Abstract
Background and Aims
The Platanthera clade dominates the North American orchid flora and is well represented in eastern Asia. It has also generated some classic studies of speciation in Platanthera sections Platanthera and Limnorchis. However, it has proved rich in taxonomic controversy and near-monotypic genera. The clade is reviewed via a new molecular phylogenetic analysis and those results are combined with brief reconsideration of morphology in the group, aiming to rationalize the species into a smaller number of larger monophyletic genera and sections.
Methods
Nuclear ribosomal internal transcribed spacer (ITS) sequences were obtained from 86 accessions of 35 named taxa, supplemented from GenBank with five accessions encompassing a further two named taxa.
Key Results
Using Pseudorchis as outgroup, and scoring indels, the data matrix generated 30 most-parsimonious trees that differed in the placement of two major groups plus two closely related species. Several other internal nodes also attracted only indifferent statistical support. Nonetheless, by combining implicit assessment of morphological divergence with explicit assessment of molecular divergence (when available), nine former genera can be rationalized into four revised genera by sinking the monotypic Amerorchis, together with Aceratorchis and Chondradenia (neither yet sequenced), into Galearis, and by amalgamating Piperia, Diphylax and the monotypic Tsaiorchis into the former Platanthera section Platanthera. After further species sampling, this section will require sub-division into at least three sections. The present nomenclatural adjustments prompt five new combinations.
Conclusions
Resolution of major groups should facilitate future species-level research on the Platanthera clade. Recent evidence suggests that ITS sequence divergence characterizes most species other than the P. bifolia group. The floral differences that distinguished Piperia, Diphylax and Tsaiorchis from Platanthera, and Aceratorchis and Chondradenia from Galearis, reflect various forms of heterochrony (notably paedomorphosis); this affected both the perianth and the gynostemium, and may have proved adaptive in montane habitats. Floral reduction was combined with lateral expansion of the root tubers in Piperia and Diphylax (including Tsaiorchis), whereas root tubers were minimized in the putative (but currently poorly supported) Neolindleya–Galearis clade. Allopolyploidy and/or autogamy strongly influenced speciation in Platanthera section Limnorchis and perhaps also Neolindleya. Reproductive biology remains an important driver of evolution in the clade, though plant–pollinator specificity and distinctness of the species boundaries have often been exaggerated.
Key words: Aceratorchis, Amerorchis, Chondradenia, Diphylax, Galearis, generic delimitation, internal transcribed spacer, Neolindleya, orchid, Piperia, phylogeny, Platanthera, Pseudorchis, speciation, Tsaiorchis
INTRODUCTION
Platanthera Rich. (the butterfly orchids) is one of the most species-rich genera of North Temperate orchids (Hapeman and Inoue, 1997), having centres of diversity in East Asia (Inoue, 1983) and North America (Luer, 1975; Sheviak, 2002). Platanthera and allied genera share with many other temperate orchid groups a long and complex taxonomic history. Victorian luminaries including Robert Brown and Joseph Hooker lumped Platanthera species into Habenaria, misled by superficial floral similarities of these genera evident in, for example, rostellum morphology (cf. Dressler, 1993). Other authors elected to divide Platanthera into several genera, each of which typically contained few (1–5) species. During the second half of the 20th century, the view most commonly expressed by morphological systematists recognized the majority of these former genera as sections of the genus Platanthera (e.g. Lysiella, Limnorchis, Blephariglottis and Tulotis). In contrast, other relevant taxa became more commonly recognized as distinct genera, notably Pseudorchis Seguier (syn. Leucorchis A. & D. Löve), Piperia Rydb. (1901a) and Diphylax Hook.f. (1869), together with the more obscure, monotypic Tsaiorchis Tang & Wang (1936). The most notorious ‘taxonomic football’ was Piperia, which was variously assigned to four other genera (Table 1b).
Table 1.
Taxonomic revisions of the former genera Amerorchis, Aceratorchis, Chondradenia, Diphylax and Tsaiorchis, plus a nomenclatural update on the former genus Piperia
| (a) Former genera Amerorchis, Aceratorchis and Chondradenia |
| Galearis rotundifolia (Banks ex Pursh) R.M.Bateman, comb. nov. |
| Basionym: Orchis rotundifolia Banks ex Pursh, Fl. Amer. Sept., 2: 588 (1814). |
| Synonyms: Habenaria rotundifolia (Banks) Richardson, In Franklin: Journey: 750 (1823); Platanthera rotundifolia (Banks) Lindl., Gen. Sp. Orchid.: 292 (1835); Amerorchis rotundifolia Hultén, Ark. Bot. ser. 2, 7: 34 (1968). |
| Galearis tschiliensis* (Schl.) P.J.Cribb, S.Gale & R.M.Bateman, comb. nov. |
| Basionym: Aceratorchis tschiliensis Schl., Rep. Spec. Nov. Regni Veg. Beih. 12: 329 (1922). |
| Synonyms: Orchis aceratorchis von Soó, Ann. Nat. Hist. Mus. Natl. Hung. 26: 350 (1929); Orchis tschiliensis (Schl.) von Soó, Ann. Nat Hist. Mus. Natl. Hung. 26: 351 (1929). |
| Galearis fauriei* (Finet) P.F.Hunt, Kew Bull. 26: 172 (1971). |
| Basionym: Orchis fauriei Finet, J. de Bot.1808: 340 (1898). |
| Synonyms: Chondradenia yatabei Maxim., Cat. Species Herb. Imp. Univ.: 287 (1886), nomen nudum ex Makino, Bot. Mag. (Tokyo)16: 8 (1902); Orchis yatabei (Maxim.) Makino. Ill. Fl. Jap.: 704 (1948); Chondradenia fauriei (Finet) T.Sawada, J. Jap. Bot. 10: 78 (1934), nomen nudum, ex F.Maekawa, Wild orch. Jap. col.: 456, 107 (1971). |
| Galearis doyonensis* (Handel-Mazzetti) P.F.Hunt, Kew Bull.26: 171 (1971). |
| Basionym: Orchis doyonensis Handel-Mazzetti, Symbolae Sinicae, Anthophyta 7: 1234 (1936). |
| Synonyms: Galeorchis doyonensis (Handel-Mazzetti) von Soó, Act. Bot. Acad. Sci. Hung. 12: 352 (1966); Chondradenia doyonensis (Handel-Mazzetti) Vermeulen, Jber. Naturwiss. Ver. Wuppertal25: 36 (1972); Platanthera roseotincta (W.W.Smith) T.Tang & F.T.Wang, Bull. Fan Mem. Inst., Bot. 10 (1940): 30 (see Lang et al., 1999). |
| (b) Former genus Piperia (supplement to revision by Bateman in Bateman et al., 2003) |
| Platanthera unalascensis (Spreng.) Kurtz, Bot. Jahrb.19: 408 (1894). |
| Basionym: Spiranthes unalascensis Spreng., Systema Vegetabilium3: 708 (1826). |
| Synonyms: Herminium unalasc(hk)ense (Spreng.) Rchb.f., Icon. Fl. Germ. Helv.13–14: 107 (1838); Platanthera foetida Geyer ex Hook.f., J. Bot. Kew Misc.7: 376 (1855); Habenaria unalasc(h)ensis (Spreng.) S.Wats., Proc. Amer. Acad. Arts12: 277 (1877); Piperia unalasc(h)ensis (Spreng.) Rydberg, Bull. Torrey Bot. Club28: 270 (1901a). |
| Platanthera ephemerantha* R.M.Bateman, nom. nov. |
| Synonyms: Piperia candida R.Morgan & Ackerman, Lindleyana5: 207 (1990); Platanthera candida (R.Morgan & Ackerman) R.M.Bateman, nom. illegit., Bot. J. Linn. Soc.142: 21 (2003) (illegitimate homonym of Platanthera candida Lindl., Gen. Spec. Orchid. Pl.: 295 (1835). |
| Etymology: Gr. ephemera (transient) and Gr. anthus (flower): ‘The flowers in Piperia candida are more completely white and more ephemeral than in any other member of the genus’ (Ackerman, 2002, p. 574). The holotype of ‘Piperia’ candida is retained, following Article 72.1a of the ICBN. |
| (c) Former genera Diphylax and Tsaiorchis |
| Platanthera urceolata (Hook.f.) R.M.Bateman, comb. nov. |
| Basionym: Diphylax urceolata Hook.f., in Hook.f. Icon., pl. xix (1889). |
| Synonym: Habenaria urceolata (Hook.f.) C.B.Clarke, J. Linn. Soc. Bot.25: 73 + t30 (1889) ex Hook.f., Fl. Brit. Ind. 6: 165 (1896). |
| Platanthera uniformis?* T.Tang & F.T.Wang, Bull. Fan Mem. Inst. Biol. Bot., 10: 28 (1940). |
| Synonym: Diphylax uniformis (T.Tang & F.T.Wang) T.Tang, F.T.Wang & K.Y.Lang, Bot. Res., Inst. Bot. (Beijing), 4: 11 (1989). |
| Platanthera contigua?* T.Tang & F.T.Wang, Bull. Fan Mem. Inst. Biol. Bot., 10: 31 (1940). |
| Synonym: Diphylax contigua (T.Tang & F.T.Wang) T.Tang, F.T.Wang & K.Y.Lang, Vasc. Pl. Hengduan Mount., 2: 2526 (1994). |
| Platanthera neottianthoides* (T.Tang & F.T.Wang) R.M.Bateman & P.J.Cribb, comb. nov. |
| Basionym: Tsaiorchis neottianthoides T.Tang & F.T.Wang, Bull. Fan Mem. Inst. Biol. Bot., 7: 131 (1936). |
* Species have not yet been subjected to molecular analysis.
Also relevant to this discussion are a small number of Eurasian species that were attributed by many authors to the dominantly European genus Orchis L., despite obvious floral and especially vegetative differences. Toward the end of the 20th century these species became more commonly assigned to the small, dominantly Eurasian genus Galearis Rafinesque (1833) and to two monotypic genera: the exclusively North American Amerorchis Hultén (1968) and the Chinese Aceratorchis Schlechter (1922). A similarly monotypic east Asian genus, Neolindleya Kraenzlin (1897–1904), was more commonly misassigned to Gymnadenia, again despite obvious floral and especially vegetative differences. Few if any authors suggested close relationships among (a) Neolindleya, (b) Amerorchis plus Galearis, (c) Aceratorchis or (d) Pseudorchis plus Platanthera; moreover, the supposed close relationship between Pseudorchis and Platanthera was frequently challenged, Pseudorchis often being attributed to Gymnadenia by authors who ignored several obvious floral differences (cf. Bateman et al., 2006; Delforge, 2006).
Over the last decade, these intuitively inferred boundaries of, and relationships among, genera have been systematically revised (and, in many cases, overturned) by sequence-based phylogenetic analyses using the rapidly evolving nuclear ribosomal internal transcribed spacers (ITS) of nuclear ribosomal DNA (nrDNA). Pridgeon et al. (1997) and Bateman et al. (1997) identified a weakly supported Platanthera clade, consisting of Pseudorchis (one species analysed) as sister to Galearis (two species) plus Platanthera (six species, acting as placeholders for putative sections within this larger genus, estimated at >85 species by Hapeman and Inoue, 1997). These generic relationships were also obtained (again with limited statistical support) by Bateman et al. (2003), in a study benefiting from substantially increased taxon sampling. Pseudorchis (two species) was again shown as basally divergent, followed by the monotypic Amerorchis, which was placed as sister to Galearis (three species). The next branch showed the monotypic Neolindleya as sister to Platanthera sensu stricto (s.s.). Lastly, the genus Platanthera proved to encompass several former genera that were already incorporated within Platanthera by most authorities (represented by eight species). However, also nested within the genus was a strongly supported monophyletic group of the three sampled species of Piperia. Consequently, all ten Piperia species recognized by Ackerman and Morgan (2002) were duly incorporated in Platanthera as a new section of the genus (Table 4 of Bateman et al., 2003).
In a parallel sequence-based project, Hapeman and Inoue (1997) produced an ITS tree for a better sampling (36 species) of Platanthera s.s., though they omitted discussion of the identity and relationships of the outgroups. Their study recognized five sections within the genus. Four of these sections (Tulotis, Lacera, Limnorchis and Blephariglottis) were well supported as monophyletic. However, the fifth (and most species-rich) section, Platanthera, was very poorly supported, and none of the relationships inferred among the sections gained support from bootstrap values or decay indices.
More recently, DNA-based studies have sought to use genetic data to help explore speciation and hybridization in more narrowly defined groups of Platanthera, most notably the P. dilatata group (section Limnorchis) in North America (Wallace, 2003, 2004, 2006) and the P. bifolia group (section Platanthera) in Eurasia in general and England in particular (Bateman, 2005; Bateman et al., 2009). The P. bifolia group is of particular significance because it generated one of the classic textbook examples of selection-mediated co-evolution between orchids and their pollinating insects (e.g. Nilsson, 1983, 1985; Maad and Nilsson, 2004; reviewed by Hapeman and Inoue, 1997; Bateman and Sexton, 2008), and has contributed to contrasting theories of instantaneous saltational speciation (summarized by Bateman and Rudall, 2006).
The present ITS analysis includes several additional species from Platanthera sections Platanthera, Limnorchis and Lacera sensu Hapeman and Inoue (1997), together with representatives of putative sections not recognized by Hapeman and Inoue (Lysiella and Lysias). It also includes two of the three commonly recognized species of the putative genus Diphylax, considered to be an upland endemic of the eastern Himalayas (Tang and Wang, 1940; Cribb, 2001). The purpose of the study is primarily to complete our revision of generic limits in the Platanthera clade, applying the principle of monophyly to the ITS data (where available) and reconsidering selected morphological characters across the clade. We also provide a better informed phylogenetic context for more focused ongoing evolutionary studies of the P. dilatata and P. bifolia aggregates. Lastly, we speculate briefly on speciation mechanisms, notably heterochrony, that appear to be operating within the Platanthera clade.
MATERIALS AND METHODS
Fieldwork and analytical materials
Data for the full ITS1–5·8S–ITS2 assembly were generated by the present authors from 86 accessions. Of these sequences, four were first published by Hapeman and Inoue (1997), nine by Pridgeon et al. (1997; also Bateman et al., 1997) and eight by Bateman et al. (2003). We downloaded from GenBank a further four ITS sequences of Platanthera section Limnorchis selected from among a larger number deposited in 2006 by Lisa Wallace, and one sequence of section Lacera from the published study of Szalanski et al. (2001). A further three sequences were derived from the forthcoming doctoral thesis of R. K. Lauri. The remaining 62 accessions were sequenced specifically for the present paper and/or its companion (Bateman et al., 2009). Most of the specimens were collected by R. Bateman, and the majority of the remainder by Y.-B. Luo (China) and Monicá Moura (Azores), though we also thank for provision of (typically single) samples M. Carine, M. Fischer, M. Hedrén, H. Lambert, R. Manuel, D. Nickrent, J. Tyler and J. Vogel.
Together, these 91 ITS sequences encompassed the following genera (sensu Bateman et al., 2003): Pseudorchis (two accessions), Neolindleya (one), Amerorchis (one), Galearis (three), Diphylax (two), Platanthera section Tulotis (one), section Lacera (three), the controversial sections Lysiella (one) and Lysias (one), section Limnorchis (11 accessions of five species) and section Piperia (three). The previously well supported section Blephariglottis was not represented, nor were three East Asian monotypic/near-monotypic genera: Aceratorchis, Chondradenia and Tsaiorchis. Platanthera section Platanthera yielded 50 sequences. These included single accessions of four molecularly distinct Oriental species. Of seven putative species of the Eurasian Platanthera bifolia group, two were represented by only one accession (P. finetiana and P. metabifolia from China) and one by just two accessions (P. holmboei: a reliable accession from Cyprus and a more ambiguous accession from Lesvos). The Azorean flora was represented by four accessions of P. micrantha and two accessions of the rarer P. azorica. In contrast, P. bifolia and P. chlorantha (the primary subjects of the companion study: Bateman et al., 2009), together with their hybrids, were collectively represented by 42 accessions: three from China, nine from Continental Europe and the remaining 30 from Britain (Bateman et al., 2009). As the P. bifolia group generated just seven closely similar ITS alleles, single exemplars only of each allele were carried forward to the parsimony analysis in order to simplify the tree-building procedure.
DNA extraction and sequencing
Total genomic DNA was extracted from silica-desiccated floral (or, less often, leaf) material from 75 specimens using the standard 2× CTAB (cetyltrimethylammonium bromide) procedure (Doyle and Doyle, 1990) except that extractions were incubated in 500 mL of CTAB buffer, 50 mL of sarkosyl and 10 mL of proteinase-K. The rapidly mutating ITS region of nrDNA (e.g. Baldwin et al., 1995; Hershkovitz et al., 1999) was amplified via polymerase chain reaction using primers ITS4 and ITS5 (White et al., 1990; Baldwin et al., 1995) and cycling parameters from the earliest well-described phylogenetic analysis of subtribe Orchidinae, conducted by Pridgeon et al. (1997). Bidirectional sequencing was carried out on an ABI 3730 capillary DNA sequencer using an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer), employing the same primers used for amplification. Available resources did not permit cloning of ITS alleles.
Tree-building methods
Together, these 91 accessions generated 40 ITS alleles representing 37 named taxa. Exemplar samples for each allele are listed in the Appendix. Where particular species or infraspecific taxa yielded more than one allele, the exemplar samples are numbered; note that the alleles typified by the Homefield Wood specimen of P. chlorantha and the Morgan's Hill specimen of P. bifolia each represent large numbers of accessions of both species. In order to accelerate the tree-building procedure and to facilitate full rather than fast bootstrap analysis, trees were constructed only from these 40 alleles. Alignment was achieved by eye, yielding a total of 634 bp. All indels were coded as bistate characters. Each differentiable gap was coded separately, thereby maximizing the number of indels recognized (52) but also maximizing the proportion of those indels that functioned only as autapomorphies (32, = 62 %). Trees were constructed in PAUP 4·0b10 (Swofford, 2001) by heuristic search using sub-tree pruning–regrafting (SPR) with MulTrees in effect, no limit on number of trees held and swapping on all trees, in order to recover all islands of most-parsimonious trees.
The primary analysis (A) identified Pseudorchis as the outgroup, following the topology of Bateman et al. (2003), and included the Galearis clade. Subsidiary experimental analyses omitted Pseudorchis, instead identifying the Galearis clade as outgroup (B), and omitted the Galearis clade (C). Each of the three analyses was conducted both with and without indels.
Robustness of nodes was explored via full bootstrap analyses using a full heuristic search with stepwise addition, permitting 1000 replicates. The large numbers of sub-optimal trees found in the primary analysis under collapse nodes with minimum length of zero, amb- (L = 30 trees, L + 1 = 969, L + 2 = approximately 10 000), limited calculation of decay index values to ≤3.
RESULTS
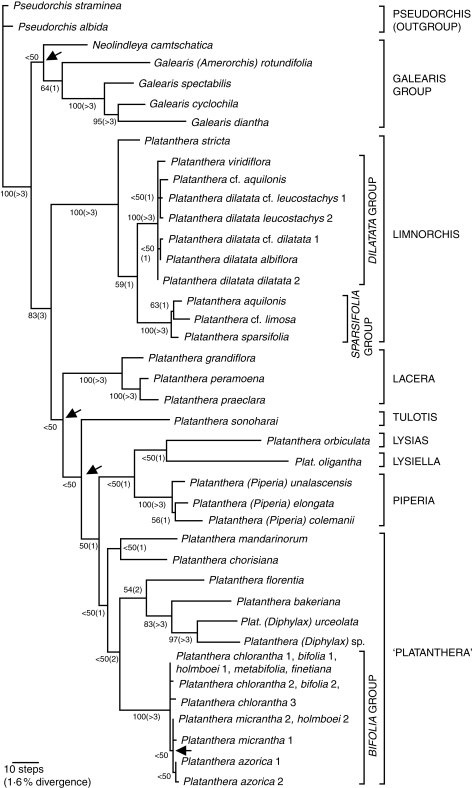
Of 692 characters, 325 were variable and 189 were potentially parsimony informative. By definition, all 52 indels were variable, but only 20 proved to be potentially parsimony informative. When indels were included, the primary analysis yielded a single island of 30 most-parsimonious trees, 671 steps in length with a consistency index of 0·55 (0·65 including autapomorphies) and a retention index of 0·80. Tree 19 is here reproduced as Fig. 1.
Fig. 1.
Preferred most-parsimonious tree of representatives of four of the five sections of Platanthera recognized by Hapeman and Inoue (1997: listed to the right), together with the former genera Piperia (Bateman et al., 2003) and Diphylax, plus the two genera that constitute the revised Galearis group; the two Pseudorchis species constitute the outgroup. Branch lengths are proportional to Acctran optimization and include autapomorphies; figures on internal branches indicate bootstrap support and (in parentheses) decay index values. The four arrows indicate nodes that collapse in the strict consensus tree.
Support for all but two of the 31 internal branches could be categorized as either strong (bootstrap >95 %, decay index >3) or weak (bootstrap support ≤65 %, decay index ≤2). The weak nodes characterized either groups of closely related species that formed near-polytomies (P. dilatata group, P. sparsiflora group, section Piperia and P. bifolia group) or character conflict among major groups distributed along the spine of the cladogram. Four nodes collapsed in the strict consensus tree (arrowed in Fig. 1). Of these nodes, that within the P. bifolia group was relatively trivial, reflecting low levels of sequence divergence (Bateman et al., 2009). However, the remaining three equivocal nodes were of greater potential importance. Possibly as a consequence of the absence of any representative of section Blephariglottis, section Lacera proved to be a serious source of instability in the central portion of the tree, occurring variously as sister to section Limnorchis, section Tulotis (P. sonoharai), or all sections other than Lacera and Limnorchis (Fig. 1). Section Tulotis was placed as sister to either section Limnorchis or all sections other than Lacera and Limnorchis (Fig. 1). Toward the base of the tree, Neolindleya could be placed as sister to either Amerorchis plus Galearis (Fig. 1) or the genus Platanthera (Efimov et al., 2009).
Several tree-building experiments (topologies not shown) were conducted to explore the robustness of the trees resulting from the primary analysis. Reanalysing the primary matrix without indels generated a similar set of most-parsimonious trees, but caused P. orbiculata to diverge earlier in the topologies of the most-parsimonious trees, shifting from its previous position as sister to P. oligantha (section Lysiella) and relocating as sister to section Lacera. Omitting Galearis and Neolindleya reduced the number of most-parsimonious topologies to 18 and similarly shifted P. orbiculata down the trees, to a location immediately above section Lacera. Omitting Pseudorchis and instead identifying the Galearis–Neolindleya clade as a multiple outgroup reduced the number of most-parsimonious topologies to 10. It not only placed P. orbiculata as sister to section Lacera but also placed this clade as sister to section Limnorchis, and identified section Tulotis as sister to this novel clade. In effect, the tree shown in Fig. 1 was re-rooted immediately above P. sonoharai. The main message of these experiments was that P. orbiculata is the greatest source of topological instability in the matrix.
DISCUSSION
The following discussion is based on, but not confined to, our preferred most-parsimonious tree based on our ITS sequences (Fig. 1). We regret that, after many years of searching, we have not yet acquired analysable DNA of the obscure (near-)monotypic Asian genera Aceratorchis, Chondradenia and Tsaiorchis. Consequently, our taxonomic treatment of these genera (Table 1) is based wholly on reconsideration of often-limited morphological evidence, and to some degree on the assumption that paedomorphosis of vegetative and/or floral organs is an iterative evolutionary theme within the Platanthera clade sensu latissimo. Further morphological and DNA-based studies are therefore desirable. Our conclusions regarding generic limits even deviate substantially from those established in Genera Orchidacearum (GO: Pridgeon et al., 2001); we recommend amalgamation into other more widespread genera of three supposed genera that were recognized in GO (Aceratorchis, Chondradenia and Diphylax) and recognition of one genus that was omitted from GO (Neolindleya).
Delimitation and relationships of genera and sections in the Platanthera clade
The Platanthera sensu lato (s.l.) clade is undoubtedly sister to Dactylorhiza s.l. plus Gymnadenia s.l., and is far more distantly related to Habenaria, despite the similar rostellar morphologies of the two genera (Bateman et al., 2003). With the notable exception of Pseudorchis, mutation rate appears to be substantially higher in the Platanthera clade than in its sister group, the Dactylorhiza s.l.–Gymnadenia s.l. clade (fig. 1c of Box et al., 2008). Within the Platanthera clade, it remains unclear whether Pseudorchis or the Galearis clade is sister to Platanthera s.s., but it is clear that Pseudorchis merits the status of genus (contra Luer, 1975; Delforge, 2006). The two ITS branches that define the dichotomy between the Galearis clade (tentatively including Neolindleya) and Platanthera s.s. are only weakly supported, despite the fact that the two clades are readily separated using morphology.
Characters listed by Hapeman and Inoue (1997) as delimiting the genus Platanthera included its broad anther, undivided stigma and fusiform tubers, though reports of exceptions for each character have increased in number as the former genera Piperia and Diphylax have successively been incorporated into the genus (Bateman et al., 2003; present study; also Lauri, 2007, who disagrees with this degree of ‘lumping’ at the genus level).
Within Platanthera, most of the sections recognized by Hapeman and Inoue (1997) survive the present analysis. However, the monophyly of section Platanthera sensu Hapeman and Inoue (1997) is challenged by the fact that the former genus Diphylax (and possibly also the former genus Piperia) is embedded deeply within it, and by the placement of P. orbiculata (attributed to section Platanthera by Hapeman and Inoue, 1997) as sister to P. oligantha of section Lysiella (Fig. 1). Admittedly, this placement is decidedly tentative. Several experiments with taxon and character sampling separated P. orbiculata from P. oligantha and placed it further down the tree. Also, P. orbiculata and P. obtusata (putatively a close relative of P. oligantha) were separated (albeit both occurring within section Platanthera) in the analyses of Hapeman and Inoue (1997). Lastly, P. orbiculata and P. oligantha are subtended by the longest branches in our tree, thus raising the possibility that their apparent sister-group status may reflect long-branch attraction.
Irrespective of their mutual relationships, valid cases can be made for recognizing the former genera Lysiella (here represented by P. oligantha) and Lysias (here represented by P. orbiculata) as sections of Platanthera (Fig. 1). The assignment of P. orbiculata to section Platanthera by Hapeman and Inoue (1997) is not supported.
The Pseudorchis clade
The ITS analysis of Bateman et al. (2003) revealed Pseudorchis to be a relatively short-branch taxon that caused topological instability within the Dactylorhiza–Gymnadenia–Platanthera clade. They noted its general morphological similarity to Platanthera, but also listed some morphological distinctions. These include spatulate leaves, the highly reduced central lobe to the labellum and a reduced gynostemium that minimizes the connective separating the two anther loculi.
Many authors consider the genus to be monotypic, whereas morphometric and allozyme analyses suggest that two closely related species may occur in Scandinavia (Reinhammar, 1995; Reinhammar and Hedrén, 1998). Although Bateman et al. (2003) considered the ITS divergence between the two putative species to be worryingly low (Fig. 1), it exceeds the level of divergence recorded here among members of the Platanthera bifolia aggregate (see below).
The Galearis clade: Galearis s.l.
The taxonomic challenges presented within the Galearis clade are analogous to those previously presented by the Eurasian Himantoglossum clade (Bateman et al., 2003), in which a core group of several species of Himantoglossum s.s. had as its unambiguous sister the possibly monotypic, morphologically similar Barlia. This clade in turn had as its unambiguous sister the unequivocally monotypic, superficially morphologically distinct Comperia, and this clade in turn had as its ambiguous sister the unequivocally monotypic, more substantially morphologically distinct Steveniella. Each former genus was separated by a moderate degree of disparity in ITS sequences. Thus, Bateman et al. (2003) chose to sink both Barlia and Comperia into Himantoglossum, but retained Steveniella as a separate genus because of its less confident phylogenetic placement and its comparatively distinct morphology (small, purple-washed leaves, central lobe of the labellum not further divided, spur apex clearly bifid, gynostemium compact).
In the case of the Galearis clade, Amerorchis mimics the role of Comperia and Neolindleya mimics the role of Steveniella. Both genera are commonly viewed as monotypic. Amerorchis is undoubted sister to Galearis s.s., though they are separated by a substantial molecular distance. However, comparison of the morphological descriptions of the two genera given in recent publications (e.g. Wood and Neiland, 2001a, b; Sheviak and Catling, 2002) showed the two supposed genera to be similar in most characters. Moreover, characters that do show some difference are those that are known to be highly homoplastic within subtribe Orchidinae (Bateman et al., 2003), notably Amerorchis more often having only one expanded leaf rather than two, and tending to have a more clearly three-lobed labellum. Gynostemium morphology differs between Amerorchis, North American Galearis s.s. (i.e. G. spectabilis, the type species of the genus) and southest Asian Galearis s.s. (Luo and Chen, 2000; Perner and Luo, 2007; R. Lauri, unpubl. res.). Amerorchis possesses an undivided bilobed bursicle and G. spectabilis a divided bursicle, whereas Asian Galearis lack a bursicle (Perner and Luo, 2007). The stigma is divided into two zones separated by the rostellum in both Amerorchis (R. Lauri, unpubl. res.) and southeast Asian Galearis s.s. (Perner and Luo, 2007), whereas it delineates a single inverted triangle in North American Galearis s.s. In addition, Amerorchis rotundifolia has a distribution in North America that largely complements that of the more southerly Galearis spectabilis, exhibiting only a narrow zone of overlap. Their successive divergence below the two Asian species of Galearis in Fig. 1 implies that the Asian species are derived relative to the North American species (Bateman et al., 2003).
Considered together, these factors arguably suggest that Amerorchis is better incorporated into Galearis than retained as a monotypic genus. The relevant combination is made here in Table 1; surprisingly, this species has not previously been placed in Rafinesque's (1833) Galearis, but rather was variously assigned to Habenaria, Orchis and (more credibly) Platanthera, before being isolated as Amerorchis as recently as 1968 (Hultén, 1968).
Luer (1975) and Sheviak and Catling (2002) recognized only one North American and one east Asian species of Galearis (excluding Amerorchis), whereas Wood and Neiland (2001b) suggested 3–6 species in total, Cribb and Gale (2009) listed five species (including the former genus Aceratorchis: see below) and Perner and Luo (2007) recognized six species in China alone. Expanding his geographical coverage, Hunt (1971) listed 11 species in Asia alone (including Aorchis sensu Vermeulen, 1972). Our analysis shows substantial molecular divergence between the two east Asian species analysed, G. cyclochila and G. diantha, both of which appear morphologically distinct from G. wardii (illustrated from China as ‘Orchis’ wardii by Chen et al., 1999, p. 306). We suspect that there are substantially more than two bona fide species in the genus Galearis.
In addition, after a decade of failing to obtain DNA samples of the rare Chinese species Aceratorchis tschilensis Schlechter (1922), we have nonetheless taken the radical step of incorporating Aceratorchis into Galearis, an action suggested tentatively by Cribb and Wood (2001) and more firmly by Cribb and Gale (2009). [Perner and Luo (2007, p. 135) went one step further in suggesting that ‘A.’ tschiliensis is conspecific with Galearis roborowskii.] Our decision to place Aceratorchis within Galearis is based partly on similarities in vegetative and gynostemium characters and partly on the hypothesis that the near-radial symmetry of the perianth of Aceratorchis and its spurless condition reflect an origin from ‘wild-type’ Galearis flowers via a heterochronic developmental shift termed pseudopeloria (Rudall and Bateman, 2002; Bateman et al., 2003; Bateman and Rudall, 2006; Mondragón-Palomino and Theissen, 2008, 2009). If so, they constitute atavistic reversals, rather than showing the fundamentally primitive morphology inferred by Chen (1982) and Chen and Tsi (1987).
This heterochronic hypothesis remains in need of explicit testing, ideally via evolutionary–developmental genetics (cf. Box et al., 2008). Nonetheless, we have applied the same logic when re-assigning to Galearis the two southeast Asian species formerly attributed to the genus Chondradenia (cf. Maximowicz, 1886; Finet, 1898; Makino, 1902; Schlechter, 1919; Handel-Mazzetti, 1936; von Soó, 1966; Hunt, 1971; Makawa, 1971; Vermeulen, 1972; Brieger et al., 1973; Wood, 2001; Perner and Luo, 2007). (Alternatively, assignment to Platanthera was suggested in the case of ‘P.’ doyonensis: Lang et al., 1999.) Nomenclaturally, these species have a chequered history, having undergone several previous generic transfers and received at least two nomina nuda (Table 1); also, morphological descriptions contrast in key features (e.g. compare Vermeulen, 1972; Wood, 2001). We still lack DNA samples and chromosome counts for either species. They closely resemble Galearis in vegetative features, having highly reduced tubers, only 1–2(–3) expanded leaves, and compact, few-flowered inflorescences characterized by large bracts. Flowers are small and unhooded. Perianth differentiation is poor, though the labellum is slightly larger than the other tepals and bears a small globose spur (also a swollen apical appendix in the case of the Japanese species). Resupination is unreliable, characterizing some but by no means all individuals. Their columns lack a bursicle (thus resembling the Asian rather than the North American species of Galearis), and the pair of pollinia are connected by a single dorsiventrally elongate viscidium (viscidial fusion has occurred in other species within subtribe Orchidinae, most notably Anacamptis pyramidalis: Darwin, 1877; Lind and Linderborg, 1989).
Despite this potential reproductive synapomorphy apparently uniting the two species, their respective geographical distributions are both highly restricted and strongly disjunct (‘Chondradenia’ fauriei occurs in Honshu Island of Japan and ‘C.’ doyonensis in Yunnan, southern-most China: Wood, 2001), suggesting that these two rare species could have arisen independently. We speculate that these species originated from within Galearis and acquired similar paedomorphic morphologies that fitted them for montane specialization, a hypothesis readily tested via molecular phylogenetics. They may be analogous to the former genus Aceratorchis in showing floral paedomorphosis, though the effects have been less radical in ‘Chondradenia’.
Neolindleya
The east Asian genus Neolindleya Kraenzlin (1899) (a genus that, to our surprise, was not recognized in Genera Orchidacearum 2: Pridgeon et al., 2001) is most commonly attributed to Gymnadenia by southeast Asian authors and to Platanthera by Russian authors, but was shown by Bateman et al. (2003) on ITS evidence to be a member of the Pseudorchis–Galearis–Platanthera clade. In their tree it was placed immediately above the Galearis plus Amerorchis clade as sister to Platanthera, albeit without bootstrap support. Here, it proved equally parsimonious to once again place Neolindleya as sister to Platanthera or as sister to Galearis s.l. (including Amerorchis: Fig. 1).
Given this molecular ambiguity, Efimov et al. (2009) re-examined the morphology of Neolindleya in an attempt to arbitrate between the two competing molecular phylogenetic placements. Morphology strongly supports placement of Neolindleya as sister to Galearis–Amerorchis, which would permit recognition of a Neolindleya–Amerorchis–Galearis clade, readily distinguished by the derived condition of extremely reduced tubers (a condition otherwise unknown in subtribe Orchidinae) and its failure to produce nectar, in contrast with Pseudorchis and Platanthera (e.g. Box et al., 2008). Neolindleya shows greater morphological divergence from Galearis s.s. than does Amerorchis, including possessing larger numbers of expanded, crenulate-margined leaves and a labellum that is long and parallel-sided with a truncated central lobe (Bateman et al., 2003). Efimov et al. (2009) noted that the combination of friable massulae and reduction to a rudimentary state of the caudicles and bursicle renders Neolindleya facultatively autogamous. They also highlighted a previously reported chromosome number of 2X = 36–38 (Sokolovskaya, 1960), which contrasts with the figure of 2X = 42 that characterizes the remainder of the Platanthera clade.
This morphological and ecological divergence, combined with its uncertain phylogenetic placement, suggests that Neolindleya is best retained as a genus separate from Galearis s.l. (Efimov et al., 2009).
The Platanthera clade: section Limnorchis
In the analysis of Bateman et al. (2003), section Limnorchis was represented by only a single placeholder, P. (hyperborea) viridiflora. Here, the 11 sequences of Platanthera section Limnorchis generated by us or by Wallace (2002) (Appendix) show moderate divergence and constitute a well-supported monophyletic group. Indeed, the morphological distinction between section Limnorchis and section Platanthera was recently reinforced by Gamarra et al. (2008), who documented significantly different seed types in the two sections. Our 11 accessions supposedly represent five species, and the five accessions of P. dilatata (all subtly molecularly different) represent three varieties sensu Luer (1975) and Sheviak (2002). The samples are resolved into three molecularly distinct groups, based respectively on P. stricta, P. dilatata and P. sparsiflora (Fig. 1). Despite their similarity in morphology (noted by Luer, 1975), we originally hypothesized that the substantial divergence between the dilatata (originally referred to hyperborea) and sparsiflora groups reflected contrasting geographical foci; the former characterizes northwestern North America, whereas the latter, which appeared relatively derived, is concentrated in the uplands of Mexico and southwestern North America. However, the subsequent inclusion in our analysis of two accessions of P. aquilonis, a species widespread in temperate North America (Sheviak, 1999), may challenge this interpretation. The accession assigned to P. aquilonis by L. Wallace was placed firmly in the southerly sparsiflora group, whereas the Alaskan accession tentatively assigned to P. aquilonis by R. Lauri was placed within the northerly P. dilatata group (Fig. 1). Further molecular and morphological study of this species group is therefore required.
The three accessions of the sparsiflora group analysed show moderate levels of both sequence divergence (Fig. 1) and morphological divergence (Sheviak, 2002). If correctly identified, the Sedona population of P. limosa found by us represents a small northward extension of the dominantly Mexican range given by Luer (1975). Morphologically, it is differentiated from the similarly but more widely distributed P. sparsiflora by its smaller flowers, narrower gynostemium and a labellum bearing a comparatively long spur and a small basal callus convergent with that commonly found in section Tulotis (Luer, 1975; Hapeman and Inoue, 1997; Sheviak, 2002). Platanthera aquilonis is facultatively autogamous (Wallace, 2006) and has a dorsiventrally compressed gynostemium; previous morphological comparisons with P. hyperborea s.s. are contradicted by the placement of Wallace's sample but encouraged by the placement of Lauri's sample. Wallace (2003) observed approximately equal separation of P. sparsiflora, P. aquilonis, P. dilatata and P. stricta in a population-level analysis that combined inter simple sequence repeats (ISSRs) and randomly amplified polymorphic DNAs (RAPDs).
Because of the exceptional taxonomic instability surrounding the delimitation of P. hyperborea and its putative allies (here represented by P. viridiflora and P. dilatata s.l.: cf. Luer, 1975; Wood and Neiland, 2001c; Sheviak, 2002), we have chosen to refer to the sister of the sparsiflora group as the dilatata group (Figs 1 and 2). Bona fide species of the dilatata group have distributions that stretch from the Aleutians in the north west southwards to California and eastwards through Canada and northeastern USA to Greenland, whereas P. hyperborea stretches to Iceland (Luer, 1975; Sheviak, 2002). Luer (1975) awarded viridiflora only varietal status under P. hyperborea, arguing that viridiflora was merely a more robust form of P. hyperborea with a longer labellum and a longer spur (greater than, as opposed to less than, 6 mm). However, Hapeman and Inoue (1997) revealed considerable ITS divergence between P. hyperborea and P. viridiflora, which did not prove to be sisters, thus strongly suggesting that viridiflora merits species-level distinction. It includes the western-most populations commonly attributed to the P. hyperborea group, occurring in Alaska, the Aleutians and Kamchatka (Luer, 1975; Sheviak, 2002). Supposed outlying populations of P. viridiflora in Hawaii have since been referred to P. holochila; they appear to be relatively distantly related to P. viridiflora within section Limnorchis (R. Lauri, unpubl. res.).
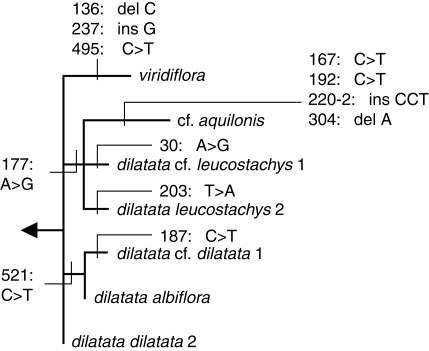
Fig. 2.
Detail of topology for the Platanthera dilatata group, highlighting the position and nature of the small number of base pair changes that separate seven closely similar ITS alleles that differ by a maximum of eight character states (cf. Fig. 1).
Certainly, the substantial divergence of P. stricta from the P. dilatata group challenges Sheviak's (2002) synonymization of P. viridiflora into P. stricta. Considerable molecular divergence between these species was also reported by Hapeman and Inoue (1997), though admittedly in our analysis it costs only one additional step to place P. stricta as sister to the P. dilatata group, despite the seven-step length of the branch separating P. stricta from the P. dilatata group (Fig. 1). The distribution of P. stricta coincides with those of P. dilatata and P. hyperborea, stretching from the Aleutians to northern California and Nevada. Morphologically similar to P. dilatata and P. hyperborea, it is best distinguished from them by its short, scrotiform spur (though the specimen analysed by us was the relatively slender-spurred ‘gracilis’ morph).
Platanthera dilatata is shown as being only subtly molecularly distinct from P. viridiflora (Fig. 2), separated by a single base pair change plus two single base pair indels. The group was considered by both Luer (1975) and Sheviak (2002) to contain three varieties, differentiated by contrasts in the amount of green pigment in their flowers, overall flower size and the relative dimensions of the floral organs, notably the spur and the gynostemium. Sheviak (2002) speculated that these varieties show a significant degree of pollinator specialization. Platanthera dilatata var. dilatata is the most widespread taxon, stretching from Siberia across North America and southward to California and Utah, whereas var. leucostachys occurs only in the western half of the distribution of the nominate race, and var. albiflora has an even more restricted distribution across the Pacific northwest. The group was represented only by var. leucostachys in the analysis of Hapeman and Inoue (1997), but here it is represented by five accessions; of these, two (both of which were tentative identifications at the varietal level) were collected by us and three were downloaded from GenBank as randomly selected exemplars of 12 sequences deposited by Wallace (2002). No two of these accessions were separated by more than 4 bp differences (i.e. levels of divergence similar to that separating P. viridiflora from P. dilatata s.l.), but equally no two accessions were identical; all exhibited at least one sequence difference (Fig. 2). The modest phylogenetic structure revealed links together the two samples of var. leucostachys but not the two samples of var. dilatata s.s., one of which clusters with var. albiflora whereas the other appears to have the potentially ancestral ITS allele (Fig. 2). Both the molecular and morphological data suggest considerable complexity of relationships, consistent with the hybridization and allopolyploidization events recently reported in the group (e.g. Wallace, 2002, 2003, 2006). Understanding of this taxonomic section has improved greatly between the treatments of Luer (1975), Hapeman and Inoue (1997) and Sheviak (2002), a trend that will probably continue during the next decade (R. Lauri, unpubl. res.; L. Wallace, unpubl. res.).
Sections Lacera and Tulotis
Members of the distinctive section Lacera, recognized only relatively recently by Hapeman and Inoue (1997), have a fringed/toothed, three-lobed labellum and, in many cases, brightly coloured flowers (the first and third features are convergent with species in the well supported section Blephariglottis: Hapeman and Inoue, 1997). Our analysis included only three species of section Lacera. At this level of species sampling, the tree strongly supports P. grandiflora (northern Appalachians) as sister to P. peramoena (mid- to southern Appalachians) plus P. praeclara (US Midwest), a topology congruent with that obtained by Hapeman and Inoue (1997). Above section Lacera we have only the Oriental P. sonoharai as the placeholder for the widespread section Tulotis (cf. Hapeman and Inoue, 1997).
Sections Lysiella and Lysias
As discussed at the beginning of this section, we maintain reservations regarding the reliability of the sister relationships shown in Fig. 1 of section Lysiella and section Lysias to each other, and when combined as sister to section Piperia. Certainly, this clade would encompass an exceptionally broad range of morphologies, though it would permit the likely origin of section Piperia within North America. Support for both the clade consisting of sections Lysiella, Lysias and Piperia and the sister clade of section Platanthera is sufficiently weak that inclusion of any of the former sections in the latter cannot confidently be refuted.
Section Piperia
Piperia was previously widely recognized as a separate genus in North America (Luer, 1975; Wood and Neiland, 2001c; Ackerman and Morgan, 2002). However, although the ITS study of Bateman et al. (2003) clearly demonstrated that it was strongly supported as monophyletic, it showed equally convincingly that the clade was nested well within the genus Platanthera (see also Lauri, 2007). Piperia was therefore relegated to a section of the genus Platanthera.
The molecular branches subtending section Piperia and the clade that it forms with sections Lysiella and Lysias are no longer than those subtending other sections of Platanthera that were previously frequently recognized as separate genera (Limnorchis, Lacera and Tulotis). However, incorporation of the genus Piperia into the genus Platanthera has met greater resistance from orchid taxonomists, primarily because Piperia is delimited by morphological synapomorphies (notably short, inconspicuous caudicles and globose rather than fusiform tubers: cf. Luer, 1975; Ackermann, 1977, 2002; Wood and Neiland, 2001c; Ackerman and Morgan, 2002; Bateman et al., 2003), whereas the genus Platanthera, as presently delimited, is not. However, we note that inconspicuous caudicles are a feature of several other genera in subtribe Orchidinae, and globose tubers are the plesiomorphic condition in the subtribe. Rather, it is the fusiform tubers of Pseudorchis and Platanthera, and their radical reduction in the Galearis clade, that constitute the most convincing synapomorphies (Box et al., 2008; Efimov et al., 2009). The globose tubers of section Piperia are ostensibly a reversion to the plesiomorphic condition within Orchidinae, though we suspect that careful ontogenetic studies would reveal developmental differences between the globose tubers in section Piperia vs. those of more distant genera such as Orchis s.s. We also note that field recognition (a critical element of any successful classification) is dependent primarily on finding reliable differences between species in one or more character states; for identification purposes, it is unimportant whether these constitute synapomorphies.
Levels of sequence divergence between the three Piperia accessions included in Fig. 1 are consistent with their status as separate species. The history of species recognition in the section post-Rydberg (1901a, b) was summarized by Bateman et al. (2003, p. 21). However, the taxonomic revision given by Bateman in Table 4 of Bateman et al. (2003) requires further revision, as he failed to consider the possibility of accidentally creating orthographic synonyms when combining species and subspecies of ‘Piperia’ into Platanthera (J. Zarucchi, pers. comm., 2003). These errors are here corrected in Table 1b. A current in-depth study by Lauri (2007) should provide a greatly improved understanding of the origin and evolution of section Piperia, as well as a much-needed test of previous hypotheses that the ten constituent species of the group originated relatively recently (Ackerman, 1977).
Section Platanthera
Section Platanthera was well sampled by Hapeman and Inoue (1997), who tentatively found the former sections Lysiella (epitomized by the circumboreal combination of P. obtusata and P. oligantha) and Lysias (epitomized by P. orbiculata) to be nested within section Platanthera. The consequently expanded section Platanthera was represented in their study by 15 species, but resolution among those species was sufficiently poor that, of the six groups that could be recognized, none had bootstrap support exceeding 50 % or a decay index >1. In addition, this study wholly lacked representatives of Piperia and Diphylax. Both former genera have since been shown to be nested within section Platanthera as broadly delimited by Hapeman and Inoue (1997) (Fig. 1), which can now be seen to constitute the largest and least resolved section within the genus.
The present analysis agrees with that of Hapeman and Inoue (1997) in suggesting that monophyly of section Platanthera is at best poorly supported, and that there is considerable molecular divergence among exclusively Asiatic species of the section (i.e. P. mandarinorum, P. florentia and P. bakeriana: cf. Inoue, 1983). The topology mirrors that of Bateman et al. (2003) in nesting (albeit with negligible bootstrap support) the Eurasian and European Platanthera bifolia aggregate within three Asian species of section Platanthera that are molecularly distinct but show only poorly resolved relationships. Moreover, the relationship of P. mandarinorum as sister to the group that extends from P. florentia to the P. bifolia group matches its placement in the topology of Hapeman and Inoue (1997), where P. hachijoensis and P. ophrydioides substitute for P. mandarinorum and P. hookeri (North American) substitutes for P. florentia.
Tentatively placed as sister to P. mandarinorum is P. chorisiana, which ranges from North Japan across the Aleutian islands to the Pacific Northwest of North America. This small boreal species produces a secund inflorescence of tiny, near-cleistogamous flowers that approach radial symmetry with a poorly developed labellar spur and gynostemium (e.g. Luer, 1975). It is therefore understandable that this species was assigned to Limnorchis by Anderson (1945), though this hypothesis is refuted by the ITS phylogeny, which is more consistent with the earlier decision of Nevski and Komarov in Komarov (1935) to separate this species as the novel genus, Pseudodiphryllum. Lastly, Fig. 1 places the Japanese P. florentia with moderate support as sister to the Sino-Himalayan P. bakeriana plus the Himalayan species of the former genus Diphylax.
The former genus Diphylax and its relatives
Diphylax was established as a genus by the younger Hooker (1889) but the taxon was soon downgraded by him to a section of Habenaria (Hooker, 1890). As summarized by Cribb (2001), Diphylax is a genus of three or four species and is confined to high elevations in the eastern Himalayas and southeastern China (Tang and Wang, 1940; Tang et al., 1989, 1994). It resembles Platanthera closely in its tuber and vegetative characters, but its flowers are relatively small, compact and held in a secund inflorescence. The perianth approaches radial symmetry, showing only poor differentiation in shape and size between the lanceolate sepals, lateral petals and labellum. The labellar spur is short and strikingly saccate. The column is bilaterally compressed, bearing distinct erect, cornute stigmatic lobes, elongate staminodes and a poorly developed rostellum. The pollinia have exceptionally short caudicles that contrast with the relatively large viscidium.
Cribb (2001) also advocated synonymization of the supposed monotypic genus Tsaiorchis neottianthoides Tang & Wang (1936) into Diphylax, convincingly arguing that the elongate, bifid, canaliculate rostellum of ‘Tsaiorchis’ is a relatively minor modification of the condition previously described in Diphylax by Hooker (1890). These new nomenclatural combinations are here enacted in Table 1c.
The Platanthera bifolia group
The P. bifolia aggregate was represented by only a single accession of P. bifolia in the study of Hapeman and Inoue (1997) and by single accessions each of P. bifolia and P. chlorantha in Bateman et al. (1997), later supplemented with a sample of an eastern Mediterranean segregate of P. chlorantha, P. cf. homboei (Bateman et al., 2003). Their study revealed only a single base pair separating P. bifolia from P. chlorantha plus P. holmboei, a level of divergence in ITS lower than that reported for any other putative species of Platanthera in any previous study.
The present study reports ITS data for 51 accessions of the P. bifolia aggregate, together representing seven putative species (including five of the six species of section Platanthera reputedly occurring in Europe plus Macaronesia: cf. Webb, 1980; Delforge, 2006). The aggregate is well supported as monophyletic in the ITS tree (Fig. 1). However, even given this greatly expanded sampling, levels of sequence divergence detected were exceptionally low, reaching a maximum in the 5 bp (0·7 % sequence divergence) that separated two Chinese accessions attributed to P. chlorantha from one of two sequences obtained from the Azorean endemic P. azorica (Bateman et al., 2009). The only apparent phylogenetic structures detected within the P. bifolia group were two single-step branches: (1) a C > T transition at position 172 separated from the Azorean endemics P. micrantha and P. azorica, plus the sequence from the accession of P. holmboei from Cyprus, and (2) another C > T transition at position 629 that separated both accessions of P. azorica from all four accessions of P. micrantha (Fig. 1). Both branches attracted predictably low statistical support.
Overall, our 51 accessions of seven putative species yielded just seven ITS alleles. Three of these seven alleles (including the two most common) were found in more than one putative species, and the apparently plesiomorphic allele was found in no less than five of the seven putative species. Moreover, several individual accessions of P. bifolia and P. chlorantha were each observed to maintain two alleles (Bateman et al., 2009).
Morphologically, the P. bifolia group shows levels of variation similar to those of most other sectional and subsectional groups within the genus Platanthera, at least in features such as overall flower size, labellum dimensions and pollinium presentation (Bateman et al., 2009). One notable feature of the group is the unusually strong differentiation evident between the one or more often two expanded basal leaves and the bract-like leaves that develop closer to the inflorescence. This feature is less noticeable in the Azorean species P. micrantha s.l. and P. azorica (e.g. Delforge, 2006), which have small, compact, green, relatively short-spurred flowers that are superficially more reminiscent of section Limnorchis than of section Platanthera. However, our data tentatively suggest that the species diverged recently, from within the P. bifolia group. Geographical proximity suggests that the most likely ancestor is P. algeriensis, a putative derivative of P. chlorantha endemic to northwestern Saharan Africa that we have not yet analysed molecularly.
Clearly, there is a strong discrepancy between the relative degrees of morphological and molecular divergence observed among species of the P. bifolia group. There are three possible explanations for this discrepancy: (1) they are not (yet) bona fide species; (2) they are bona fide species but evolved very recently; or (3) they are older species that have existed for a significant period of time but are still given collective molecular cohesion by ongoing gene flow through hybridization. These critical issues are explored at greater depth by Bateman et al. (2009).
Evolutionary trends in the Platanthera clade
In their benchmark study of the genus, Hapeman and Inoue (1997) mapped across their fairly well sampled tree of Platanthera species flower colour, dominant pollinator type and typical placement of pollinia on those pollinating insects, reporting substantial homoplasy in each of those character suites. Understandably, they emphasized floral convergence toward specific pollinators as the predominant speciation mechanism within the genus, noting that it ‘has apparently undergone a tremendous radiation [that] represents nearly all of the non-deceptive pollination syndromes found in the Orchidaceae’ (p. 436).
However, setting aside the spectacular colours and fringed labella of some North American species of sections Blephariglottis and Lacera (which are strongly positively correlated with preferred pollinators that possess colour vision), it is perhaps more remarkable how the genus has achieved those shifts of dominant pollinator using such a modest repertoire of floral changes. In the great majority of species, flower colour varies from green to ‘white’ (strictly, translucent), presumably depending on the concentration of chlorophyll in the flowers. Flower size varies considerably across the genus. Within the flower, the relative dimensions (and occasionally the postures) of the perianth segments and the shape of the gynostemium differ somewhat, but these are simple allometric shifts (Bateman and Sexton, 2008) that could readily be mapped and interpreted on a Thompson grid (Bateman et al., 2009). Thus, perhaps the most striking feature of the taxonomic diversification in the genus is that it required such a limited repertoire of morphological innovations.
We agree with Hapeman and Inoue (1997) that pre-adaptation has most probably played a significant role in the evolution of Platanthera, but we also infer a major role for heterochrony, underpinning the relatively simple changes of relative shape and sizes of the organs within the flowers (Rudall and Bateman, 2002, 2004; Bateman and Rudall, 2006; Bateman et al., 2009). Paedomorphic reduction of the perianth is evident in the former genera Aceratorchis, Chondradenia, Piperia and Diphylax, in which it is often accompanied by reduction in the relative size and complexity of the gynostemium. Overall, there are obvious similarities in the independent origins of broadly similar morphologies that led to the establishment of these four former genera, all apparently originating in montane areas (above 3000 m in the case of the three Southeast Asian groups). The trend is also evident in species more commonly assigned to the genus Platanthera in boreal species such as P. chorisiana. In addition, alteration between paedomorphosis and peramorphosis would explain the evolutionary lability of spur length evident in the group. The green flowers of many species of Platanthera could also be ascribed to retention of juvenile features (i.e. production of chlorophyll) in the mature flower.
Among the putatively adaptive floral characters, variations in spur length, caudicle length and viscidial presentation have attracted greatest attention from evolutionary biologists. This emphasis is predicated on the assumption that these features are under the strongest selection pressure, since they are the characters that, together with scent, are considered most likely to determine (or perhaps be determined by) pollinator preference (Luer, 1975; Nilsson, 1983, 1985; Catling and Catling, 1991; Hapeman and Inoue, 1997; Ackerman and Morgan, 2002; Sheviak, 2002). Work is underway to explore the comparative genetics of spur development in Orchidinae (Box et al., 2008; unpubl. res.), but studies of gynostemium development are also highly desirable, particularly if the key genes underpinning the allometric shifts in viscidial placement can be identified. Similarly, the apparent reversions to plesiomorphic ovoid tubers in the former genera Diphylax and Piperia merit evolutionary–developmental examination, to determine whether they are homologous at the genetic level with those in genera such as Orchis s.s.
Compared with some other genera of Orchidinae (e.g. Dactylorhiza: Pillon et al., 2007), chromosomal changes appear to have played a limited role in the diversification of the Platanthera clade. Although Brandham (1999) listed a wide range of chromosome counts in Platanthera, Pridgeon et al. (1997) critically appraised those reports and found that, for the range of species sequenced by them, all of the reliable reports gave only the plesiomorphic condition for Orchidinae, 2n = 42, across the Platanthera clade. To the best of our knowledge, only one relevant count has been made in the subsequent decade, specifically 2n = 42 for Galearis diantha (Luo, 2004). The notable exception to this chromosomal consistency is Platanthera section Limnorchis, where polyploidy apparently occurs frequently. Webb (1980) reported that the Icelandic P. hyperborea is tetraploid (2n = 84) and the Nordic–Siberian P. oligantha (= P. obtusata subsp. oligantha) is hexaploid (2n = 126). In North America, the origins of the allotetraploid P. huronensis (cf. Tanaka and Kanemoto, 1984; Sheviak and Bracht, 1998) have been traced to allopolyploidy events involving hybridization between the widespread, moderately closely or closely related diploid species P. dilatata and P. aquilonis (Fig. 1). At least two origins of P. huronensis with the opposite maternal and paternal parents have been inferred (Wallace, 2003, 2004). Although P. aquilonis is a facultative autogam, it continues to hybridize occasionally with P. dilatata (Wallace, 2006).
More broadly, reports of hybridization among Platanthera spp. are spread sporadically across the taxonomic sections, but the reports are uncommon and evidence is often equivocal. Co-evolutionists convinced of the effective pre-zygotic isolation of Platanthera spp. might argue that this pattern accurately reflects gene flow among the species. However, sceptics might merely argue that the relatively simple and conservative morphology of the flowers makes morphological identification of hybrids extremely challenging (Bateman and Denholm, 1983; Bateman and Sexton, 2008; Bateman et al., 2009). There have been no credible records of hybridization among members of the three genus-level groupings within the Platanthera clade s.l. (i.e. the Pseudorchis, Galearis and Platanthera clades in Fig. 1). This suggests that the levels of genetic disparity render such crosses inviable, reinforcing the validity of the recircumscribed genera.
We believe that understanding of the phylogeny of the Platanthera clade has reached a critical threshold. As noted by Hapeman and Inoue (1997), the genus Platanthera alone has the potential to provide an exceptional case study in evolutionary diversification and biogeographic expansion. It combines a considerable degree of floral diversification (traditionally ascribed to plant–pollinator co-evolution: Darwin, 1877; Catling and Catling, 1991; Wood and Neiland, 2001c) with lesser degrees of vegetative diversification and considerable degrees of sequence divergence with lesser degrees of chromosomal change. Of equal potential value is comparison with the two other groups within the Platanthera clade, namely Galearis–Neolindleya and Pseudorchis. The three groups are readily distinguished by floral characters and, in the case of Galearis–Neolindleya, by vegetative characters. Moreover, they provide valuable contrasts in the reward given to pollinators, which has been much discussed in the recent literature (cf. van der Cingel, 1995; Neiland and Wilcock, 1998; Cozzolino and Widmer, 2005; Tremblay et al., 2005). Specifically, substantial nectar is offered by Platanthera and limited nectar by Pseudorchis and at least one Chinese species of Galearis (Yu and Luo, unpubl. res.), but no nectar is provided by the remainder of the food-deceptive Galearis–Neolindleya group.
In addition, all three major clades have circumpolar distributions, a pattern broken only by the absence of Galearis s.l. from Europe. This makes it especially difficult to infer the geographic origin of the entire Pseudorchis–Neolindleya–Galaearis–Platanthera clade. Nonetheless, Platanthera and Galearis–Neolindleya constitute an excellent biogeographic comparison, given that both clades appear to have originated and diversified at the genus level in East Asia. Neolindleya remained endemic to that region (Efimov et al., 2009). Galearis reached North America (or could have originated there: Fig. 1), but it failed to undergo substantial speciation on that continent. In contrast, the present tree and that of Hapeman and Inoue (1997) both imply that Platanthera reached North America on multiple occasions, with at least some of the lineages subsequently speciating extensively. There is also equivocal evidence that reverse migrations toward East Asia occurred in Platanthera sections Limnorchis and Platanthera. Only one lineage within Platanthera section Platanthera, the P. bifolia group, spread westwards from Asia into Europe, apparently showing modest subsequent morphological divergence but remarkably little detectable molecular diversification (Bateman et al., 2009).
Further substantial progress in understanding evolution and migration in the group via phylogenetic reconstruction will require molecular analysis of all extant species and sequencing of additional nuclear and plastid regions, in the hope of clarifying the complex morphological and biogeographical patterns and improving the statistical rigour of critical nodes, particularly those separating the major species groups. Our incorporation of Aceratorchis and Chondradenia into Galearis and of Piperia, Diphylax and Tsaiorchis into Platanthera (admittedly without molecular support in the cases of the monotypic genera Aceratorchis and Tsaiorchis, and of the potentially monotypic Chondradenia) demonstrates that species comparison should not be confined to pre-delimited genera, most notably by illustrating the relative frequency with which taxa can shift from expected positions in the outgroup to better supported positions in the ingroup.
The relationships among the three major groups within the Platanthera clade, and among the eight or more major groups within the genus Platanthera itself (Fig. 1), are crucial to interpreting both character change and biogeography. Most notably, Hapeman and Inoue (1997) found the earliest divergent group within Platanthera to be the wholly Asian section Tulotis, whereas we found it to be the circumpolar section Limnorchis (as did R. Lauri, unpubl. res.). These contrasting placements lead to substantially different polarities of both character change and geographical migration. Unfortunately, at present, neither hypothesis of relationship is well supported. A collaborative effort is underway to remedy this situation.
Opportunities now exist to explore simultaneously the morphometrics, population genetics, reproductive biology and autecology of narrowly defined species groups within the Platanthera clade. Recent studies combining two or more of these approaches have shown much promise (cf. Bateman, 2001; Wallace, 2002, 2003, 2004, 2006; Holzinger and Wallace, 2004; Little et al., 2005; Lauri, 2007; Bateman et al., 2009). They also suggest that most members of the group have not been studied in sufficient detail, and hence would benefit from rigorous monographic treatment.
ACKNOWLEDGEMENTS
We thank for provision of samples M. Carine, M. Fischer, M. Hedrén, H. Lambert, R. Manuel, M. Moura, D. Nickrent, J. Tyler and J. Vogel, and Lisa Wallace for uploading several unpublished DNA sequences into GenBank. We also thank Lavinia Robba for laboratory assistance during the latter stages of the sequencing project. James Zarucchi kindly drew the attention of R.M.B. to his earlier nomenclatural errors made when combining Piperia species into Platanthera.
APPENDIX: SAMPLES YIELDING ITS SEQUENCES
Sequence sources from previous publications: 1 = Hapeman and Inoue (1997); 2 = Pridgeon et al. (1997; also Bateman et al., 1997); 3 = Bateman et al. (2003); 4 = Szalanski et al. (2001). * = incorrectly named as P. hyberborea viridis by Bateman et al. (2003). Collection numbers: EF and AF, GenBank; K, RBG Kew database; B, Bateman collection; L, Lauri collection. Parentheses indicate sequences that are identical to those generated by other listed accessions and so were excluded from parsimony analysis.
| Number | Pub | Taxon | Locality | Collector(s) |
|---|---|---|---|---|
| Related genera (7) | ||||
| Ksn | 3 | Pseudorchis straminea | ?Sweden | Liden |
| B63 | 2, 3 | Pseudorchis albida | Balvattan Hill, SE Aviemore, Rothiemurchus, C Scotland | R. Bateman |
| B578 | 3 | Neolindleya camtschatica | Ullung Island, Korea | Y. N. Lee |
| K850 | 3 | Galearis (Amerorchis) rotundifolia | Palmerston North, Lanark, Ottawa, Canada | ? |
| Ksn | 2, 3 | Galearis spectabilis | USA | Davis |
| Ksn | 2, 3 | Galearis cyclochila | ?Japan | K. Inoue |
| Ksn | 3 | Galearis diantha | China | Y. B. Luo |
| North America (18) | ||||
| B1559 | Platanthera stricta ‘gracilis’ [Limnorchis] | Mt Lassen, E Redding, N California, USA | R. Bateman | |
| B1558 | Platanthera dilatata cf. leucostachys 1 [Limnorchis] | N Mt Lassen, E Redding, N California, USA | R. Bateman | |
| EF025518 | Platanthera dilatata leucostachys 2 [Limnorchis] | USA? | L. Wallace | |
| B1092 | Platanthera dilatata dilatata 1 [Limnorchis] | Lr Supreme Trail, E Alta, Snowbird, NE Utah, USA | R. Bateman | |
| EF025524 | Platanthera dilatata dilatata 2 [Limnorchis] | USA? | L. Wallace | |
| EF025521 | Platanthera dilatata albiflora [Limnorchis] | USA? | L. Wallace | |
| EF025530 | Platanthera aquilonis [Limnorchis] | USA? | L. Wallace | |
| Lsn | Platanthera cf. aquilonis [Limnorchis] | Alaska, USA | R. Lauri | |
| B486 | Platanthera cf. limosa [Limnorchis] | W Fork Trail, Oak Creek Canyon, Sedona, Arizona, USA | R. Bateman | |
| B1091 | Platanthera sparsifolia s.s. [Limnorchis] | Mossy Cave Trail, NE Ruby's Inn, Bryce, S Utah, USA | R. Bateman | |
| Ksn | 1, 2, 3 | Platanthera grandiflora [Lacera] | USA | V. Albert |
| K9161 | Platanthera peramoena [Lacera] | USA | D. Nickrent | |
| AF301445 | 4 | Platanthera praeclara [Lacera] | USA? | ? |
| Lsn | Platanthera orbiculata [Lysias] | USA | R. Lauri | |
| K930 | 3 | Platanthera (Piperia) unalascensis | California, USA | J. Hapeman |
| Bsn | 3 | Platanthera (Piperia) colemanii | California, USA | W. Temple |
| Bsn | 3 | Platanthera (Piperia) elongata | California, USA | W. Temple |
| Lsn | Platanthera chorisiana | USA? | R. Lauri | |
| Southeast Asia (10 > 8) | ||||
| Ksn | 1, 2, 3 | Platanthera (hyperborea) viridiflora* [Limnorchis] | ?Japan | K. Inoue |
| Ksn | 1, 2, 3 | Platanthera sonoharai [Tulotis] | ?Japan | K. Inoue |
| K13087 | Platanthera mandarinorum | China | Y. B. Luo | |
| Ksn | 1, 2, 3 | Platanthera florentia | ?Japan | K. Inoue |
| K8048 | Platanthera bakeriana | China | Y. B. Luo | |
| Ksn | Platanthera (Diphylax) urceolata | China | Y. B. Luo | |
| Ksn | Platanthera (Diphylax) sp. | China | Y. B. Luo | |
| (K13038) | Platanthera finetiana | China | Y. B. Luo | |
| (K13073) | Platanthera metabifolia | China | Y. B. Luo | |
| K13071 | Platanthera chlorantha | China | Y. B. Luo | |
| Europe (8 > 7) | ||||
| H3054 | Platanthera oligantha [Lysiella] | N Norway | M. Hedrén | |
| B787 | Platanthera chlorantha | Homefield Wood, Medmenham, Bucks, UK | R. Bateman | |
| B1033 | Platanthera bifolia | Morgans Hill, SE Calne, Wilts, UK | R. Bateman | |
| (B1398) | Platanthera holmboei | Copse N crossroads, Mandria, Troodos, C Cyprus | R. Bateman | |
| B2193 | Platanthera micrantha 1 | Lagoa di Canario, San Miguel, Azores | M. Moura | |
| B2194 | Platanthera micrantha 2 | Lagoa di Canario, San Miguel, Azores | M. Moura | |
| B2195 | Platanthera azorica 1 | Lagoa di Fogo, San Miguel, Azores | M. Moura | |
| B2196 | Platanthera azorica 2 | Lagoa di Fogo, San Miguel, Azores | M. Moura | |
LITERATURE CITED
- Ackerman JD. Biosystematics of the genus Piperia Rydb. (Orchidaceae) Botanical Journal of the the Linnean Society. 1977;75:245–270. [Google Scholar]
- Ackermann JD, Morgan R. Flora of North America North of Mexico 26. Oxford: Oxford University Press; 2002. Piperia Rydberg; pp. 571–577. Flora of North America Editorial Committee. eds. [Google Scholar]
- Anderson JP. Iowa State College Journal of Science. 1945;19:187. [Platanthera chorisiana] [Google Scholar]
- Baldwin BG, Sanderson MJ, Porter JM, Wojciechowski MF, Campbell CS, Donoghue MJ. The ITS region of nuclear ribosomal DNA: a valuable source of evidence on angiosperm phylogeny. Annals of the Missouri Botanical Garden. 1995;82:247–277. [Google Scholar]
- Bateman RM. Evolution and classification of European orchids: insights from molecular and morphological characters. Journal Europäischer Orchideen. 2001;33:33–119. [Google Scholar]
- Bateman RM. Circumscribing and interpreting closely related orchid species: Platanthera, Dactylorhiza and the crucial role of mutation. Journal of the Hardy Orchid Society. 2005;2:104–111. [Google Scholar]
- Bateman RM, Denholm I. A reappraisal of the British and Irish dactylorchids, 1. The tetraploid marsh-orchids. Watsonia. 1983;14:347–376. [Google Scholar]
- Bateman RM, Rudall PJ. The good, the bad, and the ugly: using naturally occurring terata to distinguish the possible from the impossible in orchid floral evolution. Aliso. 2006;22:481–496. [Google Scholar]
- Bateman RM, Sexton R. Is spur length of Platanthera species in the British Isles adaptively optimized or an evolutionary red herring? Watsonia. 2008;28:1–21. [Google Scholar]
- Bateman RM, Pridgeon AM, Chase MW. Phylogenetics of subtribe Orchidinae (Orchidoideae, Orchidaceae) based on nuclear ITS sequences. 2. Infrageneric relationships and taxonomic revision to achieve monophyly of Orchis sensu stricto. Lindleyana. 1997;12:113–141. [Google Scholar]
- Bateman RM, Hollingsworth PM, Preston J, Luo Y-B, Pridgeon AM, Chase MW. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Botanical Journal of the Linnean Society. 2003;142:1–40. [Google Scholar]
- Bateman RM, Rudall PJ, James KE. Phylogenetic context, generic affinities and evolutionary origin of the enigmatic Balkan orchid Gymnadenia frivaldii Hampe ex Griseb. Taxon. 2006;55:107–118. [Google Scholar]
- Bateman RM, James KE, Rudall PJ. Contrast in levels of morphological versus molecular divergence between two closely related Eurasian species of Platanthera (Orchidaceae) suggests recent evolution with a strong allometric component. Annals of Botany. 2009 in press. [Google Scholar]
- Box MS, Bateman RM, Glover BJ, Rudall PJ. Floral ontogenetic evidence of repeated speciation via paedomorphosis in subtribe Orchidinae (Orchidaceae) Botanical Journal of the Linnean Society. 2008;157:429–454. [Google Scholar]
- Brandham P. Cytogenetics. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum. 1. General introduction, Apostasioideae, Cypripedioideae. Oxford: Oxford University Press; 1999. pp. 67–80. [Google Scholar]
- Brieger FG, Maatsch R, Senghas K. 42. Chondradenia. In: Brieger FG, Maatsch R, Senghas K, editors. Rudolf Schlechter: Die Orchideen 3. Vol. 4. Berlin: Paul Parey Verlag; 1973. p. 239. [Google Scholar]
- Catling PM, Catling VR. A synopsis of breeding systems and pollination in North American orchids. Lindleyana. 1991;6:187–210. [Google Scholar]
- Chen S-C. The origin and early differentiation of the Orchidaceae. Acta Phytotaxonomica Sinica. 1982;20:1–22. [Google Scholar]
- Chen S-C, Tsi Z-H. Eria medogensis, a probably peloric form of Eria coronaria, with a discussion on peloria in Orchidaceae. Acta Phytotaxonomica Sinica. 1987;25:329–339. [Google Scholar]
- Chen S-C, Tsi Z-H, Luo Y-B. Native orchids of China in colour. Beijing: Science Press; 1999. [Google Scholar]
- Chen S-C, Cribb PJ, Gale S. In: Flora of China 25: Burmanniaceae–Orchidaceae. Wu Z-Y, Raven PH, Hong D-Y, editors. Beijing: Science Press; 2009. Galearis in press. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends in Ecology and Evolution. 2005;20:487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cribb PJ. In: Genera Orchidacearum 2: Orchidoideae 1. Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Oxford: Oxford University Press; 2001. pp. 264–266. Diphylax. [Google Scholar]
- Cribb PJ, Wood JJ. In: Genera Orchidacearum 2: Orchidoideae 1. Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Oxford: Oxford University Press; 2001. pp. 244–245. Aceratorchis. [Google Scholar]
- Darwin C. The various contrivances by which orchids are fertilised by insects. 2nd edn. London: Murray; 1877. [Google Scholar]
- Delforge P. Orchids of Europe, North Africa and the Middle East. London: A. & C. Black; 2006. [Google Scholar]
- Doyle J, Doyle J. A rapid total DNA preparation procedure for fresh plant tissue. Focus. 1990;12:13–15. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland: Timber Press; 1993. [Google Scholar]
- Efimov PG, Lauri RK, Bateman RM. Neolindleya Kraenzl., an enigmatic and largely overlooked autogamous orchid from temperate East Asia. Kew Bulletin. 2009;64 in press. [Google Scholar]
- Finet A. Orchideés nouvelles ou peu connues. Journal de Botanique. 1898;12:340–344. [Google Scholar]
- Gamarra R, Golán P, Herrera I, Ortúnez E. Seed micromorphology supports the splitting of Limnorchis from Platanthera. Nordic Journal of Botany. 2008;26:61–65. [Google Scholar]
- Handel-Mazzetti H. Symbolae Sinicae: botanische Ergebnisse der Expedition der Akademie der Wissenschaftten in Wien nach südwest-China, 1914–1918. Vol. 7. Anthophyta; 1936. p. 1324. [Google Scholar]
- Hapeman JR, Inoue K. Plant–pollinator interactions and floral radiation in Platanthera (Orchidaceae) In: Givnish TJ, Sytsma KJ, editors. Molecular evolution and adaptive radiation. Cambridge: Cambridge University Press; 1997. pp. 433–454. [Google Scholar]
- Hershkovitz MA, Zimmer EA, Hahn WJ. Ribosomal DNA sequences and angiosperm systematics. In: Hollingsworth PM, Bateman RM, Gornall RJ, editors. Molecular systematics and plant evolution. London: Taylor & Francis; 1999. pp. 268–326. [Google Scholar]
- Holzinger KE, Wallace LE. Bayesian approaches for the analysis of population genetic structure: an example from Platanthera leucophaea (Orchidaceae) Molecular Ecology. 2004;13:887–894. doi: 10.1111/j.1365-294x.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- Hooker JD. Diphylax urceolata, Hook.f. Icones Plantarum. 1889;19 t1865. [Google Scholar]
- Hooker JD. The flora of British India 6: Orchideae. London: Reeve; 1890. [Google Scholar]
- Hultén E. Amerorchis nov. gen. Arkiv for Botanik Series 2. 1968;7:34–35. [Google Scholar]
- Hunt PF. Notes on Asiatic orchids: VI. Kew Bulletin. 1971;26:171–185. [Google Scholar]
- Inoue KI. Systematics of the genus Platanthera (Orchidaceae) in Japan and adjacent regions with special reference to pollination. Journal of the Faculty of Science, University of Tokyo, Section III (Botany) 1983;13:285–374. [Google Scholar]
- Komarov VL. Orchidaceae. Flora of the USSR. 1935;4:448–554. [Google Scholar]
- Kränzlin F. Orchidacearum genera et species. Berlin: Meyer & Müller; 1897–1904. [Google Scholar]
- Lang K-Y, Chen S-C, Luo Y-B, Zhu GH. Flora Reipublicae Popularis Sinicae 17. Orchidaceae 1. Beijing: Science Press; 1999. [Google Scholar]
- Lauri R. The systematic and phylogenetic study of the subgenus Piperia (Orchidaceae) and closely related Platanthera. Botanical Society of America Abstracts. 2007:241. [Google Scholar]
- Lind H, Lindeborg M. Lepidopterans as presumptive pollinators of Anacamptis pyramidalis. Entomologisk Tidsskrift. 1989;110:151–160. [Google Scholar]
- Little KJ, Dieringer G, Romano M. Pollination ecology, genetic diversity and selection on nectar spur length in Platanthera lacera (Orchidaceae) Plant Species Biology. 2005;20:183–190. [Google Scholar]
- Luer CA. The native orchids of the United States and Canada, excluding Florida. New York: New York Botanical Garden; 1975. [Google Scholar]
- Luo Y-B. Cytological studies on some representative species of the tribe Orchideae (Orchidaceae) from China. Botanical Journal of the Linnean Society. 2004;145:231–238. [Google Scholar]
- Luo Y-B, Chen S-C. The floral morphology and ontogeny of some Chinese representatives of orchid subtribe Orchidinae. Botanical Journal of the Linnean Society. 2000;134:529–548. [Google Scholar]
- Maad J, Nilsson LA. On the mechanism of floral shifts in speciation: gained pollination efficiency from tongue- to eye-attachment of pollinia in Platanthera (Orchidaceae) Biological Journal of the Linnean Society. 2004;83:481–495. [Google Scholar]
- Maekawa F. The wild orchids of Japan in colour. Tokyo: S & S; 1971. [Google Scholar]
- Makino T. Observations on the flora of Japan. Botanical Magazine (Tokyo) 1902;16:89. [Google Scholar]
- Maximowicz CJ. [Chondradenia] Catalogue of Species of the Herbarium of the Imperial University. 1886:287. [Google Scholar]
- Mondragón-Palomino M, Theissen G. MADS about the evolution of orchid flowers. Trends in Plant Science. 2008;13:51–59. doi: 10.1016/j.tplants.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Theissen G. Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. Annals of Botany. 2009;104:583–594. doi: 10.1093/aob/mcn258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiland MRM, Wilcock CC. Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany. 1998;85:1567–1581. [PubMed] [Google Scholar]
- Nilsson LA. Processes of isolation and introgressive interplay between Platanthera bifolia (L.) Rich. and P. chlorantha (Custer) Reichb. (Orchidaceae) Botanical Journal of the Linnean Society. 1983;87:325–350. [Google Scholar]
- Nilsson LA. Characteristics and distribution of intermediates between Platanthera bifolia and P. chlorantha (Orchidaceae) in the Nordic countries. Nordic Journal of Botany. 1985;5:407–419. [Google Scholar]
- Perner H, Luo Y-B. Orchids of Huanglong. Chengdu: Sichuan Art Press; 2007. [Google Scholar]
- Pillon Y, Fay MF, Hedrén M, et al. Evolution and biogeography of European species complexes in Dactylorhiza (Orchidaceae) Taxon. 2007;56 1185–1208 + E1–E17. [Google Scholar]
- Pridgeon AM, Bateman RM, Cox AV, Hapeman JR, Chase MC. Phylogenetics of subtribe Orchidinae (Orchidoideae, Orchidaceae) based on nuclear ITS sequences. 1. Intergeneric relationships and polyphyly of Orchis sensu lato. Lindleyana. 1997;12:89–109. [Google Scholar]
- Pridgeon AM, Cribb PJ, Rasmussen FN, Chase MC. Genera Orchidacearum 2: Orchidoideae 1. Oxford: Oxford University Press; 2001. [Google Scholar]
- Rafinesque CS. Herbarium Rafinesquianum. Philadelphia. [3 parts, designated as extras of Atlantic Journal.] 1833 [Google Scholar]
- Reinhammar L-G. Evidence for two distinctive species of Pseudorchis (Orchidaceae) in Scandinavia. Nordic Journal of Botany. 1995;15:469–481. [Google Scholar]
- Reinhammar L-G, Hedrén M. Allozyme differentiation between lowland and alpine populations of Pseudorchis albida s.lat. (Orchidaceae) in Sweden. Nordic Journal of Botany. 1998;18:7–14. [Google Scholar]
- Rudall PJ, Bateman RM. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: the gynostemium and labellum of orchids and other lilioid monocots. Biological Reviews. 2002;77:403–441. doi: 10.1017/s1464793102005936. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. Morphology of monocot flowers: iterative patterns and developmental constraints. New Phytologist. 2004;162:25–44. [Google Scholar]
- Rydberg PA. Studies on the Rocky Mountain flora. Part 5. Bulletin of the Torrey Botanical Club. 1901;a 28:266–284. [Google Scholar]
- Rydberg PA. The North American species of Limnorchis and Piperia, north of Mexico. Bulletin of the Torrey Botanical Club. 1901;b 28:605–643. [Google Scholar]
- Schlechter R. Orchideologiae Sino-Japonicae prodromus: eine kritische Besprechung der Orchideen Ost-Asiens. 1919;88 [Google Scholar]
- Schlechter R. Aceratorchis. Repertorium Specierum Novarum Regni Vegetabilis Beihefte. 1922;12:329. [Google Scholar]
- Sheviak CJ. The identities of Platanthera hyperborea and P. huronensis, with the description of a new species from North America. Lindleyana. 1999;14:193–203. [Google Scholar]
- Sheviak CJ. Flora of North America North of Mexico 26. Oxford: Oxford University Press; 2002. Platanthera Richard; pp. 551–571. Flora of North America Editorial Committee. eds. [Google Scholar]
- Sheviak CJ, Bracht M. New chromosome number determinations in Platanthera. Native North American Orchid Journal. 1998;4:168–172. [Google Scholar]
- Sheviak CJ, Catling PM. Flora of North America North of Mexico 26. Oxford: Oxford University Press; 2002. Galearis Rafinesque, Amerorchis Hultén; pp. 550–551. Flora of North America Editorial Committee. eds. [Google Scholar]
- Sokolovskaya AP. Geographic distribution of polyploid plant species (the research on Sakhalin Island) Vestnik Leningradskogo Universiteta, biol. 1960;21:40–58. [in Russian] [Google Scholar]
- von Soó R. Die sog. Orchis-Arten der ostasiatisch-nordamerikanischen Flora. Acta Botanica Academiae Scientiarum Hungaricae. 1966;12:351–354. [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony, version 4.0. Washington, DC: Smithsonian Institution; 2001. [Google Scholar]
- Szalanski A, Steinauer G, Bischef R, Petersen J. Origin and conservation genetics of the threatened Ute ladies-tresses, Spiranthes diluvialis (Orchidaceae) American Journal of Botany. 2001;88:177–180. [PubMed] [Google Scholar]
- Tanaka R, Kanemoto H. Chromosomes in orchids: counting and numbers. In: Arditti J, editor. Orchid biology: reviews and perspectives. III. Ithaca, NY: Cornell University Press; 1984. [Google Scholar]
- Tang T, Wang F-T. Notes on Orchidaceae of China II [Tsaiorchis] Bulletin of the Fan Memorial Institute of Biology. 1936;7:131–133. [Google Scholar]
- Tang T, Wang F-T. Contributions to the knowledge of eastern Asiatic Orchidaceae. Bulletin of the Fan Memorial Institute of Biology. 1940;10:28–31. [Google Scholar]
- Tang T, Wang F-T, Lang K-Y. The Orchidaceae of Mt. Emei in Sichuan [Diphylax] Botanical Research, Contributions from the Institute of Botany, Academia Sinica (Beijing) 1989;4:11. [Google Scholar]
- Tang T, Wang F-T, Lang K-Y. Wang W-T, editor. Vascular Plants of Hengduan Mountain. 1994;Vol. 2:2526. Diphylax. [Google Scholar]
- Tremblay R, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society. 2005;84:1–54. [Google Scholar]
- Van der Cingel NA. An atlas of orchid pollination: European orchids. Rotterdam: Balkema; 1995. [Google Scholar]
- Vermeulen P. Übersicht zur Systematik und Taxonomie der Gattung Orchis s. str. Jahresberichte des Naturwissenschlaftlichen Vereins in Wuppertal. 1972;25:22–36. [Google Scholar]
- Wallace LE. Biological investigations in the genus Platanthera (Orchidaceae): conservation issues in Platanthera leucophaea and evolutionary diversification in section Limnorchis. Ohio State University; 2002. Doctoral dissertation. [Google Scholar]
- Wallace LE. Molecular evidence for allopolyploid speciation and recurrent origins in Platanthera huronensis (Orchidaceae) International Journal of Plant Sciences. 2003;164:907–916. [Google Scholar]
- Wallace LE. A comparison of genetic variation and structure in the allopolyploid Platanthera huronensis and its diploid progenitors, Platanthera aquilonis and Platanthera dilatata (Orchidaceae) Canadian Journal of Botany. 2004;82:244–252. [Google Scholar]
- Wallace LE. Spatial genetic structure and frequency of interspecific hybridization in Platanthera aquilonis and P. dilatata (Orchidaceae) occurring in sympatry. American Journal of Botany. 2006;93:1001–1009. doi: 10.3732/ajb.93.7.1001. [DOI] [PubMed] [Google Scholar]
- Webb DA. Platanthera L.C.M. Rich. In: Tutin TG, Heywood VH, Burges NA, et al., editors. Flora Europaea 5. Cambridge: Cambridge University Press; 1980. pp. 331–332. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR protocols: a guide to methods and applications. San Diego, CA: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Wood JJ. In: Genera Orchidacearum 2: Orchidoideae 1. Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Oxford: Oxford University Press; 2001. pp. 272–274. Chondradenia. [Google Scholar]
- Wood JJ, Neiland RN. In: Genera Orchidacearum 2: Orchidoideae 1. Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Oxford: Oxford University Press; 2001a. pp. 245–247. Amerorchis. [Google Scholar]
- Wood JJ, Neiland RN. In: Genera Orchidacearum 2: Orchidoideae 1. Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Oxford: Oxford University Press; 2001b. pp. 290–292. Galearis. [Google Scholar]
- Wood JJ, Neiland RN. Platanthera. In. In: Pridgeon AM, Cribb PL, Rasmussen FN, Chase MW, editors. Genera Orchidacearum 2: Orchidoideae 1. Oxford: Oxford University Press; 2001c. pp. 345–350. [Google Scholar]