Abstract
Background and Aims
Cypripedium calceolus, although widespread in Eurasia, is rare in many countries in which it occurs. Population genetics studies with nuclear DNA markers on this species have been hampered by its large nuclear genome size. Plastid DNA markers are used here to gain an understanding of variation within and between populations and of biogeographical patterns.
Methods
Thirteen length-variable regions (microsatellites and insertions/deletions) were identified in non-coding plastid DNA. These and a previously identified complex microsatellite in the trnL-trnF intergenic spacer were used to identify plastid DNA haplotypes for European samples, with sampling focused on England, Denmark and Sweden.
Key Results
The 13 additional length-variable regions identified were two homopolymer (polyA) repeats in the rps16 intron and a homopolymer (polyA) repeat and ten indels in the accD-psa1 intergenic spacer. In accD-psa1, most of these were in an extremely AT-rich region, and it was not possible to design primers in the flanking regions; therefore, the whole intergenic spacer was sequenced. Together, these new regions and the trnL-trnF complex microsatellite allowed 23 haplotypes to be characterized. Many were found in only one or a few samples (probably due to low sampling density), but some commoner haplotypes were widespread. Most of the genetic variation was found within rather than between populations (83 vs. 18%, respectively). Two haplotypes occurred from the Spanish Pyrenees to Sweden.
Conclusions
Plastid DNA data can be used to gain an understanding of patterns of genetic variation and seed-mediated gene flow in orchids. Although these data are less information-rich than those for nuclear DNA, they present a useful option for studying species with large genomes. Here they support the hypothesis of long-distance seed dispersal often proposed for orchids.
Key words: Biogeography, Cypripedium calceolus, genome size, plastid microsatellites, population genetics, seed dispersal
INTRODUCTION
Cypripedium calceolus is one of the rarest plant species in the UK, with only one surviving original clump and some reintroduced plants present in the wild in northern England. It was formerly more widespread, although never common, and the reduction in its populations to near extinction has been attributed to the collection of plants for gardens and herbarium specimens (Nelson, 1994; Farrell, 1999). In addition to the plants in the wild, there are several plants of known UK origin in cultivation and three plants growing in semi-natural habitat which have been considered to be possible introductions from non-native material (Farrell, 1999; I. Taylor, Natural England, pers. comm.). One of these has been shown to be a member of the North American C. parviflorum complex (M. F. Fay et al., unpubl. res.), and is excluded from the present study.
Due to its extreme rarity in England, C. calceolus has been considered an important target for conservation activities ranging from site management, wardening, pollination, ex situ seed germination and, more recently, genetic fingerprinting. However, largely due to the large genome size of this species (1C = 32·4 pg, Bennett et al., 2000), the nuclear DNA-based techniques increasingly used for studies of rare and endangered species (Qamaruz-Zaman et al., 1998; Fay and Krauss, 2003), including amplified fragment length polymorphisms (AFLP, Vos et al., 1995) and random amplified polymorphic DNA (RAPD, Williams et al., 1990), have not proved effective (Fay and Cowan, 2001; Fay et al., 2002; Fay and Krauss, 2003). When the standard AFLP protocol is used with genomes as large as that in C. calceolus, levels of genetic variation are dramatically underestimated (Fay and Cowan, 2001; Fay et al., 2002, 2005; Leitch and Fay, 2008). Nuclear microsatellites, similarly, have been shown to be difficult to apply to organisms with large genomes (Garner, 2002).
To overcome this problem, Fay and Cowan (2001) investigated the use of plastid microsatellites as alternative markers. The plastid genome comprises a single, circular chromosome. Unlike the nuclear chromosomes, this exists as a single type inherited from one parent only (the female in orchids and most other angiosperms; Corriveau and Coleman, 1988) and shows relatively low levels of size variation (Chase and Palmer, 1989). The sequence of genes is well characterized, and in many cases the genes contain introns and/or are separated by intergenic spacers (IGS), both non-coding regions of DNA. At least some of these non-coding regions contain repetitive motifs, commonly consisting of either all adenine (A) or all thymine (T) residues or a combination of these two bases. When these regions contain higher numbers of repeating units (generally ten or more for single nucleotide repeats), they are referred to as plastid microsatellites. They show high levels of variability, and the length of a microsatellite can vary extensively within and between populations. They have proved especially useful in studies of biogeography, and in Europe they have been used to study biogeographical patterns in a range of orchids, including Orchis spp. (Qamaruz-Zaman, 2000; Fay et al., 2007; Bateman et al., 2008), Dactylorhiza spp. (Shipunov et al., 2004, 2005; Pillon et al., 2007) and Spiranthes romanzoffiana (Forrest et al., 2004). In addition, non-coding plastid DNA often also includes insertions/deletions (indels) due to tandem repeats of longer motifs (minisatellites) that can vary in length from a few to hundreds of base pairs (bp), for example in Sorbus (King and Ferris, 2002) and Anacamptis (Cozzolino et al., 2004).
The placement of these length-variable regions varies from taxon to taxon, and an initial survey of plastid DNA is necessary to locate them. Once suitable regions are identified, primers are designed to amplify fragments containing them. Amplification products are obtained for all individuals under study, and their length in base pairs is determined. This technique has a general advantage over multilocus genetic fingerprinting methods such as RAPD and AFLP in that only one short region is amplified per reaction, making the technique less sensitive to DNA quality and quantity. It is therefore, at least potentially, applicable to DNA samples extracted under less than ideal circumstances, e.g. from herbarium material (Fay and Cowan, 2001; Cozzolino et al., 2007).
Here we report the development of a set of length-variable fragments of plastid DNA for C. calceolus and their use in analysing patterns of genetic variation in this species. For plants in England, these data are used to infer the origin of putatively non-native material and to inform the choice of material to be used for reintroduction purposes.
MATERIALS AND METHODS
Plant materials
The plant material used in this study is listed in Table 1. Detailed localities are not given, due to the sensitivity surrounding many of the sites. New specimens were collected into silica gel using the method of Chase and Hills (1991). These included the one remaining wild plant and all living plants of known wild origin in England. Representative samples from elsewhere in Europe were included for comparative purposes. In Denmark, only two populations are known, and 34 plants were sampled from these. Because these had previously been shown to be genetically invariant (I. Kahandawala et al., unpubl. res.), however, only three representative samples are included from each population here. DNA was extracted from silica-gel dried leaf material, using a modified 2× CTAB (cetyltrimethyl-ammonium bromide) procedure (Doyle and Doyle, 1987) followed by purification on a caesium chloride gradient or columns following the manufacturers' protocols.
Table 1.
Plant material used in this study
| Origin* | Sample size | Notes |
|---|---|---|
| Austria, Steiermark (C) | 5 | |
| Denmark, Jutland (N) | 6 | Two localities (three individuals from each) |
| Estonia, Muhu (C) | 1 | |
| France, SE (W) | 3 | |
| Italy, Dolomites (C) | 1 | |
| Poland, West (C) | 4 | |
| Poland, North East (C) | 2 | |
| Spain, Pyrenees (W) | 9 | |
| Sweden, Dalarna (N) | 37 | 10 localities |
| Sweden, Jämtland (N) | 3 | |
| Switzerland, Canton Bern (C) | 6 | |
| UK, England (W) | 7 | Wild site (two shoots were both sampled)† plus five plants in semi-natural sites or cultivation |
Sample size refers to the number of individuals for which haplotype data were collected. Due to small sample sizes, for statistical analyses Austria and Italy were combined as ‘Eastern Alps’ and Estonia and Poland were combined as ‘Poland/Estonia’.
* C, N and W stand for Central, Northern and Western Europe, as used in the text.
† At the wild site, the remaining plant had two main shoots. Both of these were sampled to investigate the possibility that there might be two plants. No genetic differences were detected, and we assume that it is actually one genet.
Identification of length-variable regions
Length-variable regions flanked by conserved regions were sought in the accD-psa1 IGS and the rps16 intron. These were amplified using primers (Table 2) given in Small et al. (1998) and Oxelman et al. (1997), respectively, from three representative samples (English, Polish and Swiss) of C. calceolus and sequenced using modified dideoxy cycle sequencing with dye terminators run on an ABI 377 automated sequencer. The sequences were aligned manually in PAUP* 4·0b4 (Swofford, 2002), and variable regions were identified. Primers to amplify the length-variable regions in the rps16 intron were designed in conserved flanking regions (Table 3). For each region, one primer was labelled with a fluorescent dye to enable the amplification products to be visualized using an ABI 377 automated sequencer. Sizes (bp) were determined using GeneScan 3·1 and Genotyper 2·0 (Applied Biosystems Inc., Foster City, CA, USA). Due to the AT-rich nature of the flanking regions for the length-variable regions in accD-psa1 (see Results), primers could not be designed to amplify these regions. The entire IGS was therefore sequenced for all individuals to allow these regions to be scored. Sequences containing unique or rare indels were redone from new PCR products to test for reproducibility and to exclude possible Taq-induced artefacts. The new regions identified here and one identified by Fay and Cowan (2001) in the trnL-trnF IGS (cyp2) were scored for all individuals. [The other region in the trnL intron, orch1, used by Fay and Cowan (2001), proved to be invariant in a subset of the samples and was not used further.]
Table 2.
Primers used to amplify the accD-psa1 intergenic spacer and the rps16 intron
| Locus | Primer | Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| accD-psa1 intergenic spacer | accD-769F | GGAAGTTTGAGCTTTATGCAAATGG | Small et al. (1998) |
| psaI-75R | AGAAGCCATTGCAATTGCCGGAAA | ||
| rps16 intron | rps16-1F | CACGGGTCGCCCTCGTTCCG | Oxelman et al. (1997) |
| rps16-1R | TCGGGATCGAACATCAATTGCAAC |
Table 3.
Primer sequences used to amplify the two (A)n microsatellites in the rps16 intron and the complex microsatellite in the trnL-trnF intergenic spacer (cyp2)
| Region | Primer | Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| rps16-1 | rps16-1F | CTTGTCTCAACAATTGGATC | This paper |
| rps16-1R | CGTACTCCTAACTCAAGTTG | This paper | |
| rps16-2 | rps16-2F | CAACTTGAGTTAGGAGTACG | This paper |
| rps16-2R* | See Table 2 | Oxelman et al. (1997) | |
| cyp2 | trnL-trnF | GGTTCAAGTCCCTCTATCCC | Taberlet et al. (1991) |
| cyp2-R | CGGATCCATTTGTTAAAGAACAG | Fay and Cowan (2001) |
* The original rps16 reverse primer for amplification of the whole locus was used due to the proximity of the microsatellite region to the end of the amplified sequence.
A matrix was prepared, using different numbers for alleles of different lengths of the microsatellite regions and 1 vs. 2 (see Table 4) for the simple indels. Where two or more indel events occurred at the same position in different samples (presumed to be single indels of different lengths rather than sequential indels), the alleles were numbered from 1 to 3 or 4, as appropriate (see Table 4).
Table 4.
Description of the 14 loci, the position of the indels in the aligned accD-psa1 matrix, the alleles found and a description of alleles for the insertions/deletions
| Locus | Type | Position† | Alleles | Allele description‡ |
|---|---|---|---|---|
| 1 | accD-psa1 microsatellite (A)n | – | 1,2,3,4 | |
| 2 | accD-psa1 indel 1 | 350 | 1,2,3 | long insert–no insert–short insert |
| 3 | accD-psa1 indel 2 | 400 | 1,2,3,4 | long–middle–short–none |
| 4 | accD-psa1 indel 3 | 465 | 1,2 | normal–deletion |
| 5 | accD-psa1 indel 4 | 522 | 1,2,3 | insert–normal–deletion |
| 6 | accD-psa1 indel 5 | 630 | 1,2 | normal–insert |
| 7 | accD-psa1 indel 6 | 750 | 1,2,3 | normal–short insert–long insert |
| 8 | accD-psa1 indel 7 | 812 | 1,2 | normal–insert |
| 9 | accD-psa1 indel 8 | 895 | 1,2 | normal–deletion |
| 10 | accD-psa1 indel 9 | 1050 | 1,2 | normal–deletion |
| 11 | accD-psa1 indel 10 | 1208 | 1,2 | normal–insert |
| 12 | rps16-1 microsatellite (A)n | – | 1,2 | |
| 13 | rps16-2 microsatellite (A)n | – | 1,2,3 | |
| 14 | cyp2 microsatellite (ATn)nTn | – | 1,2,3,4 |
† Position in base pairs in aligned accD-psa1 matrix.
‡ ‘Normal’ refers to the condition found in the majority of samples. Directionality of insertions and deletions is inferred, with the commonest allele being treated as ‘normal’.
Definition of haplotypes
Haplotypes numbered 1–23 were defined by the combination of the alleles for the 14 different loci. The haplotype definitions are given in Table 5.
Table 5.
The allelic composition of haplotypes 1–23 (the alleles are described in Table 4)
| Haplotype | Alleles |
|---|---|
| H1 | 12112121111123 |
| H2 | 22112111112113 |
| H3 | 31112121111123 |
| H4 | 32112121222113 |
| H5 | 32112121112114 |
| H6 | 32112121111122 |
| H7 | 32112121111123 |
| H8 | 32112121222213 |
| H9 | 32112131111122 |
| H10 | 32113121111122 |
| H11 | 32212121111133 |
| H12 | 32322121111113 |
| H13 | 33111121111123 |
| H14 | 33112121111123 |
| H15 | 42111121111123 |
| H16 | 42112121111123 |
| H17 | 42111121111124 |
| H18 | 42112121111124 |
| H19 | 42322122111113 |
| H20 | 42322122111114 |
| H21 | 42322122121114 |
| H22 | 42412221111133 |
| H23 | 43112121111123 |
Statistical data analysis
Haplotype frequencies in the sampled populations were estimated using ARLEQUIN software (Schneider et al., 2000). Genetic diversity was estimated as the number of haplotypes, number of polymorphic sites and gene diversity using ARLEQUIN and haplotype richness following Petit et al. (1998) using the CONTRIB program. Haplotype richness was corrected for differences in sample size using the rarefaction method. To provide robust estimates, the sample size of the smallest population sample (n = 3) was used for rarefaction. The distribution of plastid DNA diversity within and among populations was studied using analysis of molecular variance (AMOVA, Excoffier et al., 1992) as implemented in ARLEQUIN, and the significance of the AMOVA was tested based on 10 000 permutations of haplotypes among populations. A comparison of the distribution of diversity for ordered vs. unordered haplotypes was carried out following Pons and Petit (1996) using the PERMUT/cpSSR software. Two different methods were used for obtaining ordered haplotype information: (1) using the number of mutation steps between haplotypes (genetic divergence estimated as NST), and (2) making use of information on repeat number of each polymorphic site (genetic divergence estimated as RST). The latter method was specifically designed for microsatellites, but the former method makes fewer assumptions about the nature and complexity of mutational patterns detected by fragment length analysis (Pons and Petit, 1996). A median-joining (MJ) network (Bandelt et al., 1999) was constructed based on plastid DNA haplotypes using the program NETWORK 4·2·0·1. (www.fluxus-engineering.com). This method is able to resolve even complex haplotype patterns (Posada and Crandall, 2001). It uses parsimony criteria to identify median vectors, i.e. consensus sequences of mutually close sequences of markers that are biologically equivalent to possible unsampled or extinct ancestral haplotypes. To reduce complexity of the network, rapidly mutating characters were downweighed in the analysis.
RESULTS
Identification of length-variable regions
Two homopolymer microsatellites (both A on the sense strand) were detected in the rps16 intron, each consisting of 11 or 12 bp in the samples studied. In the full sampling, ten indels ranging in size from 6 to 21 bp and one homopolymer microsatellite (also A on the sense strand) of 11–12 bp were found in the accD-psa1 IGS. The amplified region of the accD-psa1 IGS was exceedingly AT-rich (approx. 80 %, depending on the sample) and most of the length-variable regions were found in a fragment of 461 bp in the aligned matrix (positions 328–788), in which only one base (a G at position 581) was not A or T. In all cases where sequences were redone from new PCR products, all the same indels were found. Representative sequences are available from the first author (m.fay@kew.org).
Haplotypes recovered
All 14 length-variable regions were successfully scored for all samples, and all loci were variable. The final matrix contained information for 84 (111 if all 34 Danish samples were included) individuals. The alleles found are presented in Table 4. Twenty-three haplotypes were obtained from the combined alleles for the 14 regions (Table 5).
Distribution of haplotypes
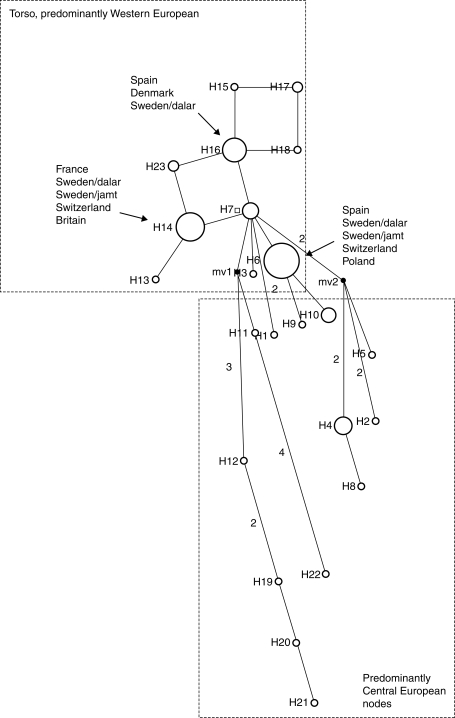
Haplotype H6 was the most common. Haplotypes H14 and H16 were also relatively common, and these three haplotypes were also widespread (see Fig. 1, Table 6). Two (H6 and H16) were found in individuals ranging from the Spanish Pyrenees to northern Sweden. Other haplotypes, at this level of sampling, were more localized, with some only being represented by one individual.
Fig. 1.
Median-joining network for plastid DNA haplotypes in Cypripedium calceolus. The haplotypes are indicated by the circles, the size of each circle being proportional to the observed frequency of each haplotype. The number of mutations required to explain transitions among haplotypes is indicated along the lines of the network, except for connections that required only a single mutation. Median vectors are labelled mv1 and mv2. Information on haplotype sharing is provided for the three most frequent types. The predominantly Western European torso of the network (upper left) and the predominantly Central European nodes (lower right) are indicated by dashed rectangles. More information on haplotype sharing is provided in Table 6. The names of the Swedish provinces of Dalarna and Jämtland are abbreviated as dalar and jamt, respectively.
Table 6.
Haplotype frequencies in nine populations of Cypripedium calceolus, determined by sequence or fragment analysis of 14 polymorphic plastid DNA sites
| Haplotype | Eastern Alps | Denmark | France | Poland/Estonia | Spain | Sweden/Dalarna | Sweden/Jämtland | Switzerland | UK |
|---|---|---|---|---|---|---|---|---|---|
| H1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| H2† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| H3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H4 | 0 | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 0 |
| H5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| H6 | 0 | 0 | 0 | 3 | 5 | 14 | 0 | 2 | 0 |
| H7 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| H8 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| H9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| H10 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| H11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H12 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| H13 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| H14 | 0 | 0 | 1 | 0 | 0 | 8 | 3 | 1 | 3 |
| H15 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| H16 | 0 | 6 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| H17 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| H18 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H19 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| H21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H22 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H23 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Distribution of genetic diversity in populations
The more diverse populations in terms of gene diversity and haplotype richness were Eastern Alps, France, Poland/Estonia and Switzerland (Table 6). If haplotype numbers and numbers of polymorphic sites are considered in addition, then Sweden/Dalarna also emerges as being among the more variable populations (eight haplotypes found among 37 specimens, eight polymorphic sites; Table 7). A high proportion of the genetic variability in the haplotype data was attributed to the within-population level (82·5 %) whereas only 17·5 % resided among populations (Table 8). Genetic divergence estimated with ordered haplotypes was not significantly different from divergence for unordered haplotypes, regardless of whether RST or NST was used to estimate divergence for ordered haplotypes. The results for the NST analysis are shown in Table 9.
Table 7.
Plastid DNA diversity in populations of Cypripedium calceolus, including for each population the number of haplotypes found (sample size), number of polymorphic sites, genetic diversity with standard error and allelic richness
| Population | No. of haplotypes (sample size) | No. of polymorphic sites | Gene diversity (±s.e.) | Allelic richness |
|---|---|---|---|---|
| Eastern Alps | 6 (6) | 9 | 1·000 (0·096) | 2·0 |
| Denmark | 1 (6) | 0 | 0·000 (0·000) | 0·0 |
| France | 3 (3) | 3 | 1·000 (0·272) | 2·0 |
| Poland/Estonia | 5 (7) | 9 | 0·857 (0·137) | 1·6 |
| Spain | 4 (9) | 3 | 0·694 (0·147) | 1·2 |
| Sweden/Dalarna | 8 (37) | 8 | 0·790 (0·044) | 1·4 |
| Sweden/Jämtland | 1 (3) | 0 | 0·000 (0·000) | 0·0 |
| Switzerland | 5 (6) | 9 | 0·933 (0·122) | 1·8 |
| Britain | 4 (6) | 4 | 0·800 (0·172) | 1·5 |
* Corrected for differences in sample size by rarefaction for n = 3, the smallest sample size among the populations studied.
Table 8.
Distribution of plastid DNA variation within and among populations of Cypripedium calceolus, estimated by Analysis of Molecular Variance (AMOVA)
| Source of variation | Sums of squares | Variance components | d.f. | Variation (%) | F-statistic | P* |
|---|---|---|---|---|---|---|
| Among populations | 24·5 | 0·244 | 8 | 17·5 | 0·175 | <0·001 |
| Within populations | 84·7 | 1·145 | 74 | 82·5 |
* The significance threshold was determined by 10 000 permutations of haplotypes among populations.
Table 9.
Analysis of plastid DNA diversity following Pons & Petit (1996), including the number of populations studied, number of haplotypes considered, and diversity and divergence statistics for unordered and ordered haplotypes
| No. of populations | No. of haplotypes | hS* | hT* | GST* | vS† | vT† | NST† |
|---|---|---|---|---|---|---|---|
| 9 | 22 | 0·675 | 0·907 | 0·256 | 0·694 | 0·905 | 0·233 |
* Unordered haplotypes: hS, within-population diversity; hT, total diversity; GST, genetic divergence.
† Ordered haplotypes: vS, within-population diversity; vT, total diversity; NST, genetic divergence. The difference in genetic divergence when estimated using unordered vs. ordered haplotype information (GST vs. NST) was not significant.
Haplotype network
The MJ network was composed of a predominantly Western European torso of closely related haplotypes (just one mutation step between nodes) and a number of predominantly Central European branches made up of more divergent haplotypes (up to four mutation steps between nodes; Fig. 1). Haplotypes H6, H14 and H16 were the most common. Each of these haplotypes was found in a central position in the network (at least three connections branching out from each haplotype), thus indicating that these are older, ancestral haplotypes that gave rise to less common, derived haplotypes found elsewhere in the network.
Haplotypes found in English individuals
Five haplotypes (H2, H10, H14, H17 and H23) were found among individuals from England (Table 6). Of these, H14 was widespread, also being found in France, Switzerland and Sweden. Haplotypes H10, H17 and H23 were also found in France and Spain, Spain, and Sweden, respectively. Haplotype H2 was only found in England in one of the putatively introduced plants (the other putative introduction shared H17 with one plant from Spain). In the MJ network, this haplotype falls on a branch with three other haplotypes: H4 and H5 (both Poland/Estonia) and H8 (Sweden/Dalarna). This supports the suggestion that this plant is non-native.
DISCUSSION
Utility of plastid DNA polymorphisms in Cypripedium calceolus
Plastid DNA polymorphisms are here shown to have the capacity to reveal genetic variability in C. calceolus, providing an alternative to the use of nuclear DNA, which in this species is compromised by the large nuclear genome size. Although data from plastid DNA are generally less information-rich due to the uniparental pattern of inheritance, in this case genetic fingerprinting using AFLP appears to have given a dramatic underestimate of levels of genetic variation (Fay and Cowan, 2001; Fay et al., 2002, 2005; Leitch and Fay, 2008). In a separate study, aiming at developing nuclear microsatellites for C. calceolus, only two out of seven which gave scorable results were polymorphic (I. Kahandawala et al., unpubl. res.), possibly indicating a similar problem.
The A/T-rich region of the accD-psa1 IGS was particularly informative in this case, and the extra effort required in terms of sequencing the fragment rather than sizing it (as for the other loci) was repaid by the increase in the number of haplotypes revealed. The percentage A/T approaches 100 % for 461 bp, making this the most A/T-rich region of DNA of which we are aware.
Distribution of genetic variation
Central European material, despite relatively sparse sampling, appears to be genetically more variable than northern and western material. In principle, the high levels of genetic diversity in the Eastern Alps and Switzerland (Table 7) are compatible with two hypotheses: (1) the presence of refugial populations in Central Europe, in regions surrounding the ice sheets that covered the Alps during the Weichselian glaciation; and (2) genetic admixture in this region involving two or more postglacial colonization routes from unsampled southern/south-eastern European refugia. A study of genetic structure with nuclear markers, including the estimation of admixture proportions for individual plants, would provide additional insight (although this may be difficult to achieve due to the problems with genome size discussed above).
The high level of genetic diversity detected in Poland/Estonia (Table 7) corroborates the findings of Kull & Paaver (1997) and Brzosko et al. (2002). The former, studying three strongly polymorphic aspartate aminotransferase loci, observed high levels of heterozygosity in seven Estonian populations of C. calceolus (Ho = 0·40–0·53). An eighth population was less genetically diverse (Ho = 0·16), which was ascribed to a founder effect. Brzosko et al. (2002) examined 11 allozyme loci in three populations from the Biebrza valley, north-eastern Poland, and assessed the genetic diversity as percentage polymorphic loci (P), mean number of alleles per locus (A), and observed (Ho) and expected (He) heterozygosity. They found diversity within populations to be relatively high compared with rare taxa and taxa with similar life histories (P = 45·5 % in all populations; A = 0·184; Ho = 0·184 on average; He = 0·156 on average). The genetic variation found between populations was even smaller (1·6 %) than in the present study.
The long branches in the predominantly Central European section of the haplotype network (Fig. 1) may be indicative of the presence of genetic material derived from a different post-glacial colonization route (or routes), possibly stemming from unsampled populations to the east of the region on which the present study was focused. Central European and eastern material remains undersampled and is an obvious focus for future studies, although the difficulty of obtaining samples due to legal protection of this species in many countries may make this problematic. This would potentially clarify the number of refugia that existed for this species during the last ice age. The variability found in Swedish material may indicate that Sweden was recolonized by individuals from two or more refugia, migrating along western and central/eastern routes.
The absence of a significant difference between the divergences for ordered haplotypes and unordered haplotypes (Table 9) indicates the absence of marked phylogeographical structure in the data, i.e. seed-mediated gene flow was more important than mutation in generating the observed genetic structure. This may obscure the geographical patterns, even if more central and eastern samples are obtained.
Long-distance dispersal of orchids
Orchid seeds are commonly said to be capable of long-distance dispersal by the wind due to their small size. Darwin (1877, pp. 278–279) stated that ‘The minute seeds within their light coats are well fitted for wide dissemination; and I have several times observed seedlings springing up in my orchard and in a newly-planted wood, which must have come from a considerable distance. This was especially the case with Epipactis latifolia; and an instance has been recorded by a good observer [Mr. Bree, in “Loudon's Mag. of Nat. Hist,” vol. ii. 1829, p. 70.] of seedlings of this plant appearing at the distance of between eight and ten miles from any place where it grew.’ Summerhayes (1951) stated that ‘the seeds are certainly very light, and easily carried for long distances by the wind’ and ‘the seeds are shaken out a few at a time, and are distributed far and wide. These seeds, as in the case with others distributed by the wind, are probably carried upwards, by ascending columns of hot air, to very considerable heights, and then transported long distances in the upper atmosphere before sinking slowly to earth again.’ In their extensive review on orchid seeds, Arditti and Ghani (2000) tabulated published dispersal distances of up to 2000 km for orchid seeds. In contrast, some authors have reported that most orchid seeds fall within only a short distance of the source plant. In Spiranthes spiralis, the vast majority of seeds were demonstrated to fall within 15 cm of the source plant (Machon et al., 2003). In Anacamptis morio, Dactylorhiza majalis and Pseudorchis albida, Jersáková and Malinová (2007) demonstrated that the proportion of seeds landing more than 1 m away from the source plant approached zero. In Orchis purpurea, Jacquemyn et al. (2007) demonstrated ‘rather limited seed dispersal distances’ (approx. 4–5 m), and in Cypripedium macranthos, Chung et al. (2009) stated that ‘most seeds fall close to the maternal plants’ on the basis of the significant fine-scale spatial genetic structure they found. Although orchid seeds do have the potential for dispersal over great distances it is thus probable that the pattern of dispersal follows a leptokurtic distribution in most (if not all) cases.
In the present study, some of the haplotypes found in C. calceolus were widely distributed, in extreme cases from the Pyrenees to northern Sweden. This and the highly homogeneous genetic structure revealed by AMOVA (Table 8) can be interpreted as support for wide-ranging seed dispersal in these light-seeded, wind-dispersed orchids, at least as an occasional (or rare) occurrence. Our data do not allow us to say whether dispersal occurred from the Pyrenees to Sweden in one or more than one step, but the hypervariable nature of the loci involved leads us to believe that it is unlikely to represent more than a few seed dispersal events. An alternative explanation is that both the Pyrenees and Sweden were colonized by individuals from geographically intermediate populations in glacial refugia. This explanation, however, still involves dispersal over substantial distances.
Implications for conservation and reintroduction
On the basis of the results presented here, English Nature (now Natural England) reached the decision that seedlings resulting from self-pollination of the two putatively introduced individuals of C. calceolus or cross pollination of these individuals with other plants in England should be excluded from the reintroduction programme for this species. Despite the extreme demographic bottleneck suffered by this species in England, a higher level of genetic diversity has been maintained than in the larger populations in Denmark. Data for nuclear microsatellites also show that the native English plants are genetically variable, whereas Danish plants are genetically homogeneous (I. Kahandawala et al., unpubl. res.). This should be taken into account for seed storage and other conservation activities. Due to the low level of sampling reported here, it is not possible to make firm recommendations about conservation activities elsewhere in Europe, but it appears that Central (and some Northern) European populations may be more diverse than those in Western Europe. Swedish populations are more diverse than those in Denmark, and this could be due to there being more than one migration route following the last glaciation. At the same time, the lack of genetic polymorphism in Danish populations could be due to a founder effect, as the present occurrence in the region of Jutland was probably founded by long-distance seed dispersal. Thus, the sampled populations (discovered as late as 1884 and 1968, respectively) are the only Danish populations known since 1767 and the only ones ever known from Jutland.
ACKNOWLEDGMENTS
This work was funded by the Royal Botanic Gardens, Kew, and English Nature (now part of Natural England) as part of the Biodiversity Action Plan for C. calceolus. We thank all colleagues who provided plant material, particularly Fred Campbell (Sweden), Marcin Zych and Maciej Romañski (Poland), José M. Iriondo (Spain), Peter Schönswetter and Andreas Tribsch (Austria) and Tiiu Kull (Estonia). We also thank Mark Chase, Phil Cribb and Ilia Leitch (Kew) and Ian Taylor (Natural England) for useful comments on this project and Olivier Maurin and Robyn Cowan for support in the laboratory.
LITERATURE CITED
- Arditti J, Ghani AKA. Tansley Review No. 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytologist. 2000;145:367–421. doi: 10.1046/j.1469-8137.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- Bandelt H-J, Forster P, Roehl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Bateman RM, Smith RJ, Fay MF. Morphometric and population genetic analyses elucidate the origin, evolutionary significance and conservation implications of Orchis × angusticruris (O. purpurea × O. simia), a hybrid orchid new to Britain. Botanical Journal of the Linnean Society. 2008;157:687–711. [Google Scholar]
- Bennett MD, Bhandol P, Leitch IJ. Nuclear DNA amounts in angiosperms and their modern uses – 807 new estimates. Annals of Botany. 2000;86:859–909. [Google Scholar]
- Brzosko E, Ratkiewicz M, Wróblewska A. Allozyme differentiation and genetic structure of the Lady's slipper (Cypripedium calceolus) island populations in north-east Poland. Botanical Journal of the Linnean Society. 2002;138:433–440. [Google Scholar]
- Chase MW, Hills HG. Silica gel: an ideal desiccant for preserving field-collected leaves for use in molecular studies. Taxon. 1991;41:215–220. [Google Scholar]
- Chase MW, Palmer JD. Chloroplast DNA systematics of the lilioid monocots: feasibility, resources, and an example from the Orchidaceae. American Journal of Botany. 1989;76:1720–1730. [Google Scholar]
- Chung JM, Park KW, Park C-S, Lee S-H, Chung MG, Chung MY. Contrasting levels of genetic diversity between the historically rare orchid Cypripedium japonicum and the historically common Cypripedium macranthos in South Korea. Botanical Journal of the Linnean Society. 2009;160:119–129. [Google Scholar]
- Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. American Journal of Botany. 1988;75:1443–1458. [Google Scholar]
- Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A. Hypervariable plastid locus variation and intron evolution in the Anacamptis palustris lineage. Genome. 2004;47:999–1003. doi: 10.1139/g04-063. [DOI] [PubMed] [Google Scholar]
- Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A. Genetic variation in time and space: the use of herbarium specimens to reconstruct patterns of genetic variation in the endangered orchid Anacamptis palustris. Conservation Genetics. 2007;8:629–639. [Google Scholar]
- Darwin C. The various contrivances by which orchids are fertilised by insects. 2nd edn. London: J. Murray; 1877. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell L. Lady's slipper orchid. In: Wigginton MJ., editor. Cypripedium calceolus L. (Orchidaceae) Peterborough: Joint Nature Conservancy Council; 1999. p. 114. British Red Data Books. 1. Vascular plants. [Google Scholar]
- Fay MF, Cowan RS. Plastid microsatellites in Cypripedium calceolus (Orchidaceae): genetic fingerprints from herbarium specimens. Lindleyana. 2001;16:151–156. [Google Scholar]
- Fay MF, Krauss SL. Orchid conservation genetics in the molecular age. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ., editors. Orchid conservation. Kota Kinabalu: Natural History Publications (Borneo); 2003. pp. 91–112. [Google Scholar]
- Fay MF, Qamaruz-Zaman F, Cowan RS, Thornton H. Molecular techniques for orchid conservation genetic studies. In: Clark J, Elliott WM, Tingley G, Biro J., editors. Proceedings of the 16th World Orchid Conference. Vancouver: Vancouver Orchid Society; 2002. pp. 152–157. [Google Scholar]
- Fay MF, Cowan RS, Leitch IJ. The effects of genome size on the quality and utility of AFLP fingerprints. Annals of Botany. 2005;95:237–246. doi: 10.1093/aob/mci017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay MF, Smith RJ, Zuiderduin K, et al. How does hybridization influence the decision making process in conservation? The genus Orchis (Orchidaceae) as a case history. Lankesteriana. 2007;7:135–137. [Google Scholar]
- Forrest AD, Hollingsworth ML, Hollingsworth PM, Sydes C, Bateman RM. Population genetic structure in European populations of Spiranthes romanzoffiana set in the context of other genetic studies on orchids. Heredity. 2004;92:218–227. doi: 10.1038/sj.hdy.6800399. [DOI] [PubMed] [Google Scholar]
- Garner TWJ. Genome size and microsatellites: the effect of nuclear size on amplification potential. Genome. 2002;45:212–215. doi: 10.1139/g01-113. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Vandepitte K, Honnay O, Roldán-Ruiz I, Wiegand T. A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea. New Phytologist. 2007;176:448–459. doi: 10.1111/j.1469-8137.2007.02179.x. [DOI] [PubMed] [Google Scholar]
- Jersáková J, Malinová T. Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytologist. 2007;176:237–241. doi: 10.1111/j.1469-8137.2007.02223.x. [DOI] [PubMed] [Google Scholar]
- King RA, Ferris C. Genome. Vol. 45. Rosaceae: Maloideae; 2002. A variable minisatellite sequence in the chloroplast genome of Sorbus L; pp. 570–576. [DOI] [PubMed] [Google Scholar]
- Kull T, Paaver T. Patterns of aspartate aminotransferase variation in relation to population size, founder effect, and phytogeographic history in Cypripedium calceolus. Proceedings of the Estonian Academy of Sciences. Biology and Ecology. 1997;46:4–11. [Google Scholar]
- Leitch IJ, Fay MF. Plant genome horizons: Michael Bennett's contribution to genome research. Annals of Botany. 2008;101:737–746. doi: 10.1093/aob/mcn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon N, Bardin P, Mazer SJ, Moret J, Godelle B, Austerlitz F. Relationship between genetic structure and seed and pollen dispersal in the endangered orchid Spiranthes spiralis. New Phytologist. 2003;157:677–687. doi: 10.1046/j.1469-8137.2003.00694.x. [DOI] [PubMed] [Google Scholar]
- Nelson EC. Historical data from specimens in the herbarium, National Botanic Gardens, Glasnevin, Dublin (DBN), especially on Cypripedium calceolus. BSBI News. 1994;67:21–22. [Google Scholar]
- Oxelman B, Liden M, Berglund D. Chloroplast rps16 intron phylogeny of the tribe Sileneae (Caryophyllaceae) Plant Systematics and Evolution. 1997;206:393–410. [Google Scholar]
- Petit RJ, El Mousadik A, Pons O. Identifying populations for conservation on the basis of genetic markers. Conservation Biology. 1998;12:844–855. [Google Scholar]
- Pillon Y, Fay MF, Hedrén M, et al. Evolution and temporal diversification of western European polyploid species complexes in Dactylorhiza (Orchidaceae) Taxon. 2007;56:1185–1208. [Google Scholar]
- Pons O, Petit RJ. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics. 1996;144:1237–1245. doi: 10.1093/genetics/144.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Intraspecific gene genealogies: grafting trees into networks. Trends in Ecology and Evolution. 2001;16:37–45. doi: 10.1016/s0169-5347(00)02026-7. [DOI] [PubMed] [Google Scholar]
- Qamaruz-Zaman F. Conservation genetics of rare and endangered British orchids. UK: University of Cambridge; 2000. PhD thesis. [Google Scholar]
- Qamaruz-Zaman F, Fay MF, Parker JS, Chase MW. Molecular techniques employed in the assessment of genetic diversity: a review focusing on orchid conservation. Lindleyana. 1998;13:259–283. [Google Scholar]
- Schneider S, Roessli D, Excoffier L. Arlequin: a software for population genetics data analysis. Version 2·000. Geneva: Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva; 2000. [Google Scholar]
- Shipunov AB, Fay MF, Pillon Y, Bateman RM, Chase MW. Dactylorhiza (Orchidaceae) in European Russia: combined molecular and morphological analysis. American Journal of Botany. 2004;91:1419–1426. doi: 10.3732/ajb.91.9.1419. [DOI] [PubMed] [Google Scholar]
- Shipunov AB, Fay MF, Chase MW. Taxonomic status of Dactylorhiza baltica (Orchidaceae) from European Russia: evidence from molecular markers and morphology. Botanical Journal of the Linnean Society. 2005;147:257–274. [Google Scholar]
- Small RL, Ryburn JA, Cronn RC, Seelanan T, Wendel JF. The tortoise and the hare: choosing between noncoding plastome and nuclear Adh sequences for phylogeny reconstruction in a recently diverged plant group. American Journal of Botany. 1998;85:1301–1315. [PubMed] [Google Scholar]
- Summerhayes VS. Wild orchids of Britain. London: Collins; 1951. [Google Scholar]
- Swofford D. PAUP* 4·0b10: Phylogenetic analysis using parsimony (* and other methods) Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research. 1990;18:6231–6235. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]