Abstract
Background
Conservation through reserves alone is now considered unlikely to achieve protection of plant species necessary to mitigate direct losses of habitat and the pervasive impact of global climate change. Assisted translocation/migration represent new challenges in the face of climate change; species, particularly orchids, will need artificial assistance to migrate from hostile environments, across ecological barriers (alienated lands such as farmlands and built infrastructure) to new climatically buffered sites. The technology and science to underpin assisted migration concepts are in their infancy for plants in general, and orchids, with their high degree of rarity, represent a particularly challenging group for which these principles need to be developed. It is likely that orchids, more than any other plant family, will be in the front-line of species to suffer large-scale extinction events as a result of climate change.
Scope
The South West Australian Floristic Region (SWAFR) is the only global biodiversity hotspot in Australia and represents an ideal test-bed for development of orchid conservation principles. Orchids comprise 6 % of all threatened vascular plants in the SWAFR, with 76 out of the 407 species known for the region having a high level of conservation risk. The situation in the SWAFR is a portent of the global crisis in terrestrial orchid conservation, and it is a region where innovative conservation solutions will be required if the impending wave of extinction is to be averted. Major threatening processes are varied, and include land clearance, salinity, burning, weed encroachment, disease and pests. This is compounded by highly specialized pollinators (locally endemic native invertebrates) and, in the most threatened groups such as hammer orchids (Drakaea) and spider orchids (Caladenia), high levels of mycorrhizal specialization. Management and development of effective conservation strategies for SWAFR orchids require a wide range of integrated scientific approaches to mitigate impacts that directly influence ecological traits critical for survival.
Conclusions
In response to threats to orchid species, integrated conservation approaches have been adopted (including ex situ and translocation principles) in the SWAFR with the result that a significant, multidisciplinary approach is under development to facilitate conservation of some of the most threatened taxa and build expertise to carry out assisted migration to new sites. Here the past two decades of orchid conservation research in the SWAFR and the role of research-based approaches for managing effective orchid conservation in a global biodiversity hotspot are reviewed.
Key words: Orchids, pollination, mycorrhiza, integrated conservation, terrestrial, threats, ex situ conservation, in situ conservation
INTRODUCTION
As we face the sixth great extinction event in the history of life on earth (Canadell and Noble, 2001), there is growing awareness that conservation of biodiversity, although underpinned by compelling economic arguments, is an intrinsic responsibility for mankind. With nearly 12·5 % of the global vascular flora facing extinction, conservation of rare and threatened plants is of international consequence (Walter and Gillet, 1998). The most threatened species are confined to hotspots, regions of biodiversity recognized for their species richness, high level of endemism and lack of adequate reserves to protect species (Barthlott et al., 1996; Myers et al., 2000). Historically, biodiversity hotspots covered approx. 12 % of the land surface, but today these areas are restricted to just 1·4 %, and, given that the majority of this loss is recent, there is an expectation that many species within these areas are already extinct or threatened with extinction (Brooks et al., 2002). It is therefore clear that conservation through reserves is unlikely to provide protection of all plant species in hotspots, and assisted migration to new, climatically buffered sites presents immediate scientific and ethical challenges. Meeting these challenges is even more critical relative to anticipated impacts of climate change on global plant biodiversity.
Orchidaceae are the most diverse of all angiosperm families, with estimates of >25 000 species (Dressler, 1993; Mabberley, 1997; Cribb et al., 2003). Orchids comprise five subfamilies and approx. 870 genera, and are considered almost ubiquitous, occurring on all vegetated continents and even some Antarctic islands (Dressler, 1981; Chase et al., 2003). Orchid distribution and abundance are distinctly skewed towards the tropics and vary between continents and within regions, following hotspots of species richness and high angiosperm endemism as described by Myers et al. (2000). Orchid-rich areas include the northern Andes of South America, Madagascar, Sumatra and Borneo for mostly epiphytic species, Indochina for both epiphytic and terrestrial species, and southwestern Western Australia as a centre of terrestrial orchid richness (Cribb et al., 2003). Orchidaceae, more than any other plant family, have a high proportion of threatened genera, with most containing threatened species. Two Australian examples are Drakaea, consisting of ten taxa, five of which are threatened (Hopper and Brown, 2007), and Caladenia, consisting of 243 taxa, 97 of which are threatened (Backhouse, 2007).
Two-thirds of orchid species are epiphytes and lithophytes, with terrestrial species comprising the remaining third, yet almost half of the extinct species according to The World Conservation Union (IUCN, 1999) are terrestrial herbaceous perennials. Terrestrial orchids thus represent a life-form class likely to experience a greater extinction risk as a result of the multiplicity of threatening processes, particularly under current climatic change scenarios. Here, we review the issues and trends in contemporary conservation of terrestrial orchid species as template taxa for understanding conservation of orchids in general, particularly if species need to be the subject of assisted migration (i.e. translocation to new locations to mitigate threatening processes, including climate change; Keel, 2007).
The orchid flora of Australia represents important scientific opportunities for deriving conservation principles. It is an island continent with a rich and highly endemic orchid flora of 1700 species; 25 % of globally extinct orchids are Australian (Koopowitz, 2001). The majority of orchids are represented by southern temperate zone terrestrials, with the balance comprising epiphytes and lithophytes in the northern and eastern rainforests and vine thickets (Dixon and Hopper, 1996; Jones, 2006). Therefore, understanding rarity in the context of extrinsic and intrinsic processes in Australian habitats might bring us closer to the drivers of global orchid extinction. A model of integrated conservation is proposed as the most effective for providing sound and timely orchid conservation.
RARITY IN TERRESTRIAL ORCHIDS
An observation of ecological significance is that organisms differ greatly in distribution and abundance; consequently, rare species may be recognized as those of low numerical abundance compared with others (Pate and Hopper, 1993). Harper (1981) classified rare species according to space, time or group relatedness. A space-dependent species may be locally abundant, but only occur in a limited number of sites, restricted due to high niche specificity or barriers reducing dispersal potential. These species are often local endemics, particularly vulnerable to threatening processes. A time-dependent rare species results from fluctuations in population numbers following adverse sporadic or cyclical events, such as drought or fire (see Koopowitz et al., 2003). Populations of a rare species, occupying a specialized niche with a limited distribution, represent group-dependent rarity associated with certain ecotypes often at ecological frontiers for species. Orchids are found in all these classes.
Although a significant literature exists on the many causes of rarity in plants, drivers of rarity in orchids are more often than not linked to their unique habitats and pollinator requirements. Ecological specialization has not only contributed to the great species diversity in Orchidaceae, but has also resulted in the high level of threat in this family (Cribb et al., 2003). However, it is the complexity of ecological specialization that makes orchids ideal model species for developing and testing conservation strategies.
A significant challenge in orchid conservation is that although the family is one of the most species-rich of all flowering plant families, there are no cases in which orchids are linked to distinct, large-scale ecosystem services. Ecosystem services such as clean water, clean air and productive soils worth trillions of dollars in a global sense (Schwartz et al., 2000) can be linked to natural biodiversity of the planet. Tangible economic benefits arise when, for example, forests around reservoirs contribute to water purification or bees contribute to pollination success in crops (Kennedy, 2006), whereas orchids, by their limited abundance and position as net ‘users’ of ecosystem services, are in themselves incapable of contributing in any significant way to global ecosystem fitness. The debate for orchid conservation therefore rests with their intrinsic value as bioindicators and early warning systems (‘pit canaries’) of ecosystem health and as research tools for devising effective conservation strategies.
EXTRINSIC AND INTRINSIC DRIVERS OF RARITY IN ORCHIDS
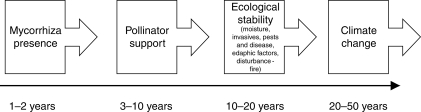
Orchid persistence is linked to abiotic (extrinsic) and biotic (extrinsic and intrinsic) factors that act in a linear sequence of interactions dependent on their level of criticality for growth, development and reproductive success (Fig. 1). For example, for most terrestrial orchids, the presence and vitality (efficacy) of mycorrhiza in soil around plants have a more immediate impact on plant persistence than some other factors, with tuberous reserves enabling plant survival for just a few years after loss of the endophyte.
Fig. 1.
The sequence of abiotic and biotic factors that have an impact upon terrestrial orchid population (or species) persistence on a continuum from immediate term impact (1–2 years for mycorrhizas) to long-term impacts for factors such as climate change.
Extrinsic factors
Extrinsic rarity in orchids is a reflection of anthropogenic threatening processes directly limiting or reducing the distribution and abundance of a species, such as collecting of wild orchids or land clearance (Koopowitz, 2001; Cribb et al., 2003; Koopowitz et al., 2003). Anthropogenic processes often also accelerate environmental and habitat change (kick-on effects), adversely impacting environmental conditions necessary for sustaining orchid populations. These include such factors as spread of disease and pests, changed fire regimes, salinization and desertification (Sahagian, 2000). Extrinsic factors with knock-on effects pose some of the most significant and pervasive of all threats to orchid conservation, particularly in the face of climate change (Dixon et al., 2003).
Over the last 50 years, organisms and ecosystems have become increasingly vulnerable to extinction, and orchids, representing approx. 10 % of all named plants, are predisposed to these risks (Koopowitz et al., 2003). Natural ecosystems are subject to a continuum from partial degradation to complete destruction caused by conversion to new land use, with estimates of man's ecological footprint equating to 2·2 ha of land per person – an ecologically unsustainable figure (Wackernargel and Rees, 1996).
In this century the most rapid growth in human population with the greatest contingent pressure on natural resources has occurred in regions where biological diversity is highest (Kennedy, 2006). The nature of threatening processes to orchids in almost all cases can be traced to human activities, including land clearing for agriculture, mining and urban development, weed invasion, grazing, altered environmental conditions and collection of plants for horticulture and ethnobotanical purposes. Orchids, along with other iconic plant groups including cacti and cycads, have suffered extraordinary deprivation at the hands of collectors and enthusiasts (IUCN, 1999). Fragmentation of habitats, removal of key species critical to the continued existence of ecosystems, increased susceptibility to fire threats, pollinator decline and introduction of feral animals are also documented to result in drastic losses in orchid populations and diversity (Sosa and Platas, 1998; Coates and Dixon, 2007).
Historically, collection of wild orchids has threatened many species with extinction (Cribb et al., 2003). Koopowitz et al. (2003) described a ‘golden age’ of plant collecting from the mid-1800s until the onset of the First World War. The jungles of Brazil, Colombia, Burma, Borneo and New Guinea were explored and exploited for Cattlya, Oncidium, Paphiopedilum, Phalaenopsis, Dendrobium and other genera of high floricultural value. International treaties, such as the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), have markedly reduced unsustainable wild collection, providing new levels of protection, but illegal collection continues, often compounded by habitat loss, particularly in areas rich in orchid species (Koopowitz et al., 2003).
In the Australian context, threatening processes for orchids generally reflect global threats, although ethnobotanical use of orchids is virtually non-existent. Many mitigating factors have resulted in extraordinary levels of threat to Australian plants. Australian has among the highest proportion of threatened species per capita, with 83 recorded plant extinctions since European settlement and 43 animal extinctions, including 19 mammal species (Beeton et al., 2006). Seven out of every hundred plant species are considered threatened by human activities, and without action many will become extinct within the next 20 years. The impact of 200 years of European settlement in Australia has been particularly pronounced in orchids, with more species being affected than in any other plant family (Government Gazette, WA, 2008). This has been due in part to centres of human development being coincident with centres of terrestrial orchid richness, particularly in the Southwest Australian Floristic Region (SWAFR) (Hopper and Gioia, 2004) and the southeastern seaboard of Australia. Unprecedented large-scale clearing of areas such as the wheatbelt within the SWAFR (Coates and Dixon, 2007) has given rise to the largest single extirpation event in Australia, with indicators of impact being among the worst for the country (Beeton et al., 2006).
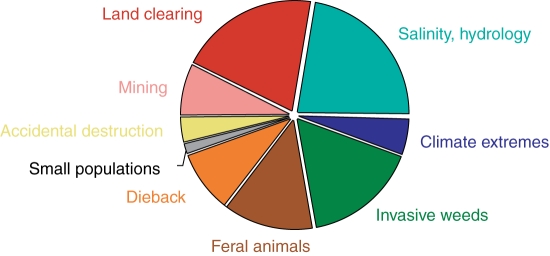
Each habitat and orchid population may experience specific threats (Fay 1992; Fig. 2). Although habitat destruction and degradation often appear to be the most immediate and significant effects, losses of unique evolutionary lineages and erosion of natural demographic and genetic processes associated with small population sizes and isolation are sure to be of consequence when considering the future of these populations (Coates, 2000; Hopper, 2000).
Fig. 2.
Major threatening processes in plant conservation in the South-West Australian Floristic Region (SWAFR; Coates and Atkins, 2001).
Intrinsic factors
Intrinsic rarity refers to limits on abundance and distribution as a result of natural factors. In the case of terrestrial orchids, for example, range and abundance may be driven by factors pertaining to the underground and above-ground life history phases of species (Woolcock and Woolcock, 1984; Clements, 1988; Dixon, 1989). The first need, represented in the underground phase, is a mycorrhizal association with a fungal endophyte (Warcup, 1971; Ramsay et al., 1986; Rasmussen, 2002). The second is effective pollination/fertilization in the above-ground phase (Stoutamire, 1983; Roberts, 2003). The great taxonomic diversity of Orchidaceae is often attributed to specialization of these two requirements, either independently or in combination, the effects of which place species on a theoretical risk continuum from low to high in the event of environmental and habitat change (Fig. 2).
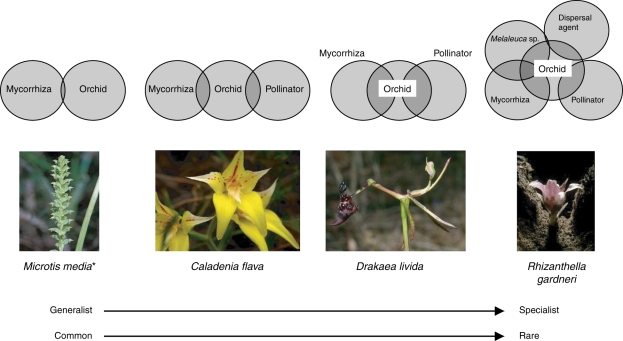
The level of biotic interdependency between a species and other organisms is now seen as increasing the risk of extinction in rare species (Brundrett, 2007). The continuum from generalist to specialist interactions in the context of rarity for four Australian terrestrial orchid species is illustrated in Fig. 3. Although this continuum may reflect interactions that are ecologically robust, factors associated with such extreme specialization may lead to significant risks. In each of these cases extinction risk is about not just the level of specialization but also the capacity of an orchid species to engage in ecological substitution, i.e. the ability to switch from one partner organism (be it a mycoheterobiont in the case of mycoheterotrophs or vectors in sexually deceptive pollination) to another under changed ecological conditions. For example, a generalist pollinator in food-deceptive Caladenia flava means less reliance on one agent for pollination and a broader range of endophyte tolerances than, for example, in Rhizanthella gardneri, for which a specialized mycorrhizal agent and obligate heteromycotrophy combined with a high level of pollinator specificity and a specialized seed dispersal agent (thought to be a native mammal now extirpated from all known locations for the orchid) may result in the orchid facing a higher level of extinction risk (Fig. 1; Dixon, 1991). Although many of these aspects have been considered from an evolutionary perspective, rarely has the level of ecological substitution been considered in conservation planning or assessment of relationships between the orchid and important critical co-associating organisms. Whereas many plants possess a broad capacity for ecological substitution, orchids are often the first organisms to disappear from a disturbed ecosystem (see Dixon et al., 2003, for case studies of specialized attributes in orchids). In devising conservation strategies, particularly assisted migration to new safe sites (that may be outside the home range for the species), it is therefore important for biologists and conservation planners to be fully aware of orchid life history traits that may have an impact on long-term sustainability.
Fig. 3.
Specialisation in temperate terrestrial orchids based on the primary biotic agents limiting abundance and distribution – the degree of overlap in components indicates the degree of biological dependency of the orchid on that factor; e.g. the ability to have more than one fungal associate or multiple pollinators means broader ecological tolerance in the face of loss of one of these factors (ecological substitution). This leads to the concept of rarity being related to the ability for ecological substitution in the event of decline or elimination of a symbiont or pollinator. The development of conservation strategies including assisted migration via translocation to new sites relies on a thorough understanding of the type and level of biotic specializations and highlights the need for conservation actions to integrate research disciplines. *Microtis media is autogamous. (derived from Ramsay et al., 1986; Dixon et al., 1990; Peakall, 1990; Brundrett et al., 2003; Bonnardeaux et al., 2007; Brundrett, 2007).
SEED GERMINATION ECOLOGY AND MYCORRHIZAL ASSOCIATIONS OF TERRESTRIAL ORCHIDS
Orchids produce vast numbers of minute seeds lacking storage reserves, such as an endosperm, found in many other angiosperms (Arditti and Ghani, 2000; Batty et al., 2000). Production of large numbers of small seeds favours high dispersal rates, plant fecundity and expression of genetic variability across geographical and ecological boundaries while minimizing parental investment per seed (Batty et al., 2002; Zettler et al., 2003). Although abundant seed is released, few germinate, and even fewer develop into mature plants. A study of Caladenia arenicola germination revealed that each capsule contained approx. 30 000 ± 2000 seeds, but <1 % germinated and developed to a stage capable of surviving the critical summer dormancy period (Batty et al., 2001a). Seed recruitment success varies from species to species, with some temperate terrestrial species possessing a requirement for stratification or ageing in soil to release dormancy (Stoutamire, 1974). This implies that seeds of some orchid species require optimal environmental windows for effective germination, with new data showing that terrestrial orchid seeds indeed may possess high levels of innate deep seed dormancy (J. Soanes, University of Western Australia (UWA), Australia, pers. comm.). In contrast, many tropical orchids germinate readily in the presence of moisture, nutrients and suitable germination temperatures, with seeds of these species generally lacking physiological barriers to germination (Rasmussen, 1995; Baskin and Baskin, 1998).
Under natural conditions, seeds of most terrestrial orchid species will germinate only in association with a compatible mycorrhizal fungus (Warcup, 1981; Ramsay et al., 1986; Arditti et al., 1990). Due to limited food reserves, orchid seeds have a complete dependency on nutrients supplied by the mycorrhizal association during early germination and seedling establishment phases (Rasmussen, 1995), although some species may substitute or acquire new mycorrhizal associates depending upon plant maturity (Bidartondo and Read, 2008). A number of authors have suggested that orchids exploit carbohydrate, mineral and water resources of the mycobiont without reciprocal benefits for the fungus (Batty et al., 2002; Gardes, 2002; Julou et al., 2005), whereas others have documented mycorrhizal interactions involving a reciprocal exchange of photosynthetic plant-derived carbon in return for access to soil nutrients such as nitrogen, phosphorus, vitamins and/or amino acids (Leake, 1994; Waterman and Bidartondo, 2008). The nature of the orchid–mycorrhizal association is thus complex, probably involving a continuum from mutualism to full exploitation by the orchid of the fungal partner.
The orchid–fungus interaction exhibits a higher degree of mycotrophic specialization than that found in other plants (Brundrett, 2004), which may be linked to rarity when the mycorrhizal association is restricted to an endophyte species limited in distribution (Waterman and Bidartondo, 2008). In addition, common species with the capacity to swap or share endophyte species may compete for the endophyte niche or potential niches of the rare species (Bidartondo and Read, 2008). Brundrett et al. (2003) found that the common and widespread opportunistic species Microtis media germinated in situ twice as frequently as five other non-opportunistic native taxa. A similar result was reported by Bonnardeaux et al. (2007) using in vitro symbiotic germination methods with orchids such as Microtis media and Disa bracteata (an invasive alien species), being compatible with a wider diversity of fungal endophytes than other taxa with less opportunistic characteristics.
The requirement for specialized mycorrhizal fungi in terrestrial orchid germination is well documented (Burgeff, 1909; Curtis, 1939; Warcup, 1971; Batty et al., 2002; Rasmussen, 2002), but the degree of dependency in mature plants is less clear. Smith and Read (1997) and Batty et al. (2002) suggested that mycorrhizal dependency varies according to the autotrophic or heterotrophic lifestyle of the orchid. As the orchid matures, dependency on its mycobiont changes to partial mycoheterotrophy with a decrease correlated with increased photosynthetic capacity. Some orchids remain non-photosynthetic throughout their lives, and as a result life-long mycoheterotrophism ensures survival (McCormick et al., 2004). Orchids, with their high degree of endophyte exploitation, are generally characterized by a range of specific associations with rarer species engaging in complex and highly specialized tripartite interactions. For example, Corallorhiza maculata and C. mertensiana associate with a range of species belonging to the ectomycorrhizal family Russulaceae over a variety of habitat types (Taylor and Bruns, 1999). In comparison, the achlorophyllous subterranean orchid Rhizanthella gardneri forms a highly specialized tripartite association with a Rhizoctonia endophyte that operates as an ectomycorrhiza on roots of a nearby myrtaceous shrub, Melaleuca uncinata (Warcup, 1985) (Fig. 3). Melaleuca uncinata habitats are severely threatened by rising salinity and drying climate in the wheatbelt of southwestern Australia, and R. gardneri is now restricted to just two locations with populations registering steady declines in flowering performance over the past two decades (Murisdawati, 2004), particularly since the frequency and intensity of droughts have increased (Nicholls, 2004). In comparison, chlorophyllous orchids sympatric with R. gardneri remain in relatively good condition, with stable population numbers. The life history traits of R. gardneri involving multiple levels of species-critical specialization may be a key factor adversely restricting its long-term prospects.
Pollination strategies
From the time of Darwin, orchid pollination has intrigued biologists due to its diversity and complexity. More than any other plant family, orchids engage in elaborate systems to lure pollinators ranging from vertebrates to invertebrates, employing the most complex deception systems known in flowering plants (Tremblay et al., 2005; Jersáková et al., 2006; see also Waterman and Bidartondo, 2008). Thus, pollination systems, in a manner similar to the level of specialization in mycorrhizal associations, may play a role in causing rarity in terrestrial orchids.
Although vegetative reproduction is well known for orchids as a strategy to overcome deficits in seed output (Dixon, 1991), sexual reproduction is the primary means by which organisms maintain genetic diversity and novelty in their progeny (Sipes and Trepedino, 1995). The pollination strategy of an orchid, be it a food reward or deception system (food or sexual), strongly influences its mating system and outcrossing capability. Although autogamy is relatively uncommon in Orchidaceae, food (38 genera) or sexual (18 genera) deceit occurs in approximately one-third of species (Cozzolino and Widmer, 2005; Jersáková et al., 2006). A result of this high proportion of deceit is a trend towards reduction in the number of pollinator species per orchid species combined with specialized habitat requirements (Roberts, 2003; Tremblay et al., 2005). As specialized pollination strategies are likely to have contributed significantly to diversification in Orchidaceae, extinction risks have also increased as disruption to pollination systems in this time of environmental change affects their long-term survival and evolutionary potential (Roberts, 2003).
Australian terrestrial orchids engage in some of the most elaborate pollination strategies known for plants and express highly divergent levels of floral specialization. Extreme specialization in pollination syndrome is found in the endemic Western Australian hammer orchids, Drakaea, of which all nine species are pollinated by different species of thynnine wasp (Peakall, 1990; R. Phillips, UWA, Australia, pers. comm.). These wasps parasitize underground larvae of native scarab beetles that in turn require root systems of particular native plants as a substrate. Thus, the orchid has a total reliance on a set of ecologically linked components; the orchid is thus vulnerable to local extirpation if any of these are lost. In these circumstances, more than with most other Australian orchids, there is limited capacity for ecological substitution, and as a consequence five hammer orchid species are critically endangered, and one, Drakaea andrewsiae, is considered extinct.
In rare orchids with pollinator limitation, it may be expected that autogamy or apomixis might be favoured as a means for overcoming these limitations (Sipes and Trepidino, 1995). Changes in breeding systems involving self-pollination are more likely to occur in species at ecological frontiers, but, at present, there are limited data supporting these concepts (Smithson and Gigord, 2001). With fewer than 15 native SWAFR orchid taxa thought to engage in agamospermy or autopollination, such a shift is not widely employed as a mechanism to adapt to pollinator-limited habitats. However, apomixis has been part of the mix of successful ecological mechanisms employed by invasive orchids. In particular, Disa bracteata in Western Australia employs autogamy, enabling this species to spread over a remarkable 20 000 km2 of habitat in <100 years since its introduction from South Africa (Hoffman and Brown 1992). Similarly, some native orchid species such as Microtis media from southern Australia use prolific seed production, thought to involve agamospermy, combined with vegetative multiplication to become garden and greenhouse weeds in many parts of Australia.
THE INTEGRATED APPROACH TO ORCHID CONSERVATION AND ASSISTED MIGRATION
Conservation practice takes place in the context of limited resources and often with a matter of urgency, particularly in biodiverse regions where adverse environmental conditions affect multiple species (Coates and Dixon, 2007). Consequently, conservation practitioners often make pragmatic decisions based on an incomplete understanding of the biology and ecology of species or systems they wish to manage. As a result, extrapolation coupled with adaptive management principles (learning by doing; Bormann et al., 2007) have become the present-day drivers of conservation and restoration programmes, often in place of hard data and theory (Falk and Holsinger, 1991). Therefore, with the premise that resource and time limitation will not allow all threatened biodiversity to be saved, there is a need for setting conservation priorities and for selecting species, habitats or ecosystems at greatest risk (Falk, 1990; Hopper, 2000). Some important considerations in development of conservation programmes (including assisted migration) and policies include: recognition of existing and future environmental threats; taxonomic distinctiveness; geographic distribution; habitat specialization; reproductive biology; evolutionary processes influencing population structure; and ex situ conservation technology.
Ex situ conservation is often viewed as the key conservation action, but preservation of germplasm off-site should only be viewed as providing an ‘emergency ward’ targeted at extinction-proofing those species under the greatest or most immediate threat. Regardless of technological options for off-site conservation such as seed and germplasm banks and in vitro propagation, in situ conservation and conservation via assisted migration (linked to leading-practice restoration ecology: see Society for Ecological Restoration International Science & Policy Working Group, 2004; Keel, 2007) are the premier approaches for global biodiversity conservation into the 21st century.
Orchids in particular are vulnerable to misinterpretation of what constitutes sustainable conservation, with a single capsule being potentially able to generate thousands of plants. Indeed, it is feasible to propagate artificially just about any species at levels that could surpass the numbers in the wild for some taxa, e.g. Asian slipper orchids (Paphiopedilum). A nationally threatened Australian terrestrial orchid, Purdie's donkey orchid (Diuris purdiei), provides an example in which the ability to germinate many thousands of seeds was construed as conservation and predicated the destruction of 70 % of the species in the wild (Dixon and Hopper, 1996). Conservation biologists must be vigilant that technological capacity for conservation is not used to mitigate directly against effective conservation in situ. Kennedy (2006), in his essay on managing common ecological resources, stated that ‘The big question in the end is not whether science can help, rather, it is whether scientific evidence can successfully overcome social, economic and political resistance’ (i.e. for the protection of natural resources).
Effective and timely protection of biodiversity must now integrate a number of disciplines rapidly to improve understanding and modification of processes contributing to rarity. Productive linkages, collaboration and partnerships between systematics, plant genetics and population biology linked to conservation biotechnology (propagation sciences) and restoration ecology will be critical for reinstating and managing restored populations (Coates and Dixon, 2007) and assisted migration of taxa. For this to be possible, conservation should involve experimentation directed at continued survival of species in both an in situ and ex situ context. Thus, development of effective conservation strategies must aim to strike a balance between the need for urgent action to avoid further loss and the search for essential information and understanding of the species or ecosystem to be conserved. The adaptive management approach (Bormann et al., 2007) provides one of the most useful mechanisms for linking research to operational expediency in management and restoration of species and ecosystems.
Integrated conservation approaches rely on melding ecological and genetic studies, in situ research and ex situ propagation (Falk, 1990; Ramsay and Dixon, 2003). This approach to conservation is widely accepted and forms the basis of seminal national documents including, for Australian biodiversity, ‘The National Strategy for the Conservation of Australia's Biological Diversity’ (Hopper, 1997). The conservation strategy emphasizes interaction of land conservation, biological management, ex situ research, propagation and (re)introduction, and habitat restoration (Hopper, 1997). Importantly today, this document provides the blue-print for understanding the basis for assisted migration of species.
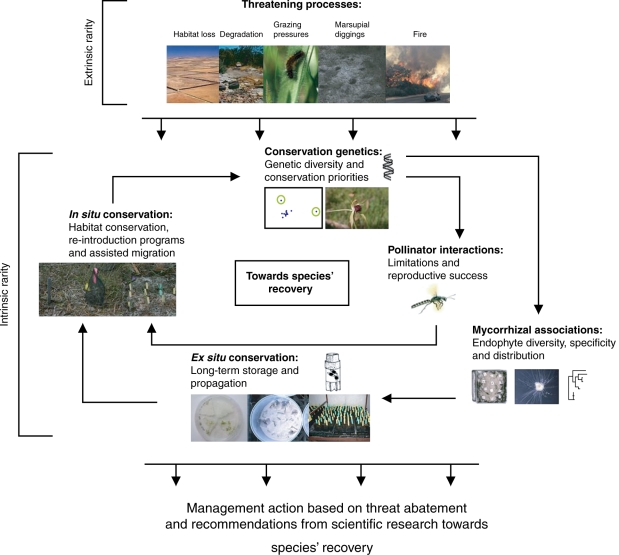
The key parts of a ‘one-stop-shop’ integrated conservation approach (Fig. 4) for Australian (in particular Western Australian) orchid conservation have been under development since 1995. They include conservation genetics, mycorrhizal associations, pollinator interactions and in situ and ex situ conservation.
Fig. 4.
The integrated orchid conservation approach for conservation and translocation (including assisted migration) of terrestrial orchids. This model was developed for the conservation and recovery of Caladenia huegelii in Western Australia; see Swarts (2007). Recommendations for effective conservation are based on deriving and applying species-relevant knowledge obtained from research across scientific and conservation disciplines. Extrinsic and intrinsic rarity is defined in the text.
Conservation genetics
Conservation genetics provides the theoretical framework and practical tools to conserve diversity and the underlying processes that drive genetic diversity in natural populations (and reconstructed populations). Until the last decade, the necessity for genetic considerations in conservation programmes was eclipsed by the need for habitat restoration and conservation (Qamaruz-Zaman et al., 1998). The advent of the molecular age has revolutionized the scale and depth of knowledge, with results available in days rather than months. Maintenance or restoration of genetic diversity is now viewed as a major goal of conservation for rare and threatened species, resulting in the use of molecular methods and phylogenetic studies in the design and application of conservation strategies (Falk and Holsinger, 1991; Hogbin et al., 2000; Hopper, 2000; Mattner et al., 2002).
Orchidaceae are characterized by a diverse range of life histories, reproductive strategies and distributions, reflecting an equally diverse variety of patterns in genetic differentiation of orchid populations (Scacchi et al., 1990; Peakall and Beattie, 1996; Sun, 1996; Gustaffson, 2000; Tremblay and Ackerman, 2001; Wallace, 2002; Forrest et al., 2004). Hamrick and Godt (1996) suggested that long-lived, outcrossing species with the capacity for long-distance seed dispersal as in Orchidaceae tend to be more genetically diverse and show less genetic differentiation among populations. Reviews by Case et al. (1998) and Forrest et al. (2004) of mean genetic differentiation in orchid population studies have found no clear trend in GST (FST analogue), with values ranging from 0·012 to 0·924. Forrest et al. (2004) recorded a mean GST estimate of 0·187 for all studies, which was higher than the averages of 0·159 and 0·087 reported earlier by Case et al. (1998) and Hamrick and Godt (1996), respectively, but still lower than that reported for other predominantly herbaceous families. Given that these data account for only a small percentage of orchid species, the large variance in GST recorded indicates that levels of genetic differentiation in individual species may be difficult to predict. Detailed knowledge of genetic diversity in orchid populations may reveal genetic consequences of pollinator behaviour, inbreeding (Peakall and Beattie, 1996), geographic isolation by fragmentation and small population sizes (Wallace, 2002). These data therefore provide knowledge fundamental to development of conservation priorities, ensuring that a mix of genotypes is used in reintroduction/translocation programmes.
Much of our knowledge of genetic diversity and population structure in orchids has come from allozyme/isozyme markers. In studies of Australian terrestrial orchids, allozyme markers have been used to confirm outcrossing and levels of gene flow consistent with pollinator movements in the sexually deceptive Caladenia tentaculata (Peakall and Beattie, 1996). Carstairs and Coates (1994) found that >95 % of allozyme diversity occurs within populations of food-deceptive Caladenia elegans and C. caesarea subsp. maritima. Allozyme studies have also demonstrated significant local genetic structure, consistent with suggestions that the majority of seed falls within 10 m of the source (Machon et al., 2003; Chung et al., 2004; Trapnell et al., 2004).
Earlier DNA-based molecular techniques were considered generally unsuitable for use in a conservation context because the required large amount of DNA necessitated the use of considerable amounts of plant tissue. The development of PCR has reversed this situation. Since then, molecular methods based on PCR have been developed or improved in the context of orchid conservation studies, e.g. DNA sequencing of a range of loci (Selosse et al., 2002; Otero et al., 2004), amplified fragment length polymorphisms (AFLPs; Hedrén et al., 2001; Smith et al., 2004), plastid microsatellites (Fay and Cowan, 2001; Fay et al., 2009) and nuclear microsatellites (Gustaffson and Sjogren-Gulve, 2002).
Nuclear microsatellites consist of simple nucleotide motifs (e.g. AAT), repeated multiple times. They are co-dominant, highly polymorphic markers able to detect higher levels of genetic diversity than allozymes (Qamaruz-Zaman et al., 1998). Although microsatellite marker development requires considerable investment of time and resources, their great variability and ease of detection validate their use in addressing questions in population genetics, gene flow and paternity analysis, particularly in a conservation context. Microsatellite markers have shown them to be an effective molecular tool with wide application, including for orchids (Gustaffson, 2000; Gustaffson and Sjogren-Gulve, 2002; Cozzolino et al., 2003; Soliva and Widmer, 2003; Mant et al., 2005).
Mycorrhizal associations
Orchid mycorrhizal endophytes are thought to persist in nature either as independent saprophytes or as biotrophs (Zettler et al., 2003). These fungi can be difficult to identify in soil, and direct isolation from orchid protocorms or infected regions of mature plants can be more effective. Orchid endophyte isolation was first attempted by Reissak in 1847 (Hadley 1982), and since then many authors (e.g. Bernard, 1909; Knudson, 1927; Curtis, 1939; Hadley, 1970; Warcup, 1981; Ramsay et al., 1986) have developed simple and highly effective methods for obtaining pure endophyte cultures.
Traditionally, morphological characters such as teleomorph stages (Warcup and Talbot, 1967), hyphal branching patterns, the presence of monilioid clusters (Sneh et al., 1991) and nucleation (Ramsay et al., 1987) were used to identify and classify mycobionts associated with host orchids. Sexual stages of orchid endophytes are rarely encountered in the field or laboratory, and broad vegetative criteria for fungal identification have consequently resulted in a taxonomy in which unrelated taxa were grouped together (Otero et al., 2002). Morphological descriptions of pure colony appearance have been the main source of information for identification of orchid endophytes. Isolation of single pelotons and subsequent culture from single hyphal tips is the most reliable method of ensuring that a single genotype has been isolated. Other methods of differentiating endophytes include pectic zymograms (Abdul Karim, 2004), hyphal anastamosis groupings (AGs; Ramsay et al., 1987), differential staining and physiological techniques that determine mycorrhizal responses to temperature, light and organic content (Dijk, 1990; Tsutsui and Tomita, 1990).
Most orchid mycorrhizal fungi that have been isolated and cultured are assigned to the form-genus Rhizoctonia, a genus of basidiomycete fungi imperfecti (Heterobasidiomycetes; Hadley, 1982; Currah and Zelmar, 1992). Rhizoctonia-like fungi include the anamorphs Ceratorhiza, Epulorhiza and Moniliopsis (Moore, 1987) and a variety of teleomorphs of Ceratobasidium, Thanatephorus, Tulsanella and Sebacina (Otero et al., 2002). Many Rhizoctonia-type species have strains forming a variety of associations in plants, including mycorrhizas with orchids and pathogens of a wide variety of crop plants, and others that are successful saprophytes with no known direct association with plants.
More recently, the use of molecular approaches to identify fungal associates has dominated research of orchid–fungal relationships. Analysis of DNA sequences permits rapid inference of taxonomic affinities of orchid endophytes from genetic databases such as GenBank (http://www.ncbi.nlm.nih.gov). DNA sequencing, mostly based on the nuclear ribosomal internal transcribed spacer (nrITS) region (e.g. Kristiansen et al., 2001; Pope and Carter, 2001; Otero et al., 2002; Bougoure et al., 2005; Bonnardeaux et al., 2007), has substantially advanced the taxonomy of Rhizoctonia spp. and other mycorrhizas associated with orchids, but generic and species concepts within associated fungal families remain largely unresolved. A significant failing of some studies is that sequencing is done by direct assay of orchid root or stem tissues without recourse to subsequent isolation and testing for germination efficacy. As shown by Huynh et al. (2004), orchid tissues can possess multiple fungal endophytes, and only by testing for germination efficacy can an endophyte be deemed mycorrhizal. Otherwise we may be screening for non-pathogenic endophytes that provide limited or no benefit to the orchid. Bidartondo and Read (2008) used direct assay and isolation procedures for orchid endophytes in combination with germination efficacy as a means of determining comparative ecological benefits of endophytes; this serves as an exemplar of approaches needed to match potential with realized ecological specificity (of wild baited protocorms; see below) rather than a sole reliance on direct molecular assessment.
Orchids are unusual in the plant kingdom (with monotropoid Ericaceae) in typically having a dedicated requirement for a fungal associate for seed germination to occur. Symbiotic germination demonstrates the compatibility of orchid seed and endophytes using a variety of methods. The term ‘physiological specificity’ can be applied to in vitro symbiotic germination, but associations occurring under these conditions should not be assumed necessarily to reflect field conditions (Zettler et al., 2003). Interactions occurring in defined orchid habitats in situ are termed ‘ecological specificity’ (Masuhara and Katsuya, 1994) and may more accurately reflect the actual specificity of the mycorrhizal association (Batty et al., 2001a). Perkins and McGee (1995) suggested that under natural conditions some orchids demonstrate a greater specificity for fungal associates than in laboratory experiments, but this remains to be tested.
Mycorrhizal associations of orchids are important in implementing recovery and restoration programmes, and genetic studies used to identify the diversity of fungi associated with certain orchids are demonstrating a marked degree of specialization (Fay and Krauss, 2003). An understanding of mycorrhizal diversity associated with species targeted for reintroduction is crucial for success of rehabilitation efforts.
Pollinator interactions
Orchid pollination, particularly from an evolutionary perspective, is relatively well understood in comparison with their other biological and ecological attributes. Charles Darwin (1862) was one of the first to explore orchid floral diversity and pollination strategies. Since Darwin, there have been many studies of pollination mechanisms, amounting to scores of research papers (see Tremblay et al., 2005), but there has been relatively little attention given to orchid pollination in a conservation context.
Given the wide range of pollination mechanisms in Orchidaceae, with many using insects and other vectors to promote outbreeding, pollinator and resource limitation are major issues in conservation planning. Roberts (2003) suggested that orchids are naturally pollinator-limited within a season but resource-limited over their lifetime. Pollination limitation or failure in plants can be attributed to absence of pollinators, failure of removed pollen to reach a recipient, poor quality or insufficient pollen quantity and dispersion of heterospecific pollen (Wilcock and Neiland, 2002), whereas resource limitation may be a reflection of the fitness costs associated with sexual reproduction in the previous season (Ackerman and Montalvo, 1990). Although orchids are ecologically adapted to these limitations, anthropogenic processes such as habitat reduction or fragmentation and overcollection may interrupt sexual reproduction by reducing pollen movement, with a subsequent decrease in fruit set and seedling recruitment. Thus, understanding limitations on reproductive success in orchids and impacts of anthropogenic and environmental change is important in conservation planning and management (Roberts, 2003).
Knowledge of pollinator behaviour and genetic consequences of the breeding system used by an orchid is advancing with the application of population genetics (e.g. Peakall and Beattie, 1996; Soliva and Widmer, 2003) and phylogenetic analyses of orchids and their pollinators (e.g. Kores et al., 2001; Mant et al., 2002). The taxonomy and ecological requirements of pollinators of terrestrial orchids remain largely unknown, restricted to in situ observations or presentation of ‘bait’ flowers to attract pollinators (Stoutamire, 1983; Peakall, 1990; Peakall and Beattie, 1996). The strategy of sexual deception is more highly developed in Australian terrestrial orchids than in almost any other orchid flora, and includes male thynnine wasps of the subfamily Thynninae. Thynnines are the pollinators of approx. 70 species in six genera, but only 20–25 % of thynnid wasp species have been described, and there are few studies of their ecology (Ridsdill-Smith, 1970; Brown et al., 1997). Given that orchids are far more reliant on their pollinators than the latter are on the orchid, careful conservation planning is required to ensure pollinator requirements such as food plants, nest sites, larval host species or larval host plants are sustained in conservation and translocation programmes (Roberts, 2003).
In situ conservation: studies for management, site selection and translocation
Preservation of natural habitats is of foremost importance to conservation, but loss of habitat and ecological function (through changes in hydrological and edaphic capability, invasive species, pests and diseases and disturbance including changed fire regimes) remain the greatest threats to the integrity of orchid populations and long-term species survival. Protection of orchid habitats requires a thorough understanding of the distribution of orchids, associated pollinators and characteristics that make the habitat unique, particularly in relation to the distribution of mycorrhizal endophytes and pollinators (Ramsay and Dixon, 2003)
There is limited understanding of how to assess and manage mycorrhizal endophytes in field situations, and most information relates to non-orchid-associated fungi, such as vesicular-arbuscular, ericoid and ecto-mycorrhizas of trees and shrubs (Dixon and Hopper, 1996). Habitat management for orchids will continue to be problematic until the distribution and ecological requirements of fungal endophytes are adequately understood, as has been facilitated recently by in situ and ex situ fungal ‘baiting’ techniques to detect mycorrhizal endophytes in field sites (Batty et al., 2001a; Brundrett et al., 2003, Bidartondo and Read, 2008).
In situ baiting uses multichambered seed packets placed in field sites to assess simultaneously the presence and distribution of mycorrhizal endophytes (with either single or multiple species). Ex situ evaluation of efficacious orchid endophytes is based on the principle that these will be preferentially located in the organic rather than the mineral fraction of soil. The process involves sieving the organic fraction to concentrate the activity of potential mycorrhizal fungi and the use of direct seed assays under controlled laboratory conditions (Brundrett et al., 2003). Fungal isolations can also be made from developing protocorms and seedlings to determine endophyte diversity and specificity (McKendrick et al., 2002; Taylor and Bruns, 2003). These methods provide an excellent medium for determining the distribution and diversity of endophytes in particular habitats, inoculum potential of microsites and sites for translocation programmes.
Translocation programmes in which propagated orchid seedlings are moved into new sites (assisted migration) or used to reinforce depleted populations or in which mature plants are transplanted as an emergency are being used as tools in orchid conservation (Zettler and McInnis, 1992). However, reintroduction should not be seen as a substitute for primary habitat conservation or community involvement in in situ management. Rather, artificial manipulation of plants in wild sites should only be undertaken in the context of enhancing the conservation status of protected habitats and developing self-sustaining populations of the threatened taxon (Maunder, 1992). This relies on established principles relating to the importance of a ‘safe site’ for reintroduction of orchids, where the presence of a compatible mycorrhizal endophyte has been confirmed and the specific plant pollinator is present (Batty et al., 2002). Given the paucity of knowledge on ecological requirements of pollinators, only crude attempts can be made to ensure the vegetation composition provides for pollinator sustenance and breeding opportunities (Dixon and Hopper, 1996). Thus, ensuring the site selected for reintroduction matches the existing habitat in its vegetation characteristics, soil composition, geology and hydrology is of critical importance for ensuring the long-term survival of reintroduced orchids. Ongoing monitoring of plant growth and development and effective control of pests and diseases are also critical for the success of recovery programmes (Ramsay and Dixon, 2003).
Monitoring performance of mycorrhizas, pollinators and plants will provide important ecological and logistical parameters that are likely to influence the long-term success of a recovery programme involving translocated orchids. Failure to consider these factors in planning for orchid recovery means that such programmes represent little more than gardening (Hobbs, 2007).
Ex situ conservation: storage and propagation
Ex situ conservation strategies such as propagation and seed banking are fundamental components in any integrated conservation approach (Cribb et al., 2003), providing long-term security (‘extinction-proofing’). The ex situ conservation principle refers to off-site selection and storage of genetically representative seeds and, where applicable, somatic tissues, regeneration of plants from the stored material, continued cultivation of species to produce conservation units (Johansen and Rasmussen, 1992; Seaton and Pritchard, 2003) and storage of ecologically competent orchid mycorrhizas.
Selection and collection of translocation materials are important parts of the long-term aim of the integrated conservation approach for creating self-sustaining populations. Modern molecular methods are increasing our technical capacity to identify and select material of genetic provenances considered most significant from a conservation perspective. Indeed, active restoration of habitats and species now requires genetic issues to be considered when germplasm is to be translocated as part of a recovery plan or to be stored in long-term off-site genebanks (Rosetto et al., 1995; Fay and Krauss, 2003). In these cases, appropriate genetic characterization is required to avoid inbreeding or outbreeding depression and maximize evolutionary potential of these new populations.
Germplasm collections, however, have little value in the context of conservation if the material is not adequately identified or curated (Schuiteman and Vogel, 2003). In the process of germplasm collection and storage, accurate identification of both orchid and mycobiont should be coupled with an understanding of the reproductive biology of species to be conserved. The collection process must involve germination assays investigating compatibility and efficacy of fungal endophytes. Long-term storage of germplasm involves the use of ultra-low temperatures [(−20 to −196 °C in liquid nitrogen (LN)] to reduce the incidence of conservation artefacts such as tissue/organ ageing, spontaneous (synthetic) genetic variation (potentially non-adaptive variation) or gradual breakdown of cellular integrity (Touchell and Dixon, 1994). Batty et al. (2001b) found that dried seed stored in LN germinated substantially better than seed freshly collected or stored at 4, 18 and 22 °C for 1 year. They also found that mycorrhizal fungi that promoted germination and growth of orchids could also be successfully stored in LN (Batty et al., 2001b), although fungi are readily stored using a variety of other methods including lyophilization and freezing (Gams, 2002).
Ex situ conservation alone is simply a means to safeguard the conservation of a species in a way that provides long-term insurance against losses in the event of extinction in the wild and a ready source of material for species recovery programmes. Terrestrial orchids (including rare and threatened species), unlike their epiphytic counterparts, have proved difficult to germinate and establish in soil on a large scale (Clements et al., 1986; Batty et al., 2006a). Until recently, there has been limited research into development of effective techniques to reintroduce terrestrial orchids into native or restored habitats (Whigham and Willems, 2003), often at considerable cost, such as the programme to reintroduce the lady's slipper orchid (Cypripedium calceolus) in England (Ramsay and Dixon, 2003). Short-term survival of outplanted orchids in field sites has been reported by a number of authors (e.g. McKendrick, 1995; Zettler and Hofer, 1998; Batty et al., 2002; Ramsay and Dixon, 2003), and recent work on SWAFR taxa by Batty et al. (2006a, b) and Scade et al. (2006) has attempted to overcome the difficulties of seedling acclimatization from in vitro to in situ conditions, tuber development and endophyte re-infection, with varying levels of success. These studies demonstrated the possibility of improving survival of reintroduced orchid seedlings with cross application to rare and endangered taxa. Further studies of this type are needed if we are to rebuild depleted orchid populations successfully and secure threatened species.
FUTURE CONSERVATION
Orchids are at the front-line of extinction, with more species under threat globally than any other plant family. With warming occurring at a greater rate than at any time in the last 10 000 years (Nicholls, 2004), even the most modest of impacts are likely to add to the perilous conservation situation of orchids.
Effective conservation of orchids is confounded by loss of habitats, natural migration routes and functionality of biotic partners (mycorrhizas and pollinators). Orchids are also acutely susceptible to changes in ecosystem equilibria involving organic content, light availability, hydrology and competition that can affect both parental survival and the ability of seedlings to germinate and survive to adulthood. In many respects, if the orchids are managed, the rest of the ecosystem may take care of itself.
The ability to conserve terrestrial orchids depends upon three key actions: (1) ensuring that design and management of natural reserves takes into account the specialized needs and attributes of orchids; (2) development of effective ex situ seed and mycorrhiza banks for orchids under immediate threat; and (3) development of approaches for restoration of terrestrial orchids. The last represents the greatest single challenge in terrestrial orchid conservation as, unlike other rare plant translocations, there is limited knowledge of the range of biological and ecological attributes that underpin terrestrial orchid growth, development and reproduction. Equally, horticultural knowledge, experience and science to underpin even basic propagation in preparation for translocation for many taxa is lacking or resides in the realm of the amateur grower. For some taxa, such as species with tripartite, mycoheterotrophic associations (e.g. the Western Australian underground orchid, Rhizanthella gardneri), the complexity of mycorrhizal associations, although manageable under controlled greenhouse conditions, may not be easily transferred to field conditions. There is much work to be done if terrestrial orchids are to be conserved and extinction averted globally.
LITERATURE CITED
- Abdul Karim NB. Biology of fungal endophytes associated with terrestrial orchids of the Kimberley. Perth: University of Western Australia; 1999. Honours Thesis. [Google Scholar]
- Ackerman J, Montalvo A. Short- and long-term limitations to fruit production in a tropical orchid. Ecology. 1990;71:263–272. [Google Scholar]
- Arditti J, Ghani AKA. Tansley review no. 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytologist. 2000;145:367–421. doi: 10.1046/j.1469-8137.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- Arditti J, Ernst R, Wing Yam T, Glabe C. The contribution of orchid mycorrhizal fungi to seed germination: a speculative review. Lindleyana. 1990;5:249–255. [Google Scholar]
- Backhouse G. Are our orchids safe down under? A national assessment of threatened orchids in Australia. Lankesteriana. 2007;7:28–43. [Google Scholar]
- Barthlott W, Lauer W, Placke A. Global distribution of species diversity in vascular plants. Erdkunde. 1996;50:317–327. [Google Scholar]
- Baskin CC, Baskin JA. Seeds: ecology, biogeography, and evolution of dormancy and germination. Bowen Hills: Australia Academic Press; 1998. [Google Scholar]
- Batty AL, Dixon KW, Sivasithamparam K. Soil seed bank dynamics of terrestrial orchids. Lindleyana. 2000;15:227–236. [Google Scholar]
- Batty AL, Dixon KW, Brundrett M, Sivasithamparam K. Constraints to symbiotic germination of terrestrial orchid seed in a mediterranean bushland. New Phytologist. 2001;a 152:511–520. doi: 10.1046/j.0028-646X.2001.00277.x. [DOI] [PubMed] [Google Scholar]
- Batty AL, Dixon KW, Brundrett M, Sivasitthamparam K. Long-term storage of mycorrhizal fungi and seed as a tool for the conservation of endangered Western Australian terrestrial orchids. Australian Journal of Botany. 2001;b 49:1–10. [Google Scholar]
- Batty AL, Dixon KW, Brundrett MC, Sivasithamparam K. Microorganisms in plant conservation and biodiversity. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. Orchid conservation and mycorrhizal associations. [Google Scholar]
- Batty AL, Brundrett MC, Dixon KW, Sivasithamparam K. New methods to improve symbiotic propagation of temperate terrestrial orchids from axenic culture to soil. Australian Journal of Botany. 2006;a 54:367–374. [Google Scholar]
- Batty AL, Brundrett MC, Dixon KW, Sivasithamparam K. In situ symbiotic seed germination and propagation of terrestrial orchid seedlings for establishment at field sites. Australian Journal of Botany. 2006;b 54:375–381. [Google Scholar]
- Beeton RJS, Buckley KI, Jones GJ, Morgan D, Reichelt RE, Trewin D. State of the environment report; Australia. Canberra: Department of the Environment and Heritage; 2006. http://www.environment.gov.au/soe/2006/index.html . [Google Scholar]
- Bernard N. L'evolution dans la symbiose, les orchidées et leures champignons commensaux. Annals of Science and Natural Botany, Series 9. 1909;9:1–196. [Google Scholar]
- Bidartondo MI, Read DJ. Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology. 2008;17:3707–3716. doi: 10.1111/j.1365-294X.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- Bonnardeaux Y, Brundrett M, Batty AL, Dixon KW, Koch J, Sivasithamparam K. Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research. 2007;111:51–61. doi: 10.1016/j.mycres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Bormann BT, Haynes RW, Martin JR. Adaptive management of forest ecosystems: did some rubber hit the road? BioScience. 2007;57:186–191. [Google Scholar]
- Bougoure JJ, Bougoure DS, Cainey JWG, Dearnaley JDW. ITS – RFLP and sequence analysis of endophytes from Acianthus, Caladenia and Pterostylis (Orchidaceae) in southeastern Queensland. Mycological Research. 2005;4:452–460. doi: 10.1017/s095375620500225x. [DOI] [PubMed] [Google Scholar]
- Brooks TM, Mittermeier RA, Mittermeier CG, et al. Habitat loss and extinction in the hotspots of biodiversity. Conservation Biology. 2002;16:909–923. [Google Scholar]
- Brown EM, Burbridge AH, Dell J, Edinger D, Hopper SD, Wills RT. Pollination in Western Australia: a database of animals visiting flowers. Perth: WA Naturalists Club; 1997. [Google Scholar]
- Brundrett MC. Diversity and classification of mycorrhizal associations. Biological Reviews. 2004;79:473–495. doi: 10.1017/s1464793103006316. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Role of symbiotic relationships in Australian terrestrial orchid conservation. Australian Plant Conservation. 2007;15:2–7. [Google Scholar]
- Brundrett MC, Scade A, Batty AL, Dixon KW, Sivasithamparam K. Development of in situ and ex situ seed baiting techniques to detect mycorrhizal fungi from terrestrial orchid habitats. Mycological Research. 2003;107:1210–1220. doi: 10.1017/s0953756203008463. [DOI] [PubMed] [Google Scholar]
- Burgeff H. Die Wurzelpilze der Orchidaceen, ihre Kultur und ihr Leben in der Pflanzen. Jena: G. Fischer; 1909. p. 220. [Google Scholar]
- Canadell J, Noble I. Challenges of a changing earth. Trends in Ecology and Evolution. 2001;16:664–666. [Google Scholar]
- Carstairs S, Coates D. Conservation genetics and population ecology of five rare and threatened Western Australian orchids. Final report to the endangered species unit. Perth: Australian Nature Conservation Agency; 1994. [Google Scholar]
- Case MA, Mlodozeniec HT, Wallace LE, Weldy TW. Conservation genetics and taxonomic status of the rare Kentucky lady's slipper: Cypripedium kentuckiense (Orchidaceae) American Journal of Botany. 1998;85:1779–1786. [PubMed] [Google Scholar]
- Chase MW, Cameron KM, Barrett RL, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenic classification. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 69–90. [Google Scholar]
- Chung MY, Nason JD, Chung MG. Spatial genetic structure in populations of the terrestrial orchid Cephalanthera longibracteata (Orchidaceae) American Journal of Botany. 2004;91:52–57. doi: 10.3732/ajb.91.1.52. [DOI] [PubMed] [Google Scholar]
- Clements MA. Orchid mycorrhizal associations. Lindleyana. 1988;3:73–86. [Google Scholar]
- Clements MA, Muir H, Cribb PJ. A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bulletin. 1986;41:437–445. [Google Scholar]
- Coates DJ. Defining conservation units in a rich and fragmented flora: implications for the management of genetic resources and evolutionary processes in south-west Australian plants. Australian Journal of Botany. 2000;48:329–339. [Google Scholar]
- Coates DJ, Dixon KW. Current perspectives in plant conservation biology. Australian Journal of Botany. 2007;55:187–193. [Google Scholar]
- Coates DJ, Atkins KA. Priority setting and the conservation of Western Australia's diverse and highly endemic flora. Biological Conservation. 2001;97:251–263. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception. Trends in Ecology & Evolution. 2005;20:487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A. Fine-scale phylogeographical analysis of Mediterranean Anacamptis palustris (Orchidaceae) populations based on chloroplast minisatellite and microsatellite variation. Molecular Ecology. 2003;12:2783–2792. doi: 10.1046/j.1365-294x.2003.01958.x. [DOI] [PubMed] [Google Scholar]
- Cribb PJ, Kell SP, Dixon KW, Barrett RL. Orchid conservation: a global perspective. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 1–24. [Google Scholar]
- Currah RS, Zelmer C. A key and notes for the genera of fungi mycorrhizal with orchids and a new species in the genus Epulorhiza. Reports of the Tottori Mycological Institute. 1992;30:43–59. [Google Scholar]
- Curtis JT. The relationships of specificity of orchid mycorrhizal fungi to the problem of symbiosis. American Journal of Botany. 1939;26:390–399. [Google Scholar]
- Darwin CR. On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Dijk E. Effects of mycorrhizal fungi on in vitro nitrogen response of juvenile orchids. Agriculture Ecosystems and Environment. 1990;29:1–4. [Google Scholar]
- Dixon KW. Seed propagation of ground orchids. In: Dixon KW, Buirchell BJ, Collins MJ, editors. Orchids of Western Australia. 2nd edn. Victoria Park: Native Orchid Study and Conservation Group Inc; 1989. pp. 18–26. [Google Scholar]
- Dixon K. Seeder/clonal concepts in Western Australian orchids. In: Wells TCE, Willems JH, editors. Population ecology of terrestrial orchids. The Hague: SPB Academic Publishing; 1991. pp. 111–124. [Google Scholar]
- Dixon KW, Hopper SD. Regional accounts – Australia. In: Hagsater E, Dumont V, editors. Orchids – status, survey and conservation action plan. Gland: IUCN/SSC Orchid Specialist Group; 1996. pp. 109–115. [Google Scholar]
- Dixon KW, Cribb PJ, Kell SP, Barrett RL, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. [Google Scholar]
- Dressler RL. The orchids, natural history and classification. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland, OR: Dioscorides Press; 1993. [Google Scholar]
- Falk DA. Integrated strategies for conserving plant genetic diversity. Annals of the Missouri Botanical Garden. 1990;77:38–47. [Google Scholar]
- Falk DA, Holsinger KE. Genetics and conservation of rare plants. New York: Oxford University Press; 1991. [Google Scholar]
- Fay MF. Conservation of rare and endangered plants using in vitro methods. In Vitro and Cell Developmental Biology. 1992;28P:1–4. [Google Scholar]
- Fay MF, Cowan RS. Plastid microsatellites in Cypripedium calceolus (Orchidaceae): genetic fingerprints from herbarium specimens. Lindleyana. 2001;16:151–156. [Google Scholar]
- Fay MF, Krauss SL. Orchid conservation genetics in the molecular age. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 91–112. [Google Scholar]
- Fay MF, Bone R, Cook P, et al. Genetic diversity in Cypripedium calceolus (Orchidaceae) with a focus on north-western Europe, as revealed by plastid DNA length polymorphisms. Annals of Botany. 2009;104:517–525. doi: 10.1093/aob/mcp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest AD, Hollingsworth MI, Hollingsworth PM, Sydes C, Bateman RM. Population genetic structure in European populations of Spiranthes romanzoffiana set in the context of other genetic studies on orchids. Heredity. 2004;92:218–227. doi: 10.1038/sj.hdy.6800399. [DOI] [PubMed] [Google Scholar]
- Gams W. Ex situ conservation of microbial diversity. In: Sivasithamparam K, Dixon KW, Barrett RL, editors. Microorganisms in plant conservation and biodiversity. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. pp. 269–283. [Google Scholar]
- Gardes M. An orchid–fungus marriage: physical promiscuity, conflict and cheating. New Phytologist. 2002;154:4–7. [Google Scholar]
- Government Gazette, WA. Wildlife Conservation (Rare Flora) Notice 2008. 2008. www.naturebase.net/component/option,com_docman/task,doc_download/gid,2125/Itemid,/
- Gustafsson S. Patterns of genetic variation in Gymnadenia conopsea, the fragrant orchid. Molecular Ecology. 2000;9:1863–1872. doi: 10.1046/j.1365-294x.2000.01086.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson S, Sjogren-Gulve P. Genetic diversity in the rare orchid, Gymnadenia odoratissima and a comparison with the more common congener, G. conopsea. Conservation Genetics. 2002;3:225–234. [Google Scholar]
- Hadley G. Non-specificity of symbiotic infection in orchid mycorrhiza. New Phytologist. 1970;69:1015–1023. [Google Scholar]
- Hadley G. Orchid mycorrhiza. In: Arditti J, editor. Orchid biology: reviews and perspectives, II. Ithaca, NY: Cornell University Press; 1982. pp. 85–115. [Google Scholar]
- Hamrick JL, Godt MJW. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1291–1298. [Google Scholar]
- Harper JL. The meaning of rarity. In: Synge H, editor. The biological aspects of rare plant conservation. New York: J. Wiley and Sons Ltd; 1981. pp. 189–203. [Google Scholar]
- Hedrén M, Fay MF, Chase MW. Amplified fragment length polymorphisms (AFLP) reveal details of polyploid evolution in Dactylorhiza (Orchidceae) American Journal of Botany. 2001;88:1868–1880. [PubMed] [Google Scholar]
- Hobbs RJ. Managing plant populations in fragmented landscapes: restoration or gardening? Australian Journal of Botany. 2007;55:371–374. [Google Scholar]
- Hoffman N, Brown A. Orchids of South-West Australia. 2nd edn. Nedlands: University of Western Australia Press; 1992. [Google Scholar]
- Hogbin PM, Peakall R, Sydes MA. Achieving practical outcomes from genetic studies of rare Australian plants. Australian Journal of Botany. 2000;48:375–382. [Google Scholar]
- Hopper SD. An Australian perspective on plant conservation biology in practice. In: Fiedler PL, Kareiva PM, editors. Conservation biology for the coming decade. New York: Chapman and Hall; 1997. pp. 255–278. [Google Scholar]
- Hopper SD. How well do phylogenetic studies inform the conservation of Australian plants? Australian Journal of Botany. 2000;48:321–328. [Google Scholar]
- Hopper SD, Brown AP. A revision of Australia's hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators. Australian Systematic Botany. 2007;20:252–285. [Google Scholar]
- Hopper SD, Gioia P. The Southwest Australian Floristic Region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution and Systematics. 2004;35:623–650. [Google Scholar]
- Huynh TT, McLean CB, Coates F, Lawrie AC. Effect of developmental stage and peloton morphology on success in isolation of mycorrhizal fungi in Caladenia formosa (Orchidaceae) Australian Journal of Botany. 2004;52:231–241. [Google Scholar]
- IUCN. IUCN guidelines for the prevention of biodiversity loss due to biological invasion. Species. 1999;31–32:28–42. [Google Scholar]
- Jersáková J, Johnson SD, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biological Reviews. 2006;81:219–235. doi: 10.1017/S1464793105006986. [DOI] [PubMed] [Google Scholar]
- Johansen B, Rasmussen H. Ex situ conservations of orchids. Opera Botanica. 1992;113:43–48. [Google Scholar]
- Jones DL. A complete guide to native orchids of Australia including the island territories. Sydney: Reed New Holland; 2006. [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse M-A. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist. 2005;166:639–653. doi: 10.1111/j.1469-8137.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Keel BG. Assisted migration as a conservation strategy for rapid climate change: investigating extended photoperiod and mycobiont distributions for Habenaria repens Nuttall (Orchidaceae) as a case study. New England: Antioch University; 2007. PhD Thesis. [Google Scholar]
- Kennedy D. Life on a human dominated planet. State of the planet 2006–2007. Washington: The American Association for the Advancement of Science; 2006. [Google Scholar]
- Knudson L. Symbiosis and asymbiosis relative to orchids. New Phytologist. 1927;26:328–336. [Google Scholar]
- Koopowitz H. Orchids and their conservation. Portland: Timber Press; 2001. [Google Scholar]
- Koopowitz H, Lavarack PS, Dixon KW. The nature of threats to orchid conservation. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 25–42. [Google Scholar]
- Kores PJ, Molvray M, Weston PH, et al. A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany. 2001;88:1903–1914. [PubMed] [Google Scholar]
- Kristiansen KA, Taylor DL, Kjoller R, Rasmussen HN, Rosendahl S. Identification of mycorrhizal fungi from single pelotons of Dactylorhiza majalis (Orchidaceae) using single-strand conformation polymorphism and mitochondrial ribosomal large subunit DNA sequences. Molecular Ecology. 2001;10:2089–2093. doi: 10.1046/j.0962-1083.2001.01324.x. [DOI] [PubMed] [Google Scholar]
- Leake JR. Tansley review no. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist. 1994;127:171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Mabberley DJ. The plant book. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Machon N, Bardin P, Mazer S, Moret J, Godelle B, Austerlitz F. Relationship between genetic structure and seed and pollen dispersal in the endangered orchid Spiranthes spiralis. New Phytologist. 2003;157:677–687. doi: 10.1046/j.1469-8137.2003.00694.x. [DOI] [PubMed] [Google Scholar]
- Mant J, Peakall R, Schiestl FP. Does selection on floral odour promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution. 2005;59:1449–1463. [PubMed] [Google Scholar]
- Mant JG, Schiestl FP, Peakall R, Weston PH. A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution. 2002;56:888–898. doi: 10.1111/j.0014-3820.2002.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Masuhara G, Katsuya K. In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon.) Ames. var. amoena (M. Beiberstein) Hara (Orchidaceae) New Phytologist. 1994;127:711–718. doi: 10.1111/j.1469-8137.1994.tb02974.x. [DOI] [PubMed] [Google Scholar]
- Mattner J, Zawko G, Rossetto M, Krauss SL, Dixon KW, Sivasithamparam K. Conservation genetics and implications for restoration of Hemigenia exilis (Lamiaceae), a serpentine endemic from Western Australia. Biological Conservation. 2002;107:37–45. [Google Scholar]
- Maunder M. Plant reintroduction: an overview. Biodiversity and Conservation. 1992;1:51–61. [Google Scholar]
- McCormick MK, Whigham DF, O'Neill J. Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytologist. 2004;163:425–438. doi: 10.1111/j.1469-8137.2004.01114.x. [DOI] [PubMed] [Google Scholar]
- McKendrick SL. The effects of herbivory and vegetation on laboratory-raised Dactylorhiza praetermissa (Orchidaceae) planted into grassland in southern England. Biological Conservation. 1995;73:215–220. [Google Scholar]
- McKendrick SL, Leake JR, Taylor DL, Read DJ. Symbiotic germination and development of the myco-heterotrophic orchid Neottia nidus-avis in nature and its requirement for locally distributed Sebacina spp. New Phytologist. 2002;154:233–247. [Google Scholar]
- Moore RT. The genera of Rhizoctonia-like fungi: Aschorhizoctonia, Ceratorhiza gen. nov., Epulorhiza gen nov., Moniliopsis and Rhizoctonia. Mycotaxon. 1987;29:91–99. [Google Scholar]
- Murasdiwati S. Perth: University of Western Australia; 2004. Mycorrhizal association, propagation and conservation of the myco-heterotrophic orchid Rhizanthella gardneri. Masters thesis. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nicholls N. The changing nature of Australian droughts. Climatic Change. 2004;63:1473–1480. [Google Scholar]
- Otero JT, Ackerman JD, Bayman P. Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. American Journal of Botany. 2002;89:1852–1858. doi: 10.3732/ajb.89.11.1852. [DOI] [PubMed] [Google Scholar]
- Otero JT, Ackerman JD, Bayman P. Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology. 2004;13:2393–2404. doi: 10.1111/j.1365-294X.2004.02223.x. [DOI] [PubMed] [Google Scholar]
- Pate JS, Hopper SD. Rare and common plants in ecosystems, with special reference to the south Western Australian flora. In: Schulze ED, Mooney HA, editors. Ecosystem function of biodiversity. New York: Springer Verlag; 1993. pp. 293–325. [Google Scholar]
- Peakall R. Responses of male Zaspilothynnus tribobatus turner wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology. 1990;4:159–167. [Google Scholar]
- Peakall R, Beattie AJ. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentaculata. Evolution. 1996;50:2207–2220. doi: 10.1111/j.1558-5646.1996.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, McGee PA. Distribution of the orchid mycorrhizal fungus Rhizoctonia solani, in relation to its host, Pterostylis acuminata, in the field. Australian Journal of Botany. 1995;43:565–575. [Google Scholar]
- Pope EJ, Carter DA. Phylogenetic placement and host specificity of mycorrhizal isolates belonging to AG-6 and AG-12 in the Rhizoctonia solani species complex. Mycologia. 2001;93:712–719. [Google Scholar]
- Qamaruz-Zaman F, Fay MF, Parker JS, Chase MW. Molecular techniques employed in the assessment of genetic diversity: a review focusing on orchid conservation. Lindleyana. 1998;13:259–283. [Google Scholar]
- Ramsay MM, Dixon KW. Propagation science, recovery and translocation of terrestrial orchids. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 259–288. [Google Scholar]
- Ramsay RR, Sivasithamparam K, Dixon KW. Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana. 1986;1:203–214. [Google Scholar]
- Ramsay RR, Sivasithamparam K, Dixon KW. Anastomosis groups among Rhizoctonia-like endophytic fungi in southwestern Australia Pterostylis species (Orchidaceae) Lindleyana. 1987;2:161–166. [Google Scholar]
- Rasmussen HN. Terrestrial orchids, from seed to mycotrophic plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant and Soil. 2002;244:149–163. [Google Scholar]
- Ridsdill Smith TJ. The behaviour of Hemithynnus hyalinatus (Hymenoptera: Tiphiidae), with notes on some other Thynninae. Journal of the Australian Entomological Society. 1970;9:196–208. [Google Scholar]
- Roberts DL. Pollination biology: the role of sexual reproduction in orchid conservation. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 113–136. [Google Scholar]
- Rossetto M, Weaver PK, Dixon KW. Use of RAPD analysis in devising conservation strategies for the rare and endangered Grevillea scapigera (Proteaceae) Molecular Ecology. 1995;4:321–329. doi: 10.1111/j.1365-294x.1995.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Sahagian D. Global physical effects of anthropogenic hydrological alterations: sea level and water redistribution. Global and Planetary Change. 2000;25:39–48. [Google Scholar]
- Scacchi R, De Angelis G, Lanzara P. Allozyme variation among and within eleven Orchis species (fam. Orchidaceae), with special reference to hybridizing aptitude. Genetica. 1990;81:143–150. [Google Scholar]
- Scade A, Brundrett MC, Batty AL, Dixon KW, Sivasithamparam K. Survival of transplanted terrestrial orchid seedlings in urban bushland habitats with high or low weed cover. Australian Journal of Botany. 2006;54:383–389. [Google Scholar]
- Schuiteman A, de Vogel E. Taxonomy for conservation. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 55–68. [Google Scholar]
- Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ. Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia. 2000;122:297–305. doi: 10.1007/s004420050035. [DOI] [PubMed] [Google Scholar]
- Seaton PT, Pritchard HW. Orchid germplasm collection, storage and exchange. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 227–258. [Google Scholar]
- Selosse M-A, Weiss M, Jean-Luc J, Tillier A. Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring tree ectomycorrhizae. Molecular Ecology. 2002;11:1831–1844. doi: 10.1046/j.1365-294x.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- Sipes SD, Tepedino VJ. Reproductive biology of the rare orchid, Spiranthes diluvialis: breeding system, pollination, and implications for conservation. Conservation Biology. 1995;9:929–938. [Google Scholar]
- Smith SD, Cowan RS, Gregg KB, Chase MW, Maxted N, Fay MF. Genetic discontinuities among populations of Cleistes (Orchidaceae, Vanilloideae) in North America. Botanical Journal of the Linnean Society. 2004;145:87–95. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 1997. [Google Scholar]
- Smithson A, Gigord LDB. Are there fitness advantages in being a rewardless orchid? Reward supplementation experiments with Barlia robertiana. Proceedings of the Royal Society. 2001;268:1435–1441. doi: 10.1098/rspb.2001.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneh B, Burpee L, Ogoshi A. Identification of Rhizoctonia species. St Paul, MN: American Phytopathological Society Press; 1991. [Google Scholar]
- Society for Ecological Restoration International Science & Policy Working Group. The SER international primer on ecological restoration. 2004. www.ser.org. & Tucson: Society for Ecological Restoration International.
- Soliva M, Widmer A. Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution. 2003;57:2252–2261. doi: 10.1111/j.0014-3820.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Sosa V, Platas T. Extinction and persistence of rare orchids in Veracruz, Mexico. Conservation Biology. 1998;12:451–455. [Google Scholar]
- Stoutamire WP. Terrestrial orchid seedlings. In: Withner CL, editor. The orchids: scientific studies. New York: John Wiley & Sons; 1974. pp. 101–108. [Google Scholar]
- Stoutamire WP. Wasp-pollinated species of Caladenia (Orchidaceae) in south-western Australia. Australian Journal of Botany. 1983;31:383–394. [Google Scholar]
- Sun M. Effects of population size, mating system, and evolutionary origin on genetic diversity in Spiranthes sinensis and S. hongkongensis. Conservation Biology. 1996;10:785–795. [Google Scholar]
- Swarts N. Perth: University of Western Australia; 2007. Integrated conservation of the rare and endangered terrestrial orchid Caladenia huegelii H. G. Reichb. PhD Thesis. [Google Scholar]
- Taylor DL, Bruns TD. Population, habitat and genetic correlates of mycorrhizal specialization in the ‘cheating’ orchids Corallorhiza maculata and C. mertensiana. Molecular Ecology. 1999;8:1719–1732. doi: 10.1046/j.1365-294x.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- Touchell DH, Dixon KW. Cryopreservation for seedbanking of Australian species. Annals of Botany. 1994;74:541–546. [Google Scholar]
- Trapnell DW, Hamrick JL, Nason JD. Three-dimensional fine-scale genetic structure of the neotropical epiphytic orchid, Laelia rubescens. Molecular Ecology. 2004;13:1111–1118. doi: 10.1111/j.1365-294X.2004.02148.x. [DOI] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD. Gene flow and effective population size in Lepanthes (Orchidaceae): a case for genetic drift. Biological Journal of the Linnean Society. 2001;72:47–62. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society. 2005;84:1–54. [Google Scholar]
- Tsutsui K, Tomita M. Suitability of several carbohydrates as the carbon sources for symbiotic seedling growth of two orchid species. Lindleyana. 1990;5:134–139. [Google Scholar]
- Wackernagel M, Rees WE. Our ecological footprint: reducing human impact on the earth. Gabriola Island: New Society Publishers; 1996. [Google Scholar]
- Wallace L. Examining the effects of fragmentation on genetic variation in Platanthera leucophaea (Orchidaceae): inferences from allozyme and random amplified polymorphic DNA markers. Plant Species Biology. 2002;17:37–49. [Google Scholar]
- Walter KS, Gillet H. The 1997 IUCN Red List of threatened plants. Gland and Cambridge: World Conservation Union; 1998. [Google Scholar]
- Warcup JH. Specificity of mycorrhizal association in some Australian terrestrial orchids. New Phytologist. 1971;70:41–46. [Google Scholar]
- Warcup JH. The mycorrhizal relationships of Australian orchids. New Phytologist. 1981;87:371–381. [Google Scholar]
- Warcup JH. Rhizanthella gardneri (Orchidaceae), its Rhizoctonia endophyte and close association with Melaleuca uncinata (Myrtaceae) in Western Australia. New Phytologist. 1985;99:273–280. [Google Scholar]
- Warcup JH, Talbot PHB. Perfect states of rhizoctonias associated with orchids. New Phytologist. 1967;66:631–641. [Google Scholar]
- Waterman RJ, Bidartondo MI. Deception above, deception below: linking pollination and mycorrhizal biology of orchids. Journal of Experimental Botany. 2008;59:1085–1096. doi: 10.1093/jxb/erm366. [DOI] [PubMed] [Google Scholar]
- Whigham DF, Willems JH. Demographic studies and life-history strategies of temperate terrestrial orchids as a basis for conservation. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 137–158. [Google Scholar]
- Wilcock C, Neiland M. Pollination failure in plants: why it happens and when it matters. Trends in Plant Science. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- Woolcock CE, Woolcock DT. Australian terrestrial orchids. Melbourne: Thomas Nelson; 1984. [Google Scholar]
- Zelmer CD, Currah RS. Symbiotic germination of Spiranthes lacera (Orchidaceae) with naturally occurring endophyte. Lindleyana. 1997;12:142–148. [Google Scholar]
- Zettler LW, Hofer CJ. Propagation of the little club-spur orchid (Platanthera clavellata) by symbiotic seed germination and its ecological implications. Environmental and Experimental Botany. 1998;39:189–195. [Google Scholar]
- Zettler LW, McInnis JTH. Propagation of Platanthera integrilabia (Correll) Luer, an endangered terrestrial orchid, through symbiotic seed germination. Lindleyana. 1992;7:154–161. [Google Scholar]
- Zettler LW, Sharma J, Rasmussen FN. Mycorrhizal diversity. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 205–226. [Google Scholar]