Abstract
Background and Aims
Phylogenetic relationships of subtribes Cranichidinae and Prescottiinae, two diverse groups of neotropical terrestrial orchids, are not satisfactorily understood. A previous molecular phylogenetic study supported monophyly for Cranichidinae, but Prescottiinae consisted of two clades not sister to one another. However, that analysis included only 11 species and eight genera of these subtribes. Here, plastid and nuclear DNA sequences are analysed for an enlarged sample of genera and species of Cranichidinae and Prescottiinae with the aim of clarifying their relationships, evaluating the phylogenetic position of the monospecific genera Exalaria, Ocampoa and Pseudocranichis and examining the value of various structural traits as taxonomic markers.
Methods
Approx. 6000 bp of nucleotide sequences from nuclear ribosomal (ITS) and plastid DNA (rbcL, matK-trnK and trnL-trnF) were analysed with cladistic parsimony and Bayesian inference for 45 species/14 genera of Cranichidinae and Prescottiinae (plus suitable outgroups). The utility of flower orientation, thickenings of velamen cell walls, hamular viscidium and pseudolabellum to mark clades recovered by the molecular analysis was assessed by tracing these characters on the molecular trees.
Key Results
Spiranthinae, Cranichidinae, paraphyletic Prescottia (with Pseudocranichis embedded), and a group of mainly Andean ‘prescottioid’ genera (the ‘Stenoptera clade’) were strongly supported. Relationships among these clades were unresolved by parsimony but the Bayesian tree provided moderately strong support for the resolution (Spiranthinae–(Stenoptera clade-(Prescottia/Pseudocranichis–Cranichidinae))). Three of the four structural characters mark clades on the molecular trees, but the possession of a pseudolabellum is variable in the polyphyletic Ponthieva.
Conclusions
No evidence was found for monophyly of Prescottiinae and the reinstatement of Cranichidinae s.l. (including the genera of ‘Prescottiinae’) is favoured. Cranichidinae s.l. are diagnosed by non-resupinate flowers. Lack of support from parsimony for relationships among the major clades of core spiranthids is suggestive of a rapid morphological radiation or a slow rate of molecular evolution.
Key words: Cranichideae, Cranichidinae, matK-trnK, molecular phylogenetics, nrITS, Orchidaceae, Prescottiinae, resupination, trnL-trnF
INTRODUCTION
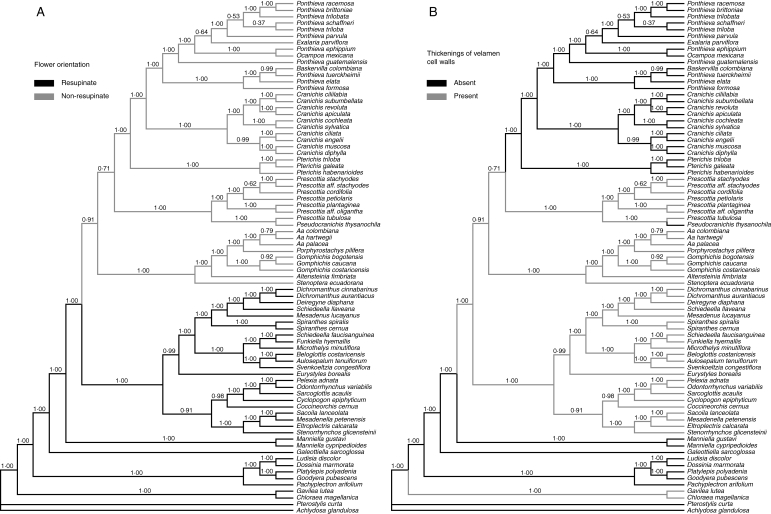
Circumscription of subtribe Cranichidinae Lindl. has varied among the several orchid classifications published during the last century in whether or not some of its constituent genera are placed in a distinct subtribe, Prescottiinae Dressler (e.g. Schlechter 1911, 1926; Brieger, 1974–75; Dressler, 1974, 1981; Chase et al., 2003; Pridgeon et al., 2003; contra Dressler, 1990, 1993; Szlachetko, 1995). Dressler (1990, 1993) segregated the genera Aa Rchb.f., Altensteinia Kunth, Gomphichis Lindl., Myrosmodes Rchb.f., Porphyrostachys Rchb.f., Prescottia Lindl. ex Hook. and Stenoptera C.Presl in Prescottiinae, distinguishing them from Cranichidinae by the possession of velamen of the Spiranthes type (after Porembski and Barthlott, 1988), a laminar rostellum, soft pollinia and lack of a hamular viscidium (Rasmussen, 1982). In contrast, Cranichidinae sensu stricto (s.s.) have a velamen of the Calanthe type, a pointed rostellum, brittle pollinia and a hamular viscidium. However, Prescottiinae lack unique distinctive features, and those separating them from Cranichidinae are shared, in various combinations, with subtribes Galeottiellinae Salazar & M.W.Chase, Manniellinae Schltr. and Spiranthinae Lindl., probably representing symplesiomorphies of ‘core spiranthids’ sensu Salazar et al. (2003) and Chase (2003). On the other hand, Cranichidinae and Prescottiinae are unique in Cranichideae in having non-resupinate flowers (Fig. 1), and this feature was the reason to group their component genera in Cranichidinae sensu lato (s.l.) in the first place (e.g. Lindley, 1840, in part; Schlechter, 1911, 1926; Brieger, 1974–75; Dressler, 1981).
Fig. 1.
Flowers of representative species previously assigned to Prescottiinae (A–D) and Cranichidinae (E–H): (A) Altensteinia fimbriata (Ecuador, Salazar 6789); (B) Prescottia plantaginea (Brazil, Salazar 6350); (C) Prescottia tubulosa (Mexico, Reyes 5767); (D) Pseudocranichis thysanochila (Mexico, Reyes 5523); (E) Ponthieva formosa (Mexico, Salazar 6539); (F) Ponthieva ephippium (Mexico, Salazar 6440); (G) Ponthieva fertilis (formerly Exalaria parviflora; Ecuador, Salazar 7641); (H) Ponthieva (Ocampoa) mexicana (Mexico, Salazar 6474).
Salazar et al. (2003) carried out a phylogenetic assessment of tribe Cranichideae based on nucleotide sequences of plastid and nuclear ribosomal (nrITS) DNA. In their combined analysis, four main clades of ‘core spiranthids’ received moderate to strong internal support, namely Cranichidinae s.s., Spiranthinae, Prescottia and a group encompassing predominantly high-Andean genera Aa, Gomphichis, Porphyrostachys and Stenoptera, assigned to Prescottiinae by Dressler (1990, 1993) and here referred to as the ‘Stenoptera clade’. However, the Stenoptera clade and Prescottia were not sisters; instead the former diverged first and Prescottia was weakly supported as sister to Cranichidinae. Prescottia/Cranichidinae were in turn weakly supported as collective sisters of Spiranthinae (Salazar et al., 2003, fig. 6).
Recently, Figueroa et al. (2008) assessed the phylogenetic relationships of 26 species of Cranichideae with the aim of exploring the evolution and systematic value of several anatomical characters of the root, including some attributes used by previous authors to define so-called velamen types (Porembski and Barthlott, 1988). They did so by analysing cladistically three structural attributes in combination with nucleotide sequences of a nuclear (nrITS) and a plastid DNA region (matK-trnK). Their analysis recovered a single most-parsimonious tree (MPT) with the same four main clades of core spiranthids as in Salazar et al. (2003). Cranichidinae were sister to a clade in which paraphyletic Prescottia (with Pseudocranichis embedded) was in turn the sister of a group consisting of Aa/Altensteinia (representatives of the Stenoptera clade) and Spiranthinae. With the exception of Prescottia/Pseudocranichis, which received weak bootstrap support (BS), all these main clades were strongly supported. Relationships among the four main clades lacked BS >50 % (Figueroa et al., 2008, fig. 4), but the three structural characters (thickenings of secondary walls of velamen cells, lamellate tilosomes and supraendodermal spaces) marked monophyletic groups recovered by the combined analysis.
Fig. 4.
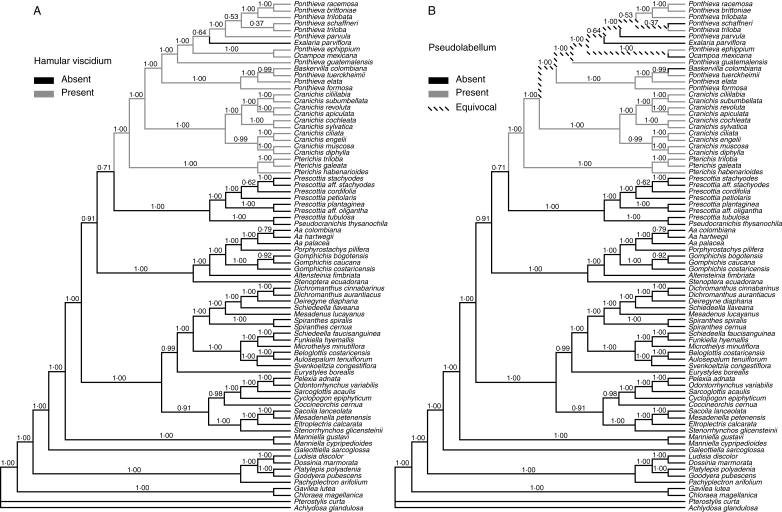
Optimization of hamular viscidium and pseudolabellum on the Bayesian tree of Fig. 2B (see text).
Together, Cranichidinae s.s. and Prescottiinae include about 210 species in 17 genera (Pridgeon et al., 2003), contributing significantly to the terrestrial orchid diversity of the neotropics. However, they are still one the least studied orchid groups. A better understanding of their phylogenetic relationships will provide a more objective basis for their classification and a background for addressing questions on various aspects of their evolution. One such question concerns the evolution of structural characters; for instance, as stated above, Cranichidinae s.s. and Prescottiinae differ from all other subtribes of Cranichideae in their non-resupinate flowers, but it is not clear whether this condition represents a uniquely derived, shared feature or a parallelism in these groups, given the lack of support for their relationships (Chase, 2003; Salazar et al., 2003; Figueroa et al., 2008).
Previous phylogenetic analyses of Cranichideae (Salazar et al., 2003; Figueroa et al., 2008) have included only a few representatives of Cranichidinae s.s. and Prescottiinae. In this study, the phylogenetic relationships of Cranichidinae and Prescottiinae are assessed by analysing a broader taxonomic sample of both groups with the same DNA regions used by Salazar et al. (2003), namely plastid genes matK and rbcL, plastid trnK intron, trnL intron and trnL-trnF intergenic spacer and the nuclear ribosomal (nr) ITS region. The aims were: (a) evaluate subtribal and generic limits and relationships of Cranichidinae s.s. and Prescottiinae; (b) clarify the systematic position of the monospecific genera Exalaria Garay & G.A.Romero-González, Ocampoa A.Rich. & Galeotti and Pseudocranichis Garay; and (c) gain insight into the value of various structural traits as taxonomic markers, including flower orientation and thickenings of the wall of velamen cells, hamular viscidia and the ‘pseudolabellum’ (a broad surface on the lower side of the flower formed by the expanded, approximate petals, whereas the true labellum is inconspicuous and stands in an upright position; Dressler, 1993).
MATERIALS AND METHODS
Taxonomic sample
Exemplars of 45 species/14 genera belonging to subtribes Cranichidinae and Prescottiinae were analysed, together with 23 species of Spiranthinae. Twelve additional species that represent all remaining subtribes of Cranichideae according to Chase (2003), namely Achlydosinae M.A.Clem. & D.L.Jones (formerly Megastylidinae Schltr., in part), Chloraeinae Rchb.f., Galeottiellinae, Goodyerinae, Manniellinae and Pterostylidinae Pfitz., were used as outgroups following previous phylogenetic studies (Kores et al., 1997, 2001; Cameron et al., 1999; Salazar et al., 2003). A list of the taxa analysed with voucher information and GenBank accessions is provided in Appendix 1.
Molecular methods
Extraction, purification, amplification and sequencing of DNA were carried out following standard procedures explained in Salazar et al. (2003) and Figueroa et al. (2008). For all DNA regions analysed, both DNA strands were sequenced and then edited and assembled with Sequencher versions 3·1 to 4·6 (GeneCodes Corp.). Alignment of sequences was done by visual inspection, using as templates the alignments of Salazar et al. (2003) and trying to maximize sequence similarity (Simmons, 2004). No data were excluded from the analyses due to unambiguous alignment, and the individual gap positions were treated as missing data.
Phylogenetic analyses
A previous assessment of phylogenetic relationships of Cranichideae (Salazar et al., 2003) showed that separate analyses of rbcL and the trnL-trnF, matK-trnK and nrITS regions recovered similar relationships, and no instances of conflicting resolution among different datasets obtaining strong internal support occurred. Furthermore, the combined analysis of all the datasets enhanced resolution and increased the proportion of clades that obtained strong support from the various measures of support applied. Therefore, in this study it was decided to analyse all datasets in combination to maximize resolution and support.
A parsimony analysis was conducted in PAUP* version 4·02b for Macintosh (Swofford, 2002) and consisted of a heuristic search with 1000 random sequences of taxon addition for the starting trees, tree–bisection–reconnection (TBR) branch swapping and the ‘MULTREES’ option on (storing multiple trees), saving all MPTs. All characters were treated as unordered and equally weighted. Internal support for clades was evaluated by 300 bootstrap replicates (Felsenstein, 1985), each with 20 random sequences of taxon addition and TBR branch swapping, saving up to 20 shortest trees from each addition replicate. Various alternative resolutions were examined by means of the ‘Constraints’ option in PAUP*, i.e. constraining the analysis to enforce monophyly of specific groups to examine the effect on tree length and consistency and retention indices.
A model-based phylogenetic analysis of the combined matrix using Bayesian Markov Chain Monte Carlo inference was also carried out as implemented in MrBayes version 3·1·2 (Ronquist et al., 2005). A six-parameter model of molecular evolution with gamma distribution and a proportion of invariant sites fit best the rbcL, matK, trnL intron, trnL-trnF intergenic spacer and nrITS data sets according to the Akaike information criterion (Akaike, 1974) in Modeltest version 3·7 (Posada and Crandall, 1998). In the case of the trnK intron, a six-parameter model with gamma distribution but with no invariant characters was selected. These models were accordingly assigned to two partitions in MrBayes. Two parallel analyses, each consisting of four Markov chains, were run for 1000 000 generations, sampling from the trees every 100 generations. In both runs, stationarity was reached around generation 70 000 and the first 150 000 generations were discarded as the ‘burn-in’. A summary Bayesian tree was calculated from the remaining 8500 trees from each run. Both runs yielded topologically identical trees with most clades being supported by a high posterior probability (PP). The trees from both analyses (17 000 trees) were then pooled into a single summary tree, and the discussion will be based on that tree.
Four morphological characters (flower orientation, thickenings of velamen cell walls, hamular viscidium and pseudolabellum) were optimized on the molecular trees using the program MacClade version 4·02 (Maddison and Maddison, 2001).
RESULTS
Parsimony analysis
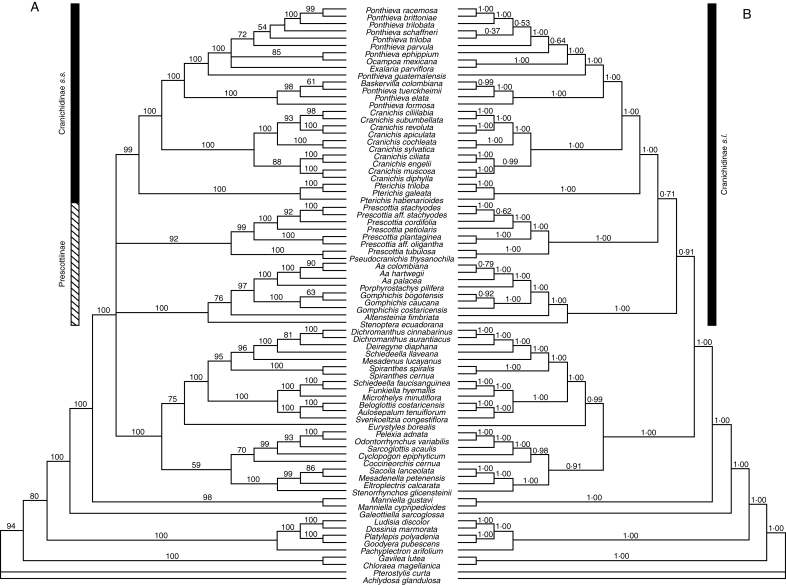
The combined dataset comprised 5944 aligned nucleotide positions, of which 2103 were variable and 1381 were potentially parsimony informative. The heuristic search found six MPTs with a length of 5841 steps, consistency index (CI) excluding uninformative characters = 0·43 and retention index (RI) = 0·74. In the strict consensus of the six trees (Fig. 2A), the core spiranthids as defined in Salazar et al. (2003) are strongly supported as monophyletic and consist, in successive branching order, of Galeottiella (Galeottiellinae), Manniella (Manniellinae) and a polytomy formed by Spiranthinae (BS 100), the Stenoptera clade (BS 100), paraphyletic Prescottia with Pseudocranichis thysanochila embedded (BS 92) and Cranichidinae (BS 99).
Fig. 2.
Phylogenetic relationships of Cranichidinae and Prescottiinae inferred from combined analyses of rbcL, matK-trnK, trnL-trnF and nrITS. (A) Strict consensus of the six MPTs found by the parsimony analysis (numbers above branches are bootstrap proportions). (B) Bayesian summary tree (numbers above branches are posterior probabilities). Bars indicate taxonomic limits of Cranichidinae and Prescottiinae.
Strongly supported Spiranthinae encompass three major clades, identified by Salazar et al. (2003) as the Stenorrhynchos, Pelexia and Spiranthes clades. Relationships within Spiranthinae are unchanged with respect to previous analyses by Salazar et al. (2003, q.v.) and will not be dealt with further here. Within the Stenoptera clade, S. ecuadorana is sister of the rest, and Altensteinia fimbriata is sister (BS 76) to a strongly supported group formed by monophyletic Gomphichis sister to Porphyrostachys pilifera/Aa. In the Prescottia/Pseudocranichis clade, Prescottia tubulosa and Pseudocranichis thysanochila are strongly supported as sister to the remaining species of Prescottia. With the exclusion of Pseudocranichis, Cranichidinae s.s. are strongly supported as monophyletic, with Pterichis Lindl. being sister to the other members. These other members form two strongly supported clades: Cranichis Sw. and a group with Baskervilla colombiana, Exalaria parviflora and Ocampoa mexicana nested among species of Ponthieva R.Br. Baskervilla colombiana occupies a derived position in a subclade that also includes, in succession, Ponthieva formosa, P. elata and P. tuerckheimii. The other major subclade of Ponthieva includes P. guatemalensis as the sister of a trichotomy formed by Exalaria parviflora, P. ephippium/Ocampoa mexicana, and a clade comprising P. triloba, P. schaffneri, P. trilobata, P. parvula and P. racemosa/P. brittoniae.
Enforcing monophyly for the group with non-resupinate flowers (i.e. Cranichidinae s.l.) in a parsimony analysis by means of a constraint tree in PAUP* resulted in two MPTs only two steps longer (with the same CI and RI) than the six MPTs from the unconstrained analysis.
Bayesian analysis
Relationships recovered by the Bayesian analysis for the most part mirror those of the parsimony analysis, but the tree is fully resolved (Fig. 2B). Spiranthinae, the Stenoptera clade, Prescottia/Pseudocranichis and Cranichidinae are all strongly supported (PP 1·00). Spiranthinae are sister to a moderately supported group (PP 0·91) comprising the Stenoptera clade as the sister of a group that includes Prescottia/Pseudocranichis, which in turn is sister of Cranichidinae s.s. (PP 0·71). Internal relationships of these groups are similar to those recovered in the parsimony analysis. However, the topology of the Bayesian tree matched none of the six MPTs found by parsimony.
DISCUSSION
Relationships among the four major clades of ‘core spiranthids’
The lack of supported resolution for the relationships among Spiranthinae, Cranichidinae, Prescottia and the Stenoptera clade noted by Salazar et al. (2003) was also observed in the parsimony analysis. In the consensus tree, these four clades form a polytomy (Fig. 2A). However, in the Bayesian tree, Spiranthinae are sister to the rest with a moderately high posterior probability (PP 0·91) and the Stenoptera clade diverges next, with paraphyletic Prescottia (including Pseudocranichis) as sister to Cranichidinae (PP 0·71; Fig. 2B). None of the MPTs of the parsimony analysis matches the topology of the Bayesian tree. Instead, each of the following resolutions was recovered by two of the six parsimony cladograms: (a) (Stenoptera clade–(Prescottia/Pseudocranichis–(Cranichidinae-Spiranthinae))); (b) (Stenoptera clade–(Cranichidinae–(Prescottia/Pseudocranichis–Spiranthinae))); and (c) (Prescottia/Pseudocranichis–(Stenoptera clade–(Cranichidinae-Spiranthinae))).
The parsimony analysis constrained to enforce monophyly of Cranichidinae s.l. resulted in two cladograms only two steps longer that the six MPTs of the unconstrained analysis. Therefore, the topology recovered by the Bayesian analysis is not substantially worse (in terms of parsimony steps) than the three (unsupported) resolutions recovered by the parsimony analysis. Cranichidinae s.l. can be unambiguously diagnosed by the non-resupinate flowers, and this requires a single transition from resupination to non-resupination in Cranichideae (Fig. 3A), since with the exception of a few species of Spiranthinae (e.g. Aracamunia liesneri, Cyclopogon glabrescens) and a few genera of Goodyerinae such as Hetaeria Bl. and Macodes Lindl., resupination is uniform in the tribe. Flower orientation is important for pollination (van der Pijl and Dodson, 1966), and transitions between resupination and non-resupination might have important evolutionary consequences, e.g. promoting divergence between lineages by adaptation to different types of pollinator. We believe that, in the absence of evidence on the contrary, a phylogenetic hypothesis that minimizes the number of transitions between these two conditions (such as that of Fig. 2B) is to be preferred.
Fig. 3.
Optimization of flower orientation and thickenings of velamen cell walls on the Bayesian tree of Fig. 2B (see text).
The lack of support for relationships among Spiranthinae, Cranichidinae, Prescottia and the Stenoptera clade in the study of Salazar et al. (2003) led Chase (2003) to adopt a conservative approach and resurrect Cranichidinae in the broad sense, i.e. putting back the genera transferred to Prescottiinae by Dressler (1990, 1993). Chase (2003) stated that this was a compromise solution pending more data, which may be less misleading than recognizing more and more narrowly circumscribed subtribes. At least the results of the present Bayesian analysis support his approach, since Cranichidinae s.l. are recovered as monophyletic. We have considered the alternative option, i.e. creation of a new subtribe for the Stenoptera clade, thus restricting Prescottiinae to include only Prescottia/Pseudocranichis. However, we are unaware of any morphological attributes diagnostic for the Stenoptera clade, and it seems pointless to propose a new undiagnosable subtribe, which only complicates further the nomenclature of these groups. Therefore, we support the merging of ‘Prescottiinae’ with Cranichidinae s.s. proposed by Chase (2003) until compelling phylogenetic evidence clearly demonstrates otherwise.
In discussing relationships between Cranichidinae and Prescottiinae, Salazar et al. (2003) stated that a hamulus, a diagnostic feature of Cranichidinae s.s., is also present in the prescottioid genus Gomphichis, but subsequent observations (A. Álvarez, Missouri Botanical Garden, Ecuador Program, Quito, Ecuador, pers. comm., 2007; see also Rasmussen, 1982) indicated that this may not be the case, and further study is required to determine the nature of the viscidium in that genus. On the other hand, the differences in velamen characteristics noted by Porembski and Barthlott (1988) between one prescottioid species, Aa palacea (as Altensteinia palacea) and two species of Cranichidinae s.s., namely Ponthieva schaffneri (as Cranichis schaffneri) and P. petiolata, have been confirmed for various other taxa by Figueroa et al. (2008). Their study showed that in Spiranthinae and most representatives of ‘Prescottiinae’ analysed (except Pseudocranichis thysanochila) secondary walls of velamen cells bear conspicuous thickenings, which are absent in members of Cranichidinae s.s. (and Pseudocranichis) examined, as well as in the species of Goodyera, Ludisia (both Goodyerinae) and Manniella (Manniellinae) they used as outgroups. In their phylogenetic tree, Cranichidinae were sister to the two prescottioid clades plus Spiranthinae, and thus absence of thickenings was interpreted as the plesiomorphic condition, with their presence representing a synapomorphy of the Prescottiinae/Spiranthinae grade (Figueroa et al., 2008, fig. 4, and 5A). Nevertheless, the relationships recovered by the present Bayesian analysis imply a different scenario, in which thickenings of velamen cell walls are synapomorphic for the whole Spiranthinae/Cranichidinae s.l. clade, with the absence of thickenings being best interpreted as a reversion (secondary loss) diagnostic of what might be termed ‘core Cranichidinae’ and evolving independently in Pseudocranichis thysanochila (Fig. 3B).
Lack of clear patterns of support for relationships among the four major clades of core spiranthids in the parsimony analysis discussed above contrasts with the otherwise strongly supported relationships at lower and higher hierarchical levels of the phylogenetic tree (Fig. 2A and B) and might be suggestive of a rapid morphological differentiation (i.e. rapid enough, in a geological timeframe, not to allow for the accumulation of nucleotide substitutions between successive divergences) or to a slower rate of molecular evolution. However, our studies have so far included only DNA sequence data, and it is necessary to conduct cladistic analyses of as many structural characters as possible to contrast results of the molecular trees. This would allow us to evaluate whether those portions of the evolutionary history of core spiranthids that have not been resolved clearly using only DNA sequences correspond to the appearance of structural changes that may have promoted rapid lineage divergence (cf. Bateman, 1999). One promising candidate for such a role as promoter of divergence is the change in flower orientation from resupinate to non-resupinate, which may have given these species access to previously unexploited types of pollinators. However, much more work is required on both assembling and analysing structural datasets for these orchids and investigating factors underlying such apparently radical changes as switching of flower orientation, to say nothing of a better understanding of pollination of these groups.
Internal relationships of the three major clades of Cranichidinae s.l.
Stenoptera clade
This group received strong support in the analyses of Salazar et al. (2003) and also in this study (Fig. 2). Figueroa et al. (2008) analysed only one species each of Aa and Altensteinia, which likewise formed a strongly supported group. No obvious features diagnosing this clade are known, but, as currently recognized, the genera it includes (Aa, Altensteinia, Gomphichis, Myrosmodes, Porphyrostachys and Stenoptera) are each clearly defined by floral characters. In both the present parsimony and Bayesian analyses, Stenoptera ecuadorana was sister to the rest, followed by Altensteinia fimbriata. Monophyletic Gomphichis was recovered as sister to a clade consisting of Porphyrostachys pilifera plus Aa. Porphyrostachys pilifera is distinctive among the group for its relatively large, bright red flowers with two white blotches on the labellum, which is funnel-shaped and adnate at its base to the prominent column foot (thus forming a deep floral tube). The remaining genera, as well as the other species of Porphyrostachys (P. parviflora) have flowers that vary in size and colour but are always much smaller and less showy than in P. pilifera, the only member of Cranichideae outside Spiranthinae that appears to be pollinated by hummingbirds (van der Pijl and Dodson, 1966).
Although no representatives of Myrosmodes were included in the combined analyses because it was not possible to sequence plastid DNA reliably from the degraded sample available to us, a preliminary heuristic search, in which an ITS sequence of Myrosmodes cochleare Garay (GenBank accession AM419768) was included, placed this species as the strongly supported sister of Aa (results no shown), in agreement with their shared possession of lateral inflorescences, scarious bracts and a lacerate-fimbriate labellum.
Prescottia and Galeoglossum (including Pseudocranichis)
As noted by Vargas (1997) and Salazar et al. (2003), Prescottia tubulosa differs from the other members of the genus in various attributes, such as the absence of functional leaves at flowering time and the slightly concave labellum with incurved (‘involute’) lateral margins (instead of calceolate). Salazar et al. (2003) suggested a close relationship between P. tubulosa and Pseudocranichis thysanochila based on similarities in labellum and column morphology (P. thysanochila was not available for molecular study at that time). The present analyses corroborate a sister-group relationship between P. tubulosa and P. thysanochila that makes Prescottia paraphyletic (see also Figueroa et al., 2008). Monophyly can be achieved either by sinking Pseudocranichis in Prescottia or by removing Prescottia tubulosa from the latter. Here we argue for the second approach, noting that the earliest generic name available for the clade that includes P. tubulosa and P. thysanochila is Galeoglossum A.Rich. & Galeotti (Salazar, 2009). Thus redelimited, Galeoglossum (including Pseudocranichis) is restricted to the floristically distinctive, seasonally dry/cool pine–oak forests occurring throughout the major mountain ranges of Mexico and Guatemala (Hágsater et al., 2005; Salazar et al., 2006). Galeoglossum is readily distinguished from Prescottia by a labellum with incurved lateral margins but open apically (not calceolate) and provided with a distinct apical lobule, the saddle-shaped stigma with two receptive areas separated by a central sterile area and the hairpin-shaped, slender pollinia. A review of the floral morphology and taxonomy of Galeoglossum, including the required new combinations, will be published elsewhere (Salazar, 2009).
The remaining species of Prescottia analysed here form a strongly supported clade with two subgroups, the first of which consists of P. plantaginea (the type species of the genus) and P. aff. oligantha. Both these species, as with most of the remaining 20-odd species of the genus, are restricted to Brazil. The second clade includes the long-petioled, broad-leaved species P. petiolaris, P. cordifolia, P. stachyodes and P. aff. stachyodes. All these species occur in continuously moist or wet tropical and cloud forests; the range of widespread P. stachyodes includes southern Mexico, but its habitat preferences are amply distinct from those of Galeoglossum. The six species of Prescottia s.s. sampled for this study encompass a good deal of the morphological variation recognized within the genus (cf. Hoehne, 1945; Vargas, 1997). All species of Prescottia s.s. have in common a calceolate labellum lacking apical lobulation, a single receptive stigmatic area located on the ventral surface of the column and ovate pollinia.
‘Core’ Cranichidinae
As stated earlier, Cranichidinae sensu Dressler (1993) are paraphyletic because Pseudocranichis thysanochila is strongly supported as sister of Prescottia tubulosa, but once the former species is excluded their monophyly is strongly supported by the present data and the unique possession of a hamular viscidium (except Exalaria parviflora; cf. Rasmussen, 1982; Szlachetko and Rutkowski, 2000) (Figs 1G and 4A). The position of Pterichis as sister to the rest in the analyses of Salazar et al. (2003) is confirmed here. The three species of Pterichis analysed in this study form a strongly supported clade in which P. habenarioides is sister to P. triloba/P.galeata. As currently delimited, Pterichis is a predominantly Andean genus encompassing about 20 species, one of which is found in Costa Rica and Panama and another in Jamaica. However, the single species of the genus Fuertesiella Schltr. (F. pterichoides), found in Cuba and Hispaniola, is morphologically similar to the species of Pterichis, and further study might demonstrate that Fuertesiella should be synonymized with Pterichis. No suitable material of Fuertesiella has so far been available for molecular analysis.
The strongly supported sister group of Pterichis consists of two clades. The first clade is Cranichis (BS 100, PP 1·00), which is fully resolved with all of its subclades receiving strong support. The group formed by Cranichis engelii/C. ciliata and C. diphylla/C. muscosa consists of species widespread in the neotropics (except for C. engelii, restricted to Andean Colombia, Ecuador and Venezuela), whereas its sister group includes only Mesoamerican taxa. Recently González (1996) proposed a new genus, Nezahualcoyotlia, for the Mexican endemic Cranichis gracilis on the basis of differences in lobulation of the clinandrium, projection of the lower margin of the stigma, size of the anther relative to the column, fusion of veins of the floral bracts and coloration of the leaves (among others). Many of these characters show gradual variation among the species, and some, such as the veining of the floral bracts, have not been adequately described for most species of the genus, thus making comparisons difficult. Although it was not possible to obtain suitable material for DNA analysis, in our view C. gracilis shows the basic floral structure of Cranichis and should be retained in this genus until there is convincing phylogenetic evidence to the contrary.
The second major clade of core Cranichidinae includes species of Ponthieva mingled with some species currently placed in other genera. Therefore, as currently delimited, Ponthieva is polyphyletic. There are two groups containing species of Ponthieva. The first one includes Baskervilla colombiana in a derived position within the grade formed by Ponthieva formosa (Fig. 1E), P. elata and P. tuerckheimii. The last three species represent a chiefly Andean group that differs from ‘typical’ members of Ponthieva in various morphological features, such as the possession of a fleshy rhizome, pollinia of two different sizes and a pair of basal labellum lobes of variable size but similar in position to the basal ‘flaps’ characteristic of the labellum of Baskervilla Lindl. These structural features agree with the DNA sequences in the present study and suggest that all these species might be grouped under Baskervilla. However, the sampling in this clade is too sparse, and making nomenclatural changes seems inadvisable at this time.
The second group of Ponthieva species encompasses all other species of Ponthieva analysed, including the type species of the genus, P. racemosa, but has both Exalaria parviflora and Ocampoa mexicana embedded among them. Ocampoa was originally proposed to include the single species, O. mexicana, characterized by a long C-shaped labellum claw and strongly oblique lateral sepals. Schlechter (1918) sank Ocampoa in the synonymy of Cranichis without discussing his rationale, and most subsequent flora writers have followed Schlechter (e.g. Williams, 1951; McVaugh, 1985), but contemporary Mexican orchid students have resurrected Ocampoa on account of its unique suite of floral characters (González, 1995; Hágsater et al., 2005; Soto, 2008) (Fig. 1H). Nevertheless, the present data firmly place O. mexicana in the clade that includes the type of the genus Ponthieva, with O. mexicana being sister to the ‘typical’ P. ephippium (BS 87, PP 1), in spite of its unusual labellum and lateral sepal morphology. On the other hand, Garay and Romero-González (1999) segregated the Andean species previously known as Cranichis fertilis into a monotypic new genus, Exalaria, combining the latter with the specific epithet of the earliest name of the species, [Ophrys] parviflora (which for priority reasons cannot be used in Cranichis). Exalaria was distinguished from Cranichis mainly by its short, broadly triangular, excised rostellum and wingless clinandrium in contrast to the pointed rostellum and a more or less conspicuous wing or flap on each side of the column of typical Cranichis. Garay and Romero-González (1999) also proposed that the New Caledonian endemic Coilochilus neocaledonicus is the closest relative of Exalaria. However, phylogenetic analyses of plastid DNA sequences (Kores et al., 2000, 2001) have shown that Coilochilus is sister to Cryptostylis (subtribe Cryptostylidinae, tribe Diurideae). Alternatively, an extremely divergent (and probably paralogous) ITS sequence relates it to subfamily Epidendroideae (Clements et al., 2002). Furthermore, Coilochilus neocaledonicus and Exalaria parviflora differ sharply in vegetative morphology, and the purported similarity between them is restricted to the overall appearance of the minute flowers (Fig. 1G). Such similarity likely resulted from extreme reduction of all floral parts undergone independently by these two distantly related, apparently self-pollinating species (Bower, 2001; G. A. Salazar, pers. obs.). As in the case of Ocampoa mexicana, the embedding of Exalaria parviflora in the clade that includes the type of Ponthieva sustains its inclusion in Ponthieva (see Appendix 2).
Ponthieva is customarily distinguished from other genera by the basal adnation of petals and labellum to the column. In addition, the petals are often distinctly broadened above the narrow base forming an obliquely triangular-ovate blade, and the two petals are close to one another forming a pseudolabellum (Dressler, 1993), whereas the inconspicuous true labellum stands in an upright position (Fig. 1E, F). In many instances, the petals adhere to the dorsal sepal at their apices. Neither Exalaria nor Ocampoa shows these features, which may be an indication of a different pollination mechanism (and likely autopollination in the former). There are other species of Ponthieva in which one or more of the above-mentioned ‘diagnostic’ features may be absent; for instance, in P. schaffneri the petals are free from the column, and they are narrowly oblanceolate-spathulate and do not form a pseudolabellum. Mapping of this last character on the Bayesian tree (Fig. 4B) reveals variation even among closely related species. All the above suggests that flower morphology is labile in the whole ‘Ponthieva complex’ and emphasizes the need for detailed comparative studies of floral morphology and development in this group, coupled with pollination studies. Dressler (1993) noted that the labellum of P. racemosa produces oil instead of nectar and suggested that this species might be pollinated by oil-gathering anthophorid bees, but otherwise there is no published information on pollination of any representative of core Cranichidinae.
No material of Nothostele Garay, Pseudocentrum Lindl. and Solenocentrum Schltr. has been available for molecular study. Nothostele includes a single species restricted to the Brazilian Plateau that was originally placed in Spiranthinae by Garay (1982), but the non-resupinate flowers, pointed rostellum and four clavate pollinia with hamular viscidium (Szlachetko and Rutkowski, 2000) support its inclusion in Cranichidinae. Pseudocentrum and Solenocentrum, on the other hand, are found in southern Central America and the Andes and include about six and two species, respectively. Plants are similar to those of Baskervilla, but their flowers differ from the latter in having distinct floral ‘spurs’, which in Pseudocentrum is formed by the partially connate sepals and in Solenocentrum by the labellum.
CONCLUSIONS
Although the present analyses included most of the genera currently recognized in Cranichidinae s.l., there are still some important gaps, including the puzzling Brazilian genus Nothostele. The inclusion of Pseudocentrum, Solenocentrum and other representatives of Baskervilla and its look-alikes in Ponthieva would permit attainment of a clearer picture of generic limits and establish a framework to investigate evolution of floral morphology in the complex by means of detailed comparative (including developmental) studies. This sort of study would also benefit greatly from data on the natural pollination of the taxa to attain a better understanding of the functional role of the floral structures.
Lack of clear patterns of support for the divergence of the four core spiranthid clades in the present parsimony analysis suggests the possibility of a rapid succession of lineage divergences or a slowdown in the rate of nucleotide substitution. Phylogenetic analyses based on morphological characters, both by themselves and in combination with DNA sequence data, might improve resolution and shed light on the kind of structural changes that accompanied, if not promoted, the early divergence of these groups.
ACKNOWLEDGEMENTS
We thank Miguel A. Soto, Günter Gerlach, Rolando Jiménez, Jerónimo Reyes, Marco A. López, Andrés Maduro, Andrea Niessen, José Portilla, Franco Pupulin, Botanical Garden Munich-Nymphenburg, Herbario AMO, Jardín Lankester of the Universidad de Costa Rica and the Royal Botanic Gardens, Kew, for plant material; Robert L. Dressler, Michael Fay and Cássio van den Berg for useful criticisms to the manuscript; the Curators of AMO, K, MEXU, NY and W for facilitating access to their collections; and the staff of the Molecular Systematics Section, Jodrell Laboratory, Royal Botanic Gardens, Kew, and Laura Márquez Valdelamar (Instituto de Biología, Universidad Nacional Autónoma de México) for assistance with DNA sequencing. Consejo Nacional de Ciencia y Tecnología, México (Apoyo Complementario a Proyectos de Investigación Científica para Investigadores en Proceso de Consolidación 2006) supported the work by G.A.S.
APPENDIX 1
Taxa studied, voucher information and GenBank accessions
| GenBank accession |
|||||
|---|---|---|---|---|---|
| Taxon | Voucher | rbcL | trnL-F | matK | ITS |
| Subtribe Achlydosinae M.A.Clem. & D.L.Jones | |||||
| Achlydosa glandulosa (Schltr.) M.A.Clem. & D.L.Jones | New Caledonia, Clements D-285, CANB | AJ542401 | AJ544506 | AJ543950 | AJ539525 |
| Subtribe Chloraeinae Rchb.f. | |||||
| Chloraea magellanica Hook.f. | Chile, Ryan 1, K (spirit) | AJ542403 | AJ544504 | AJ543948 | AJ539523 |
| Gavilea lutea (Pers.) M.N.Correa | Chile, Ryan 3, K (spirit) | AJ542402 | AJ544505 | AJ543949 | AJ539524 |
| Subtribe Cranichidinae Lindl. | |||||
| Baskervilla colombiana Garay | Colombia, Niessen 5, MEXU (spirit) | AM778157 | AM412714 | AM900826 | AM419791 |
| Cranichis apiculata Lindl. | Mexico, Ruiz 21, MEXU | AM778148 | AM412717 | AM900819 | AM419784 |
| Cranichis ciliata (Kunth) Kunth | Mexico, Salazar 7375, MEXU (spirit) | AM778142 | AM412724 | AM900811 | AM419776 |
| Cranichis ciliilabia C.Schweinf. | Mexico, Soto 8735, MEXU (spirit) | AJ542419 | AJ544488 | AJ543934 | AJ539506 |
| Cranichis cochleata Dressler | Mexico, Salazar et al. 6547, MEXU | AM778146 | AM412719 | AM900817 | AM419782 |
| Cranichis diphylla Sw. | Venezuela, Munich Bot. Gard. 92/3063, M | AM778144 | AM412722 | AM900813 | AM419778 |
| Cranichis engelii Rchb.f. | Ecuador, Schott s.n., K (spirit) | AM778145 | AM412721 | AM900814 | AM419779 |
| Cranichis muscosa Sw. | Costa Rica, Pupulin 1792, USJ | AM778143 | AM412723 | AM900812 | AM419777 |
| Cranichis revoluta F.Hamer & Garay | Mexico, Soto 10097, AMO | AM778147 | AM412718 | AM900818 | AM419783 |
| Cranichis subumbellata A.Rich. & Galeotti | Mexico, Suárez 2094, MEXU (spirit) | AM778149 | AM412720 | AM900815 | AM419780 |
| Cranichis sylvatica A.Rich. & Galeotti | Mexico, Suárez 2443, MEXU (photograph) | AM778150 | AM412734 | AM900816 | AM419781 |
| Exalaria parviflora (C.Presl) Garay & G.A.Romero | Ecuador, Chase O-401, K | AF074137 | AJ409392 | AJ310013 | AJ000137 |
| Ocampoa mexicana (A.Rich.& Galeotti) Schltr. | Mexico, López s.n., MEXU | AM778156 | AM412715 | AM900825 | AM419790 |
| Ponthieva brittoniae Ames | Mexico, Álvarez 4142, MEXU | AM778153 | AM412712 | AM900822 | AM419787 |
| Ponthieva elata Schltr. | Colombia, Salazar s.n., MEXU (photograph) | AM778158 | AM412708 | AM900827 | AM419792 |
| Ponthieva formosa Schltr. | Mexico, Salazar et al. 6250, MEXU | AM778159 | AM412707 | AM900828 | AM419793 |
| Ponthieva ephippium Rchb.f. | Mexico, Salazar et al. 6440, MEXU | AM778155 | AM412709 | AM900824 | AM419789 |
| Ponthieva guatemalensis Rchb.f. | Central America (cultivated specimen), Salazar s.n., MEXU (spirit) | AM778152 | AM412713 | AM900821 | AM419786 |
| Ponthieva parvula Schltr. | Mexico, Soto 10021, AMO | AM778151 | AM412710 | AM900820 | AM419785 |
| Ponthieva racemosa (Walt.) C.Mohr | Mexico, Salazar 6049, MEXU | AJ542417 | AJ544490 | AJ543936 | AJ539508 |
| Ponthieva schaffneri (Rchb.f.) E.W.Greenw. | Mexico, Salazar 6051, MEXU | AJ542418 | AJ544489 | AJ543935 | AJ539507 |
| Ponthieva triloba Schltr. | Mexico, Soto 10022, AMO | AM778154 | AM412711 | AM900823 | AM419788 |
| Pontieva trilobata (L.O.Williams) L.O.Williams | Mexico, Nava et al. 1747, MEXU | AM901012 | AM901010 | AM901011 | AM901013 |
| Ponthieva tuerckheimii Schltr. | Mexico, Salazar et al. 6512, MEXU | AM778160 | AM412716 | AM900829 | AM419794 |
| Pterichis galeata Lindl. | Ecuador, Schott s.n, K (spirit) | AM778162 | AM412732 | AM900831 | AM419796 |
| Pterichis habenarioides Schltr. | Colombia, Aldana 12, COL | AJ542416 | AJ544491 | AJ543937 | AJ539509 |
| Pterichis triloba (Lindl.) Schltr. | Ecuador, Schott s.n, K (spirit) | AM778161 | AM412733 | AM900830 | AM419795 |
| Subtribe Galeottiellinae Salazar & M.W.Chase | |||||
| Galeottiella sarcoglossa (A.Rich. & Galeotti) Schltr. | Mexico, Jiménez 2334, AMO | AJ542407 | AJ544500 | AJ543945 | AJ539518 |
| Subtribe Goodyerinae Klotzsch | |||||
| Dossinia marmorata (Lindl.) E.Morr. | Tropical Asia (cultivated specimen),, Munich Bot. Gard. 94/1190, M | AJ542405 | AJ544502 | AJ543947 | AJ539521 |
| Goodyera pubescens (Willd.) R.Br. | USA, Chase 212, NCU | AF074174 | AM419815 | AJ543954 | AJ539519 |
| Ludisia discolor (Ker-Gawl.) A.Rich. | Tropical Asia (cultivated specimen), Salazar 6354, K (spirit) | AJ542395 | AJ544466 | AJ543911 | AJ539483 |
| Pachyplectron arifolium Schltr. | New Caledonia, Chase 529, K | AJ542404 | AJ544503 | AJ310051 | AJ539522 |
| Platylepis polyadenia Rchb.f. | Madagascar, Salazar 6352, K (spirit) | AJ542406 | AJ544501 | AJ543946 | AJ539520 |
| Subtribe Manniellinae Schltr. | |||||
| Manniella cypripedioides Salazar, T.Franke, Zapfack & Benkeen | Cameroon, Salazar et al. 6323, YA | AJ542409 | AJ544498 | AJ543943 | AJ539516 |
| Manniella gustavi Rchb.f. | Cameroon, Etuge 4515R, YA | AJ542408 | AJ544499 | AJ543944 | AJ539517 |
| Subtribe Prescottiinae Dressler | |||||
| Aa colombiana Schltr. | Colombia, Aldana 2, ANDES | AM778133 | AM412731 | AM900802 | AM419766 |
| Aa hartwegii Garay | Ecuador, Schott s.n., K (spirit) | AM778134 | AM412730 | AM900803 | AM419767 |
| Aa palacea (Kunth) Rchb.f. | Ecuador, Chase 535, K | AJ542410 | AJ544497 | AJ309989 | AJ539515 |
| Altensteinia fimbriata Kunth | Ecuador, Salazar 6789, MEXU (spirit) | AM778132 | AM412737 | AM900801 | AM419765 |
| Gomphichis bogotensis Renz | Colombia, Bello 86, ANDES | AJ542412 | AJ544495 | AJ543941 | AJ539513 |
| Gomphichis caucana Schltr. | Colombia, Díaz 159, ANDES | AM778136 | AM412736 | AM900805 | AM419770 |
| Gomphichis costaricensis (Schltr.) Ames, F.T.Hubb. & C.Schweinf. | Costa Rica, Soto s.n., AMO | AM778135 | AM412729 | AM900804 | AM419769 |
| Porphyrostachys pilifera Rchb.f. | Peru, Whalley s.n., K (photograph) | AJ542411 | AJ544496 | AJ543942 | AJ539514 |
| Prescottia cordifolia Lindl. | Panama, Salazar et al. 6225, PMA | AM778138 | AM412727 | AM900807 | AM419772 |
| Prescottia aff. oligantha (Sw.) Lindl. | Brazil, da Silva 877, MG | AJ519445 | AJ519451 | AJ519449 | AJ519447 |
| Prescottia petiolaris Lindl. | Peru, Munich Bot. Gard. 00/2013, M | AM778137 | AM412728 | AM900806 | AM419771 |
| Prescottia plantaginea Lindl. | Brazil, Salazar 6350, K (spirit) | AJ542414 | AJ544493 | AJ543939 | AJ539511 |
| Prescottia stachyodes (Sw.) Lindl. | Mexico, Salazar 6092, MEXU | AM778139 | AM412735 | AM900808 | AM419773 |
| Prescottia aff. stachyodes (Sw.) Lindl. | Mexico, Salazar et al. 7312, MEXU | AM778140 | AM412726 | AM900809 | AM419774 |
| Prescottia tubulosa (Lindl.) L.O.Williams | Mexico, Salazar 6054, MEXU | AJ542415 | AJ544492 | AJ543938 | AJ539510 |
| Pseudocranichis thysanochila (B.L.Rob. & Greenm.) Garay | Mexico, Tenorio 17900, MEXU | AM778141 | AM412725 | AM900810 | AM419775 |
| Stenoptera ecuadorana Dodson & C.Vargas | Ecuador, Salazar 6357, K (spirit) | AJ542413 | AJ544494 | AJ543940 | AJ539512 |
| Subtribe Pterostylidinae Pfitz. | |||||
| Pterostylis curta R.Br. | Australia, Chase 572, K | AJ542400 | AJ544507 | AJ543951 | AJ539526 |
| Subtribe Spiranthinae Lindl. | |||||
| Aulosepalum tenuiflorum (Greenm.) Garay | Mexico, Salazar 6017, MEXU | – | – | AJ543919 | – |
| Aulosepalum tenuiflorum (Greenm.) Garay | Mexico, Salazar et al. 6150, MEXU | AJ542433 | AJ544474 | – | AJ539591 |
| Beloglottis costaricensis (Rchb.f.) Schltr. | Mexico, Soto 8129, MEXU | AJ542432 | AJ544475 | AJ543920 | AJ539492 |
| Coccineorchis cernua (Lindl.) Garay | Panama, Salazar et al. 6249, MEXU (spirit) | AJ542422 | AJ544485 | AJ543930 | AJ539502 |
| Cyclopogon epiphyticus (Dodson) Dodson | Ecuador, Salazar 6355, K | AJ542425 | AJ544482 | AJ543927 | AJ539499 |
| Deiregyne diaphana (Lindl.) Garay | Mexico, Salazar et al. 6172, MEXU | AJ542440 | AJ544467 | AJ543912 | AJ539484 |
| Dichromanthus aurantiacus (La Llave & Lex.) Salazar & Soto Arenas | Mexico, Salazar 6351, K (spirit) | AJ542439 | AJ544468 | AJ543913 | AJ539485 |
| Dichromanthus cinnabarinus (La Llave & Lex.) Garay | Mexico, Linares 4469, MEXU | AJ542438 | AJ544469 | AJ543914 | AJ539486 |
| Eltroplectris calcarata (Sw.) Garay & H.R.Sweet | Brazil, Soares s.n., K (photograph) | AJ519446 | AJ519452 | AJ519450 | AJ519448 |
| Eurystyles borealis A.H.Heller | Mexico, Soto 9149, AMO | AJ542427 | AJ544480 | AJ543925 | AJ539497 |
| Funkiella hyemalis (A.Rich. & Galeotti) Schltr. | Mexico, Salazar et al. 6128, MEXU | AJ542429 | AJ544478 | AJ543923 | AJ539495 |
| Mesadenella petenensis (Standl. & L.O.Williams) Garay | Mexico, Salazar 6069, MEXU | AJ542421 | AJ544486 | AJ543931 | AJ539503 |
| Mesadenus lucayanus (Britt.) Schltr. | Mexico, Salazar 6043, MEXU | AJ542436 | AJ544471 | AJ543916 | AJ539488 |
| Microthelys minutiflora (A.Rich. & Galeotti) Garay | Mexico, Salazar et al. 6129, MEXU | AJ542430 | AJ544477 | AJ543922 | AJ539494 |
| Odontorrhynchus variablis Garay | Chile, Wallace 130/85, CANB | AJ542426 | AJ544481 | AJ543926 | AJ539498 |
| Pelexia adnata (Sw.) Poit. ex Spreng. | Mexico, Salazar 6012, MEXU | AJ542423 | AJ544484 | AJ543929 | AJ539501 |
| Sacoila lanceolata (Aubl.) Garay | Brazil, Da Silva 874, MG | AJ542441 | AJ544529 | AJ543933 | – |
| Sacoila lanceolata (Aubl.) Garay | Panama, Förther 2545, M | – | – | – | AJ539504 |
| Sarcoglottis acaulis (J.E.Sm.) Schltr. | Trinidad, Salazar 6356, K (spirit) | AJ542424 | AJ544483 | AJ543928 | AJ539500 |
| Schiedeella faucisanguinea (Dod) Burns-Bal. | Mexico, Jiménez s.n., AMO | AJ542428 | AJ544479 | AJ543924 | AJ539496 |
| Schiedeella llaveana (Lindl.) Schltr. | Mexico, Salazar 6073, MEXU | – | AJ544470 | – | – |
| Schiedeella llaveana (Lindl.) Schltr. | Mexico, Salazar 6105, MEXU | AJ542437 | – | AJ543915 | AJ539487 |
| Spiranthes cernua (L.) Rich. | USA, Nickrent 4188, MEXU | AJ542435 | AJ544472 | AJ543916 | AJ539489 |
| Spiranthes spiralis (L.) Cheval. | UK, Bateman s.n., K (spirit) | AJ542434 | AJ544473 | AJ543918 | AJ539490 |
| Stenorrhynchos glicensteinii Christenson | Mexico, Salazar 6090, MEXU | AJ542420 | AJ544487 | AJ543532 | AJ539505 |
| Svenkoeltzia congestiflora (L.O.Williams) Burns-Bal. | Mexico, Salazar 6143, MEXU | AJ542431 | AJ544476 | AJ543921 | AJ539493 |
APPENDIX 2
Nomenclatural changes
Ponthieva fertilis (F.Lehm. & Kraenzl.) Salazar, comb. nov.
Basionym: Goodyera fertilis F.Lehm. & Kraenzl., Bot. Jahrb. Syst. 26: 498. 1899.
Other synonyms: Cranichis fertilis (F.Lehm. & Kraenzl.) Schltr., Repert. Sp. Nov. Regni Veg. Beih. 8: 115. 1921; Ophrys parviflora Presl, Reliq. Haenk. 2: 92. 1827, non Ponthieva parviflora Ames & C.Schweinf., 1936; Exalaria parviflora (Presl) Garay & G.A.Romero, Harvard Papers in Botany 4: 480. 1999 (for a complete synonymy of this species refer to Garay and Romero-González, 1999).
Ponthieva mexicana (A.Rich. & Galeotti) Salazar, comb. nov.
Basionym: Ocampoa mexicana A.Rich. & Galeotti, Ann. Sci. Nat., Bot., ser. 3, 3: 31. 1845.
Synonym: Cranichis mexicana (A.Rich. & Galeotti) Schltr., Beih. Bot. Centralbl. 36: 430. 1918.
LITERATURE CITED
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Bateman RM. Integrating molecular and morphological evidence of evolutionary radiations. In: Hollingsworth PM, Bateman RM, Gornall RJ, editors. Molecular systematics and plant evolution. London: Taylor & Francis; 1999. pp. 432–471. [Google Scholar]
- Bower C. Pollination (of Coilochilus) In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera orchidacearum. Vol. 2. Oxford: Oxford University Press; 2001. p. 118. Orchidoideae. Part 1. [Google Scholar]
- Brieger FG. Unterfamilie: Neottioideae. In: Brieger FG, Maatsch R, Senghas K, editors. Rudolf Schlechter, Die Orchideen; ihre Beschreibung, Kultur, und Züchtung. 3rd. edn. Berlin: Paul Parey; 1974–75. pp. 254–358. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, et al. A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences. American Journal of Botany. 1999;86:208–224. [PubMed] [Google Scholar]
- Chase MW. Phylogenetics (of Cranichidinae) In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera orchidacearum. Vol. 3. Oxford: Oxford University Press; 2003. p. 23. Orchidoideae. Part 2. Vanilloideae. [Google Scholar]
- Chase MW, Freudenstein JV, Cameron KM, Barrett RL. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- Clements MA, Jones DL, Sharma IK, et al. Phylogenetics of Diurideae (Orchidaceae) based on the internal transcribed spacer (ITS) regions of nuclear ribosomal DNA. Lindleyana. 2002;17:135–171. [Google Scholar]
- Dressler RL. Classification of the orchid family. In: Ospina M, editor. Proceedings of the 7th World Orchid Conference. Medellín: Editorial Bedout; 1974. pp. 259–279. [Google Scholar]
- Dressler RL. The orchids: natural history and classification. Cambridge: Harvard University Press; 1981. [Google Scholar]
- Dressler RL. The Spiranthoideae: grade or subfamily? Lindleyana. 1990;5:110–116. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland, OR: Dioscorides Press; 1993. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Salazar GA, Zavaleta A, Engleman M. Root character evolution and systematics in Cranichidinae, Prescottiinae and Spiranthinae (Orchidaceae, Cranichideae) Annals of Botany. 2008;101:509–520. doi: 10.1093/aob/mcm328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay LA. A generic revision of the Spiranthinae. Botanical Museum Leaflets (Harvard University) 1982;28:277–425. [Google Scholar]
- Garay LA, Romero-González GA. Schedulae orchidum II. Harvard Papers in Botany. 1999;4:475–488. [Google Scholar]
- González R. Retour du genre Ocampoa (Orchidaceae) L'Orchidophile. 1995;118:169–175. [Google Scholar]
- González R. Nezahualcoyotlia (Cranichidinae, Orchidaceae), nuevo género del occidente de México. Boletín del Instituto de Botánica, Universidad de Guadalajara. 1996;4:65–71. [Google Scholar]
- Hágsater E, Soto MA, Salazar GA, Jiménez R, López MA, Dressler RL. Orchids of Mexico. Mexico City: Instituto Chinoin; 2005. [Google Scholar]
- Hoehne FC. Flora Brasilica, São Paulo. Vol. 12. São Paulo: Departamento de Botânica do Estado; 1945. pp. 152–337. Part 2. [Google Scholar]
- Kores PJ, Cameron KM, Molvray M, Chase MW. The phylogenetic relationships of Orchidoideae and Spiranthoideae (Orchidaceae) as inferred from rbcL plastid sequences. Lindleyana. 1997;12:1–11. [Google Scholar]
- Kores PJ, Weston PH, Molvray M, Chase MW. Phylogenetic relationships within the Diurideae (Orchidaceae): inferences from plastid matK sequences. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 449–456. [Google Scholar]
- Kores PJ, Molvray M, Weston PH, et al. A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany. 2001;88:1903–1914. [PubMed] [Google Scholar]
- Lindley J. The genera and species of orchidaceous plants. Tribe VI. Neottieae. London: J. Ridgway; 1840. pp. 441–524. [Google Scholar]
- Maddison RD, Maddison WP. Sunderland, MA: Sinauer Associates; 2001. MacClade 4, version 4.02. [Google Scholar]
- McVaugh R. Orchidaceae. In: Anderson WR, editor. Flora Novo-Galiciana: a descriptive account of the vascular plants of Western Mexico. Vol. 16. Ann Arbor: The University of Michigan Press; 1985. pp. 1–363. [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1966. [Google Scholar]
- Porembski S, Barthlott W. Velamen radicum micromorphology and classification of Orchidaceae. Nordic Journal of Botany. 1988;8:117–137. [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera orchidacearum. Vol. 3. Oxford: Oxford University Press; 2003. Orchidoideae. Part 2. Vanilloideae. [Google Scholar]
- Rasmussen FN. The gynostemium of the neottioid orchids. Opera Botanica. 1982;65:1–96. [Google Scholar]
- Ronquist F, Huelsenbeck JP, van der Mark P. 2005. MrBayes 3·1 manual, draft 5/17/2005. Program documentation and manual. Website http://morphbank.ebc.uu.se/mrbayes/ (accessed 17 May 2005)
- Salazar GA. DNA, morphology, and the systematics of Galeoglossum (Orchidaceae, Cranichidinae) In: Pridgeon AM, editor. Proceedings of the Second Scientific Conference on Andean Orchids. Loja: Universidad Técnica Particular de Loja (in press); 2009. [Google Scholar]
- Salazar GA, Chase MW, Soto MA, Ingrouille M. Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA sequences. American Journal of Botany. 2003;90:777–795. doi: 10.3732/ajb.90.5.777. [DOI] [PubMed] [Google Scholar]
- Salazar GA, Reyes J, Brachet C., Pérez J. Orquídeas y otras plantas nativas de la Cañada, Cuicatlán, Oaxaca, México. Mexico City: Universidad Nacional Autónoma de México; 2006. [Google Scholar]
- Schlechter R. Die Polychondreae (Neottiinae Pfitz.) und ihre systematische Einteilung. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1911;54:375–410. (Leipzig) [Google Scholar]
- Schlechter R. Kritische Aufzaehlung der bischer aus Zentral-Amerika bekanntgewordenen Orchideen; E. Aufzaehlung der Gattungen und Arten, part 1 (Selenipedium – Isochilus) Beihefte zum Botanischen Centralblatt. 1918;36:421–458. [Google Scholar]
- Schlechter R. Das System der Orchidaceen. Notizblatt des Botanischen Garten und Museums zu Berlin-Dahlem. 1926;88:563–591. [Google Scholar]
- Simmons MP. Independence of alignment and tree search. Molecular Phylogenetics and Evolution. 2004;31:874–879. doi: 10.1016/j.ympev.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Soto MA. Hágsater E, Soto MA, editors. Ocampoa mexicana. Icones orchidacearum. 2008;10 pl. 1055. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods) Sunderland, MA: Sinauer Associates; 2002. Version 4. [Google Scholar]
- Szlachetko DL. Systema orchidalium. Fragmenta Floristica et Geobotanica (Supplement) 1995;3:1–152. [Google Scholar]
- Szlachetko DL, Rutkowski P. Gynostemia orchidalium. I. Apostasiaceae, Cypripediaceae, Orchidaceae (Thelymitroideae, Orchidoideae, Tropidioideae, Spiranthoideae, Neottioideae, Vanilloideae) Acta Botanica Fennica. 2000;169:1–379. [Google Scholar]
- Vargas CA. Phylogenetic analysis of Cranichideae and Prescottiinae (Orchidaceae), with some taxonomic changes in Prescottiinae. USA: University of Missouri; 1997. MSc Thesis. [Google Scholar]
- Williams LO. The Orchidaceae of Mexico. Ceiba. 1951;2:1–321. [Google Scholar]