selective base pairs on each primer in the selective amplification, was modified by incorporating an additional base on one primer of each pair. For this second amplification, 12 primer combinations were tested, of which three pairs with the following selective bases were chosen for the full study: -CTAT/-ACT, -CTAA/-AGG and -CTAA/-ACC.
AFLP profiles were manually scored as presence/absence. Bands with evidence of small, unscorable peaks were discarded from all samples. Bands ranging from 50 to 500 bp were scored. To reduce genotyping error, AFLP profiles were scored twice, and all samples were blind to the individual who scored them. The following statistical analyses were performed using GenAlEx 6·1 (Peakall and Smouse, 2006). The calculation of genetic distances followed the method of Peakall et al. (1995) as outlined in Maguire et al. (2002) as:
where n is the total number of polymorphic bands and 2nxy is the number of markers shared by two individuals. This gives a Euclidean metric as required for subsequent analysis of molecular variance (AMOVA). Genetic distance matrices for each AFLP primer set on their own and the total data set (three primer sets combined) were calculated. Mantel tests were performed (999 permutations) to test whether genetic patterns detected by one AFLP primer set were congruent with the patterns detected by the other primer sets. The overall genetic diversity for each population and each region was calculated. Genetic structure was tested by AMOVA on the genetic distance matrix (999 permutations). AMOVA output nomenclature follows that of Excoffier et al. (1992) in that variation was summarized both as the proportion of the total variance and as ϕ-statistics (F-statistic analogues). Genetic differentiation was tested for (a) within populations; (b) between populations; and (c) between Ireland and Italy. In addition, a non-hierarchical AMOVA was performed to test population differentiation in Ireland and Italy separately. Based on output of the individual genetic distance matrix, a principle coordinate (PCO) analysis was performed to group individuals. In addition, an UPGMA tree was produced using POPGENE 1·3·2 (Yeh et al., 2000) to analyse relationships among populations.
RESULTS
Vegetation
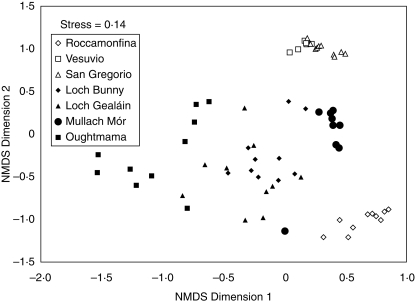
A total of 70 quadrats were analysed (30 in Italian populations; 40 in Irish populations) with a total of 80 vascular plant species found among all quadrats surveyed. NMDS analysis determined that a 2-D solution had the least stress (stress = 0·14) of vegetation data, with a sum of 82·5 % of the variation explained by this output. This analysis revealed that N. maculata occurred in areas with a different species composition in Italian and Irish populations (Fig. 2). The MRPP analysis revealed significant differences between Irish and Italian vegetation communities (T = –20·78; A = 0·129; P < 0·001). In addition, there were significant differences within Italian (T = –15·19; A = 0·547; P < 0·001) and Irish populations (T = –18·65; A = 0·304; P < 0·001), indicating that N. maculata occupies different vegetation communities in both regions. There was a clear dichotomy in Italian populations, with the Roccamonfina population clearly separated from the San Gregorio and Vesuvio populations (Fig. 2). Species diversity and soil property values for each population are given in Table 1, with a wide range of values given for each. Available P and pH are negatively correlated (Spearman rank correlation; rs = –0·867; P = 0·005); both total C (rs = 0·812; P = 0·01) and total N (rs = 0·711; P = 0·032) are positively correlated with organic matter content (LOI). Mean Shannon diversity values (H) ranged from 0·356 to 2·149 per population, highlighting the wide range in species richness of vegetation associated with N. maculata.
Fig. 2.
NMDS plot of sample scores of vegetation community data on axes 1 and 2. Open symbols indicate quadrats from Italian populations; filled symbols indicate quadrats from Irish populations. Axis 1 accounts for 39·2 % of the variation and axis 2 accounts for 43·3 % of the variation.
Breeding system
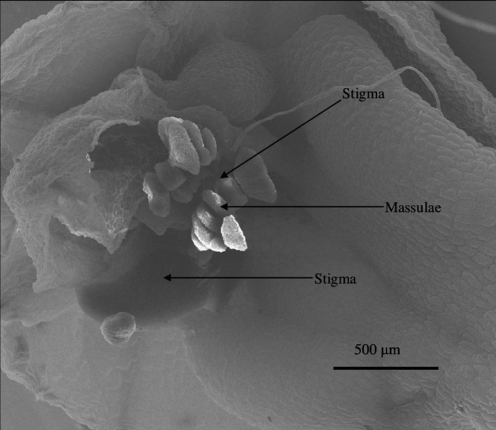
No differences in seed set were found according to either autogamy or xenogamy treatments or unmanipulated flowers (F1,56 = 0·128; P = 0·72) in either centre or edge populations. All flowers produced fruit. However, flowers that were emasculated failed to set fruit and produced no seed. Capsules contained an estimated mean of 1355 (s.e. ±52·5) seeds. This indicates that N. maculata is fully self-compatible and autonomously autogamous although not agamospermous, because it requires pollen on its stigma in order to set seed. However, pseudogamous apomixis may occur (where pollen does not participate in embryo formation). Some flowers were observed in both Irish and Italian populations which had self-pollinia on the stigma before flower opening (Fig. 3).
Fig. 3.
SEM image depicting the massulae of N. maculata already on the stigma of an unopened flower from Roccamonfina observed by K.J.D. and S.C.
The diabetic test for the presence of sugars was positive, indicating the presence of a small reward for potential flower visitors at the base of the labellum. All flowers tested in all populations had both viable pollinia and receptive stigmas.
Molecular data
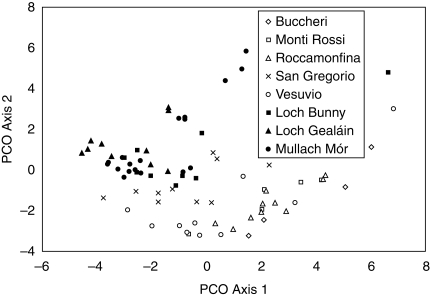
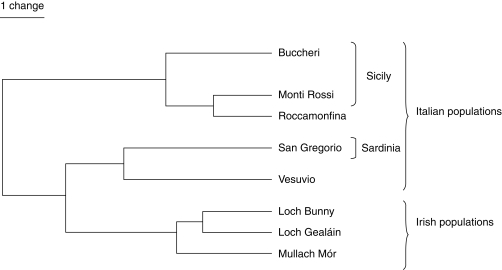
A total of 223 interpretable bands was produced: 90 -CTAT/-ACA, 78 -CTAA/-ACC and 55 -CTAA/-AGG among the 79 N. maculata individuals surveyed. Overall, 219 bands were polymorphic (98·2 %). Mantel tests revealed significant relationships between primer combinations -CTAT/-ACA and -CTAA/-ACC (rxy = 0·339; P < 0·001), -CTAA/-AGG and -CTAA/-ACC (rxy = 0·193; P < 0·001), but there were no relationships between primer combinations -CTAT/-ACA and -CTAA/-AGG (rxy = 0·112; P = 0·069). However, genetic diversity was similar within both Irish and Italian populations (Table 2), with similar overall diversity in both Ireland (0·238 ± 0·012) and Italy (0·248 ± 0·013). AMOVA revealed significant genetic differences within populations, between populations and between regions (Table 3). In contrast, the PCO analysis output (Fig. 4) and both a Neighbor-Joining and UPGMA analysis (results not shown) on individuals show no clear differentiation of populations. However, the PCO analysis shows separation of Irish and Italian regions. On the other hand, the UPGMA tree based on overall population data (Fig. 5) shows a distinction between Irish and Italian populations, which supports the AMOVA results. Different ϕ-values were obtained for both Ireland and Italy (ϕPT Ireland = 0·103; ϕPT Italy = 0·185), indicating that Irish populations have lower differentiation.
Table 2.
Details of sample populations used for molecular analysis, numbers of individuals sampled per population and AFLP banding patterns and genetic diversity within populations
| Population | Location | No. of individuals analysed | Total no. of bands | No. of private bands | Mean genetic diversity (± s.e.) |
|---|---|---|---|---|---|
| Buccheri | Sicily | 4 | 121 | 3 | 0·137 (0·013) |
| Monti Rossi | Sicily | 5 | 136 | 5 | 0·129 (0·012) |
| San Gregorio | Sardinia | 10 | 130 | 0 | 0·131 (0·012) |
| Roccamonfina | Italy | 10 | 172 | 8 | 0·188 (0·013) |
| Vesuvio | Italy | 10 | 174 | 8 | 0·244 (0·013) |
| Loch Bunny | Ireland | 10 | 144 | 1 | 0·220 (0·015) |
| Loch Gealáin | Ireland | 10 | 162 | 2 | 0·146 (0·013) |
| Mullach Mór | Ireland | 20 | 157 | 4 | 0·212 (0·014) |
Table 3.
Results of AMOVA for the AFLP data set based on three primer combinations
| Source | d.f. | SS | MS | Estimated variation | % Variation | Statistic | Value | P |
|---|---|---|---|---|---|---|---|---|
| Within populations | 71 | 1556·0 | 35·3 | 21·92 | 72 % | ϕPT | 0·283 | 0·001 |
| Among populations | 6 | 353·7 | 58·9 | 4·02 | 13 % | ϕPR | 0·155 | 0·001 |
| Among regions | 1 | 252·9 | 252·9 | 4·65 | 15 % | ϕRT | 0·152 | 0·001 |
| Total | 78 | 2162·6 | 30·58 |
Fig. 4.
Principal coordinate (PCO) plot of the first and second axes for the full data matrix based on three AFLP primers. Open symbols indicate Italian populations; filled symbols indicate Irish populations. Axis 1 accounts for 42·72 % of the variation and axis 2 accounts for 24·61 % of the variation.
Fig. 5.
Rooted UPGMA tree depicting relationships between the populations studied based on Nei's genetic distance (Nei, 1972).
DISCUSSION
Vegetation and habitats of N. maculata
Neotinea maculata occupies a different vegetation community in all populations studied. The present analysis showed that N. maculata occurs in a wide range of vegetation communities and that it occurs in relatively species-rich and species-poor habitats. Isolated individuals have been recorded at the margins of woodland in the Burren region in the west of Ireland, which is similar to the common Mediterranean habitat of this species. However, most flowering populations of N. maculata in Ireland occur in grassland with limestone outcrops (K. J. Duffy, pers. obs.). In Ireland, N. maculata is considered to be a member of the Lusitanian species suite where it belongs to a group of species that occur only in the west of Ireland and the Mediterranean (Mitchell and Ryan, 2001). Because orchid recruitment from seed is generally dependent on appropriate soil mycorrhizas (Rasmussen, 2002), the distribution of suitable mycorrhizal fungi in habitats may also explain the disjunct European distribution of N. maculata. Indeed, N. maculata has mycorrhizae taxa such as Tulasnella, Leptodontidium and Ceratobasidium associated with its roots from samples examined from both Irish and Mediterranean populations; however, the precise role of mycorrhizal symbionts in germination of seeds and seedling development has yet to be established in this species (M. I. Bidartondo, pers. comm., 2008). In addition, the presence of suitable local microsite conditions for seedling establishment has been shown to be important for orchids (Jacquemyn et al., 2007; Jersakova and Malinova, 2007) and needs to be established for N. maculata. Suitable microsite conditions can include abiotic conditions such as soil characteristics, like those examined in this study, in addition to suitable mycorrhizas. The present data suggest that N. maculata can grow in a wide range of soil types independently of region. This is similar to N. ustulata, which can also occupy different habitats with varying soil chemical properties (Tali et al., 2004).
Breeding system and pollination
Neotinea maculata is a fully autogamous species that can produce fruit and seed in the absence of pollinators. This pollination system is believed to be an evolutionary ‘dead-end’ (Stebbins, 1957; Takebayashi and Murrell, 2001). However, it may have advantages such as an escape from a dependency on pollinators (Morgan and Wilson, 1999; Kalisz et al., 2004). In addition, an equivalent quantity of seed was produced when cross-pollinated compared with selfed, and therefore N. maculata probably benefits from occasional cross-pollination, coupled with autonomous self-pollination. Pseudogamous apomixis may occur in this species; however, given the high levels of polymorphism in the AFLP data, it is unlikely. During daytime field observations of pollinator visitation over the course of this study in both Irish and Italian populations of N. maculata, only one syrphid in the Vesuvio population was seen visiting an N. maculata inflorescence; the visit lasted approx. 5 s and the fly did not visit any other flowers in the vicinity. No pollinarium removal was observed. With such infrequent visitation, autonomous autogamy may evolve if pollinators are unreliable in delivering pollen (Kalisz and Vogler, 2003; Kalisz et al., 2004). It was noticed that some flowers of N. maculata had massulae on their stigma before flower opening, which effectively means these flowers are cleistogamous, a reproductive strategy that may provide reproductive assurance at the population level (Berg and Redbo-Torstensson, 1998; Lu, 2002). However, the timing of pollen drop onto the stigma (whether some individuals delay selfing to allow outcrossing and some individuals automatically self-pollinate in order to provide reproductive assurance for the population) requires further investigation. In addition, the role of the rostellum and when it breaks down during anthesis in both early and late self-pollinating individuals should be investigated further. In comparison, N. ustulata has an average fruit set of 20·9 % in Estonian populations, with each capsule containing between 2000 and 4000 seeds (Tali et al., 2004). Other members of Neotinea, such as N. ustulata, are food-deceptive and obligate outcrossers, whereas N. maculata is the only rewarding autogamous species. The evolution of autogamy is normally associated with colder habitats in northern latitudes (Tremblay et al., 2005). It would be interesting to investigate when Irish populations became isolated from Mediterranean populations and if autogamy evolved separately in both regions or spread from one region to another.
Genetic differentiation and diversity in N. maculata
The genetic variability revealed by the AFLP markers was high, as 98·2 % of the bands scored were polymorphic. The values of genetic differentiation obtained in this study are similar to those in other orchid studies (mean GST value among all species = 0·187; Forrest et al., 2004). Although there is significant variation between populations in Ireland and Italy, the resulting genetic differentiation is lower than that in congeneric N. ustulata (mean FST = 0·51; Tali et al., 2006). Neotinea ustulata is a food-deceptive species, and these generally have lower population differentiation than rewarding species (average GST = 0·2–0·3 for rewarding species; GST 0·1–0·15 for deceptive species; Cozzolino and Widmer, 2005). The genetic diversity values within populations of N. maculata were found to range from 0·129 to 0·244. In comparison, Pillon et al. (2007) found lower diversity in the endangered Liparis loeselii (range: 0·017–0·146; mean = 0·038). Given its strong self-compatibility and autonomous autogamous pollination system, N. maculata has probably purged its genetic load as high levels of polymorphism were observed among AFLP markers found in this study (Charlesworth and Charlesworth, 1987). The comparatively lower diversity values may be due to a high level of autonomous selfing in N. maculata, which does not occur, for example, in N. ustulata (Tali et al., 2004). The lack of resolution in the PCO analysis based on all individuals highlights the fact that many individuals share bands and, although there is some evidence of population separation, most of the variation (72 %) is explained within populations. However, when grouped into discrete populations and regions, both UPGMA and AMOVA revealed significant genetic differentiation between Ireland and Italy and between all populations. It would be worthwhile developing specific microsatellite markers that may be useful in revealing fine-scale population structure and detection of alleles, which dominant markers such as AFLP cannot detect (Selkoe and Toonen, 2006; Meudt and Clarke, 2007). Future work should focus on sampling more populations across the range of N. maculata to test whether such populations are genetically distinct.
Conservation issues
The ‘abundant centre distribution’ hypothesis is a widely held view in biogeographical ecology (Sagarin and Gaines, 2006). However, this hypothesis may not hold true, given that, in a review of studies addressing this biogeographic question, only 39 % of 145 independent empirical tests support the abundant centre hypothesis using lenient criteria (Sagarin and Gaines, 2002). Indeed, Sagarin and Gaines (2006) pointed out that many studies fail to examine the entire range of focal species, which can lead to potential errors in interpretation. As the entire distribution of N. maculata was not examined here, a generalized statement cannot be made regarding its fitness over its geographical range. However, it can be said that N. maculata is not affected by a distribution that is more abundant in the centre of its range, because its population genetic diversity is similar at the edge and core of its distribution, and populations can occupy different vegetation communities in both regions. In addition, this study shows that N. maculata does not require pollinators for successful seed set at either the centre or edge of its distribution, and it does not suffer from deleterious effects of self-pollination, in terms of fruit and seed output. However, given (a) the unique vegetation communities in Ireland (e.g. it co-flowers with alpine plants such as Dryas octopetala and Gentiana verna in some populations); (b) the level of genetic differentiation compared with Italian populations; and (c) the small number of populations and individuals flowering within populations, Irish populations of N. maculata merit conservation attention. These genetic data support the hypothesis that N. maculata is native to Ireland and does not represent a recent introduction. Conservation measures should be in the form of annual monitoring to gauge flowering fluctuations in natural populations and ensure populations are not extirpated. For instance, in N. ustulata, dormancy has been shown to decrease adult survival (Shefferson and Tali, 2007) and therefore effects of dormancy on the survival of Irish populations of N. maculata should be investigated. Much work needs to be done to understand the factors governing the distribution and abundance of orchid species. Neotinea maculata offers a potential model species for further investigation of the role of mycorrhizal ecology in determining orchid species distribution. It is suggested that isolation and identification of mycorrhizal taxa associated with N. maculata are important. Specifically, this could be achieved by seed baiting in the field (e.g. Rasmussen and Whigham, 1993) and examination of the roots of mature individuals in various populations across the range of the orchid. This ideally should be coupled with in vitro germination of individuals from those populations (e.g. Rasmussen, 2002) to determine the role of mycorrhizal taxa in germination and development in this species.
CONCLUSIONS
Neotinea maculata is an autogamous self-pollinating orchid species. However, it was found that it does not suffer negative effects resulting from self-pollination in populations examined at both the centre and edge of its distribution. It occurs in different vegetation communities, each with varying soil properties among populations examined in this study. AFLP analysis revealed that populations in the centre and edge of its distribution share similar levels of genetic diversity; however, there are significant genetic differences among all populations and between Italy and Ireland. In Ireland, it flowers in smaller populations than in Italy and is restricted in its distribution. Therefore, Irish populations deserve specific conservation attention, as habitat destruction and management will have greater effects on population persistence than in Italian populations.
ACKNOWLEDGEMENTS
We are grateful to Antonio Croce for valuable help with plant identification in Italy and fieldwork, Pierluigi Cortis for hospitality and logistical support in Sardinia, Brendan Sayers for discussions and help with locating populations of N. maculata in Ireland, Naomi Kingston for early discussions, and Maria Rosaria Barone Lumaga for providing the scanning electron microscope image. We thank Trevor Hodkinson, Maeve Harbourne, Atchara Teerawantanon, Robyn Cowan and Yohan Pillon for assistance with molecular work, and Siobhan McNamee and Mark Kavanagh for help with soil analysis. We are grateful to two anonymous reviewers and Ilia Leitch for their help in improving the manuscript. We thank Vesuvio National Park for issuing collecting licences. Work was carried out on Irish populations of N. maculata under Department of the Environment, Heritage and Local Government licence number 8/2004. This work was funded by a National Parks and Wildlife Service research grant, a University of Dublin, Trinity College Start-Up Grant (both awarded to J.C.S.), and a University of Dublin, Trinity College Postgraduate Award, a David A. Webb Memorial Prize and an Oleg Polunin Memorial Prize (all awarded to K.J.D.) all of which are gratefully acknowledged.
LITERATURE CITED
- Alexander MP. A versatile stain for pollen, fungi, yeast, and bacteria. Stain Technology. 1980;55:13–18. doi: 10.3109/10520298009067890. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. Ecology and evolution of plant mating. Trends in Ecology and Evolution. 1996;11:73–79. doi: 10.1016/0169-5347(96)81046-9. [DOI] [PubMed] [Google Scholar]
- Berg H, Redbo-Torstensson P. Cleistogamy as a bet-hedging strategy in Oxalis acetosella, a perennial herb. Journal of Ecology. 1998;86:491–500. [Google Scholar]
- Blackburn TM, Gaston KJ, Quinn RM, Gregory RD. Do local abundances of British birds change with proximity to range edge? Journal of Biogeography. 1999;26:493–505. [Google Scholar]
- Brown JH. On the relationship between abundance and distribution of species. American Naturalist. 1984;124:255–279. [Google Scholar]
- Busch JW. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae) American Journal of Botany. 2005;92:1503–1512. doi: 10.3732/ajb.92.9.1503. [DOI] [PubMed] [Google Scholar]
- Carey PD, Watkinson AR, Gerard FFO. The determinants of the distribution and abundance of the winter annual grass Vulpia ciliata ssp. ambigua. Journal of Ecology. 1995;83:177–187. [Google Scholar]
- Caughley G, Grice D, Barker R, Brown B. The edge of the range. Journal of Animal Ecology. 1988;57:771–785. [Google Scholar]
- Channell R, Lomolino MV. Dynamic biogeography and conservation of endangered species. Nature. 2000;403:84–86. doi: 10.1038/47487. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Chase MW, Hills HG. Silica gel: an ideal material for field preservation of leaf samples. Taxon. 1991;40:215–220. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination: European orchids. Rotterdam: A. A. Balkema; 1995. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends in Ecology and Evolution. 2005;20:487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dafni A. Pollination ecology: a practical approach. Oxford: IRL Press at Oxford University Press; 1992. [Google Scholar]
- Dafni A, Maues MM. A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction. 1998;11:177–180. [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochemical Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Dressler RL. The orchids: natural history and classification. London: Harvard University Press; 1981. [Google Scholar]
- Ellstrand NC, Elam DR. Population genetic consequences of small population size: implication for plant conservation. Annual Review of Ecology and Systematics. 1993;24:217–242. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes – application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto JA, Eckhart VM, Geber MA. Reproductive assurance and the evolutionary ecology of self-pollination in Clarkia xantiana (Onagraceae) American Journal of Botany. 2001;88:1794–1800. [PubMed] [Google Scholar]
- Fay MF, Cowan RS, Leitch IJ. The effects of nuclear DNA content (C-value) on the quality and utility of AFLP fingerprints. Annals of Botany. 2005;95:237–246. doi: 10.1093/aob/mci017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan NS, Peakall R, Clements MA, Otero JT. Conservation of taxonomically difficult species: the case of the Australian orchid. Microtis angusii. Conservation Genetics. 2006;7:847–859. [Google Scholar]
- Forrest AD, Hollingsworth ML, Hollingsworth PM, Sydes C, Bateman RM. Population genetic structure in European populations of Spiranthes romanzoffiana set in the context of other genetic studies on orchids. Heredity. 2004;92:218–227. doi: 10.1038/sj.hdy.6800399. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Kunin WE. Rare–common differences: an overview. In: Kunin WE, Gaston KJ, editors. The biology of rarity: causes and consequences of rare–common differences. London: Chapman and Hall; 1997. pp. 12–29. [Google Scholar]
- Griggs RF. Observations on the behavior of some species at the edges of their ranges. Bulletin of the Torrey Botanical Club. 1914;41:25–49. [Google Scholar]
- Hall CAS, Stanford JA, Hauer FR. The distribution and abundance of organisms as a consequence of energy balances along multiple environmental gradients. Oikos. 1992;65:377–390. [Google Scholar]
- Hanski I. Dynamics of regional distribution – the core and satellite species hypothesis. Oikos. 1982;38:210–221. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Vandepitte K, Honnay O, Roldan-Ruiz I, Wiegand T. A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea. New Phytologist. 2007;176:448–459. doi: 10.1111/j.1469-8137.2007.02179.x. [DOI] [PubMed] [Google Scholar]
- Jersakova J, Malinova T. Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytologist. 2007;176:237–241. doi: 10.1111/j.1469-8137.2007.02223.x. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology. 2003;84:2928–2942. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- de Lange PJ, Norton DA. The ecology and conservation of Kunzea sinclairii (Myrtaceae), a naturally rare plant of rhyolitic rock outcrops. Biological Conservation. 2004;117:49–59. [Google Scholar]
- Lawton JH. Range, population abundance and conservation. Trends in Ecology and Evolution. 1993;8:409–413. doi: 10.1016/0169-5347(93)90043-O. [DOI] [PubMed] [Google Scholar]
- Leimu R, Mutikainen P, Koricheva J, Fischer M. How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology. 2006;94:942–952. [Google Scholar]
- Lesica P, Allendorf FW. When are peripheral populations valuable for conservation? Conservation Biology. 1995;9:753–760. [Google Scholar]
- Lu YQ. Why is cleistogamy a selected reproductive strategy in Impatiens capensis (Balsaminaceae)? Biological Journal of the Linnean Society. 2002;75:543–553. [Google Scholar]
- Maguire TL, Peakall R, Saenger P. Comparative analysis of genetic diversity in the mangrove species Avicennia marina (Forsk.) Vierh. (Avicenniaceae) detected by AFLPs and SSRs. Theoretical and Applied Genetics. 2002;104:388–398. doi: 10.1007/s001220100724. [DOI] [PubMed] [Google Scholar]
- McCune B, Mefford MJ. PC-ORD. Multivariate Analysis of Ecological Data version 4. Gleneden Beach, OR: 1999. [Google Scholar]
- McCune B, Grace JB. Analysis of ecological communities. Gleneden Beach, OR: MjM Software Design; 2002. [Google Scholar]
- Meudt HM, Clarke AC. Almost forgotten or latest practice? AFLP applications, analyses and advances. Trends in Plant Science. 2007;12:106–117. doi: 10.1016/j.tplants.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Mitchell F, Ryan M. Reading the Irish landscape. Dublin: Town House and Country House; 2001. [Google Scholar]
- Morgan MT, Wilson WG. Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution. 2005;59:1143–1148. [PubMed] [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Nei M. Genetic distance between populations. American Naturalist. 1972;106:283–292. [Google Scholar]
- Neiland MRM, Wilcock CC. Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany. 1998;85:1657–1671. [PubMed] [Google Scholar]
- Nelson Hayes C, Winsor JA, Stephenson AG. A comparison of male and female responses to inbreeding in Cucurbita pepo subsp. texana (Cucurbitaceae) American Journal of Botany. 2005;92:107–115. doi: 10.3732/ajb.92.1.107. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. Orchid pollination biology. Trends in Ecology and Evolution. 1992;7:255–259. doi: 10.1016/0169-5347(92)90170-G. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE, Huff DR. Evolutionary implications of allozyme and RAPD variation in diploid populations of dioecious buffalograss. Buchloe dactyloides. Molecular Ecology. 1995;4:135–147. [Google Scholar]
- Peakall R, Smouse PE. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon Y, Qamaruz-Zaman F, Fay MF, Hendoux F, Piquot Y. Genetic diversity and ecological differentiation in the endangered fen orchid (Liparis loeselii) Conservation Genetics. 2007;8:177–184. [Google Scholar]
- Preston CD, Pearman DA, Dines TD. New atlas of the British and Irish flora. Oxford: Oxford University Press; 2002. [Google Scholar]
- R Core Development Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. URL http://www.R-project.org . [Google Scholar]
- Rabinowitz D, Cairns S, Dillon T. Seven forms of rarity and their frequency in the flora of the British Isles. In: Soulé ME, editor. Conservation biology: the science and scarcity of diversity. Sunderland, MA: Sinauer Associates; 1986. [Google Scholar]
- Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant and Soil. 2002;244:149–163. [Google Scholar]
- Rasmussen HN, Whigham DF. Seed ecology of dust seeds in-situ – a new study technique and its application in terrestrial orchids. American Journal of Botany. 1993;80:1374–1378. [Google Scholar]
- Rathcke BJ, Jules ES. Habitat fragmentation and plant–pollinator interactions. Current Science. 1993;65:273–277. [Google Scholar]
- Sagarin RD, Gaines SD. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecology Letters. 2002;5:137–147. [Google Scholar]
- Sagarin RD, Gaines SD. Recent studies improve understanding of population dynamics across species ranges. Oikos. 2006;115:386–388. [Google Scholar]
- Selkoe KA, Toonen RJ. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Letters. 2006;9:615–629. doi: 10.1111/j.1461-0248.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Shefferson RP, Tali K. Dormancy is associated with decreased adult survival in the burnt orchid. Neotinea ustulata. Journal of Ecology. 2007;95:217–225. [Google Scholar]
- Smith SD, Cowan RS, Gregg KB, Chase MW, Maxted N, Fay MF. Genetic discontinuities among populations of Cleistes (Orchidaceae, Vanilloideae) in North America. Botanical Journal of the Linnean Society. 2004;145:87–95. [Google Scholar]
- Stebbins GL. Self fertilization and population variability in the higher plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Takebayashi N, Morrell PL. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany. 2001;88:1143–1150. [PubMed] [Google Scholar]
- Tali K, Foley MJ, Kull T. Orchis ustulata L. Journal of Ecology. 2004;92:174–184. [Google Scholar]
- Tali K, Fay MF, Bateman RM. Little genetic differentiation across Europe between early-flowering and late-flowering populations of the rapidly declining orchid Neotinea ustulata. Biological Journal of the Linnean Society. 2006;87:13–25. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society. 2005;84:1–54. [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, et al. AFLP – a new technique for DNA-fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetich JA, Waite TA. Spatial patterns of demography and genetic processes across the species' range: null hypotheses for landscape conservation genetics. Conservation Genetics. 2003;4:639–645. [Google Scholar]
- Yeh FC, Yang R, Boyle T. POPGENE, Microsoft windows-based freeware for population genetic analysis, Version 1·32. Edmonton; 2000. [Google Scholar]
- Young A, Boyle T, Brown T. The population genetic consequences of habitat fragmentation for plants. Trends in Ecology and Evolution. 1996;11:413–418. doi: 10.1016/0169-5347(96)10045-8. [DOI] [PubMed] [Google Scholar]