Abstract
Cumulative dissipated energy (CDE) was used with Infiniti® Vision System (Alcon Labs) as an energy delivery guide to compare four different phaco techniques and phaco settings. The supracapsular phaco technique and burst mode is known for efficiency and surgery is faster compared with the old phaco unit. In this study, we found that supracapsular phaco with burst mode had the least CDE in both cataract and nuclear sclerosis cataract with the new Infiniti® unit. We suggest that CDE can be used as one of the references to modify technique and setting to improve outcome for surgeons, especially for new surgeons.
Keywords: CDE (cumulative dissipated energy), cataract surgery, phacoemulsification, supracapsular, burst mode, Divide–Conquer
Introduction
Cumulative dissipated energy (CDE) is a built-in measurement of the Alcon Infiniti® Vision System (Alcon Labs, Hünenberg, Switzerland), a phacoemulsification unit which is specifically designed for surgeons to monitor the energy delivered during phacoemulsification. There are many different phaco machines using different measurements to assess the energy delivered in phacoemulsification. Less CDE in phacoemulsification during cataract surgery translates to less energy used and is considered better for cornea recovery.1–4 The purpose of this study is to find which phacoemulsification technique and setting had the least CDE (without considering the surgeon’s expertise). This study introduces the CDE as a measurement of surgical efficiency in hope of improving surgical outcomes. CDE data is not currently widely utilized for this purpose. However, it was used to compare the energy delivered in conventional phaco versus torsional phaco.5 According to our study, a high CDE reading is equated with statistically significant cornea endothelium cell loss with one surgeon, one technique, and one setting.
Objective
Our objective is to evaluate the use of CDE as a measurement in the comparison of different OZil® (Alcon Labs) settings, techniques, and surgeon efficiency in phacoemulsification at one cataract surgical center using the same phaco machine (Infiniti®, OZil® Torsional handpiece; Alcon Labs). We hypothesized that supracapsular phaco technique and burst mode will use the least CDE.
Study design
This is a retrospective experimental design study of 140 randomly selected patients treated during a one-month period by four different surgeons that practiced different techniques and OZil® settings. All four surgeons have practiced phacoemulsification for over 20 years and each average 250 to 500 cases per year. Patients in this sample varied in age from 32 to 95 years old and consisted of 64 male and 76 females. Patient medical records of CDE from April 17, 2008 through to May 15, 2008 were reviewed at Surgical Suite, Honolulu, Hawaii. Patient demographic and CDE data was transcribed from the charts into an Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet, and then stratified by surgeon. Analyses were done on the main cohort of data, which included all types of cataract patients. A secondary analysis was done on a subset cohort that included only nucleus sclerosis cataract patients.
Results
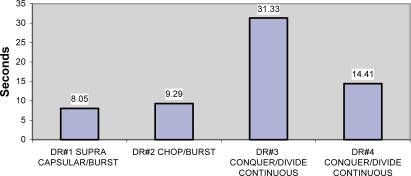
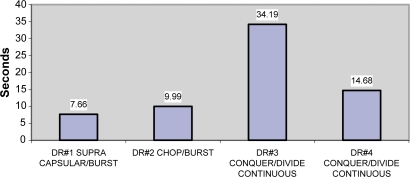
The mean average time in both data cohorts show noticeable differences in that one surgeon using supranucleus phaco technique and burst mode has the least CDE. One surgeon who used Divide–Conquer/OZil®-continuous mode had a much higher CDE compared to the other three, and was even higher than another surgeon with the same technique and continuous mode Table 1, Figures 1 and 2). The exclusion of all cataracts but nuclear sclerosis showed increase in CDE time for all surgeons except #1. The burst mode showed lower energy in surgeons #1 and #2. Surgeon #3 showed significantly higher energy by CDE.
Table 1.
Cohort, all cataracts
| Surgeon | Technique | N total | Seconds mean (SD) |
|---|---|---|---|
| 1 | Supracapsular/OZil®-burst | 24 | 8.05 (7.25) |
| 2 | Chop/OZil®-burst | 39 | 9.29 (8.27) |
| 3 | Divide–Conquer/OZil®-continuous | 22 | 31.33 (19.45) |
| 4 | Divide–Conquer/OZil®-continuous | 55 | 14.41 (11.07) |
Figure 1.
CDE for all cataract patients.
Notes: The exclusion of all cataracts but nuclear sclerosis shows increase in CDE time for all surgeons except #1. Also the burst mode showed lower energy in #1 and #2. Again, #3 shows significantly higher energy by CDE.
Abbreviation: CDE, cumulative dissipated energy.
Figure 2.
CDE for nuclear sclerosis patients.
Abbreviation: CDE, cumulative dissipated energy.
A one-way analysis of variance was done on the main data cohort using SPSS software (version 12.0.1; SPSS Inc, Chicago, IL, USA). Analysis of variance for overall test of homogeneity detected a significant difference across all four strata (f = 24.06; p < 0.001) and further regression analysis showed the same difference (f = 7.64; p = 0.006). Comparisons of individual groups were done to identify the specific statistical differences. Surgeons #3 and #4 both used the Divide–Conquer/OZil®-continuous technique and were statistically different from all other surgeons as shown in Table 2. A p-value <0.05 was considered statistically significant.
Table 2.
Comparisons between individual groups
| Surgeon | Technique | Surgeon | Technique | p-value |
|---|---|---|---|---|
| 1 | Supracapsular/OZil®-burst versus | 2 | Chop/OZil®-burst | p = 0.548 |
| 3 | Divide–Conquer/OZil®-continuous | 1 | Supracapsular/OZil®-burst versus | p < 0.001 |
| 3 | Divide–Conquer/OZil®-continuous | 2 | Chop/OZil®-burst | p < 0.001 |
| 3 | Divide–Conquer/OZil®-continuous | 4 | Divide–Conquer/OZil®-continuous | p < 0.001 |
| 4 | Divide–Conquer/OZil®-continuous | 1 | Supracapsular2/OZil®-burst versus | p = 0.012 |
| 4 | Divide–Conquer/OZil®-continuous | 2 | Chop/OZil®-burst | p < 0.001 |
| 4 | Divide–Conquer/OZil®-continuous | 3 | Divide–Conquer/OZil®-continuous | p < 0.001 |
Notes: Dependant variable, seconds; independent variable, strata.
Discussion
Study outcomes such as pachymetry, endothelium cell count, clarity of cornea post-operation and the surgeon factor (level of expertise) were not included in the study. This study shows that in one single surgical center with the same phaco machine and torsional mode, there was a variation in the average CDE reading because of the OZil® setting, technique, and surgeon expertise factors. Even though the surgeon expertise factor is difficult to unify, all four surgeons have similar qualifications and experience. It was impossible to have all four surgeons unify their setting and technique. Low CDE measurements may correlate to more efficient energy delivery, faster surgery,6,7 and less endothelium cell loss. Using CDE measurements as a guide may improve technique and instrument-setting abilities. There may be many confounding factors to link CDE as an accurate indicator. Nevertheless, for any surgery there is a need to have some type of gauge for quality assessment.
Conclusion
The study shows that the surgeon using supracapsular with burst mode had the least CDE compared to the others. There was a nonstatistical significance in CDE between supracapsular and burst modes in surgeon #1 and chop and burst modes in surgeon #2. The CDE can be used as a reference to monitor the efficiency of OZil® setting, technique, and surgeon expertise factors. In the future, we could better utilize CDE measurement for studies and teaching related to modern phacoemulsification in cataract surgery.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pirazzoli B, D’Eliseo D, Ziosi M, Acciarri R. Effects of phacoemulsification time on the corneal endothelium using phacofracture and phaco chop techniques. J Cataract Refract Surg. 1996;22(7):967–969. doi: 10.1016/s0886-3350(96)80200-8. [DOI] [PubMed] [Google Scholar]
- 2.Kreisler KR, Mortenson SW, Mamalis N. Endothelial cell loss following “modern” phacoemulsification by a senior resident. Opthalmic Surg. 1992;23(3):158–160. [PubMed] [Google Scholar]
- 3.Colvard DM, Kratz RP, Mazzocco TR, Davidson B. Endothelial cell loss following phacoemulsification in the pupillary plane. Am Intra-Ocular Implant Soc J. 1981;7(4):334–336. doi: 10.1016/s0146-2776(81)80030-4. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Hayashi H, Hakao F, Kayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):1079–1084. doi: 10.1016/s0886-3350(96)80121-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Zeng M, Liu X, et al. Torsional mode versus conventional ultrasound mode phacoemulsification. J Cataract Refract Surg. 2007;33(2):287–292. doi: 10.1016/j.jcrs.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Badoza D, Fernández Mendy J, Ganly M. Phacoemulsification using the burst mode. J Cataract Refract Surg. 2003;29(6):1101–1105. doi: 10.1016/s0886-3350(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 7.Maloney WF, Dillman DM, Nichamin LD. Supracapsular phacoemulsification: a capsule-free posterior chamber approach. J Cataract Refract Surg. 1997;23(3):323–328. doi: 10.1016/s0886-3350(97)80173-3. [DOI] [PubMed] [Google Scholar]