Abstract
Objectives: to evaluate the association between dehydroepiandosterone (DHEA) and physical frailty in older adults.
Design: cross-sectional analysis of baseline information from three separate studies in healthy older men, women and residents of assisted living.
Setting: academic health centre in greater Hartford, CT, USA.
Participants: eight hundred and ninety-eight adults residing in the community or assisted living facility.
Measurements: participants had measurement of frailty (weight loss, grip strength, sense of exhaustion, walking speed and physical activity) and serum DHEAS levels.
Results: overall, 6% of the individuals in the study were classified as frail, 58% intermediate frail and 35% were not frail. In the bivariate analysis, there were differences between categories of frailty across age, gender and by DHEAS levels. In an ordinal logistic regression model, with frailty as a dependent measure, we found that age, DHEAS and interactions of age and BMI and DHEAS and BMI were predictive of more frailty characteristics.
Conclusion: we found an association between frailty and DHEAS levels. Whether the association is due to similar conditions resulting in lower DHEA levels and more susceptibility to frailty or whether lower DHEA levels have an impact on increasing frailty cannot be addressed by cross-sectional analysis. Gender did not impact the association between DHEAS and frailty, but obesity (BMI > 30 kg/m2) attenuated the association between higher DHEA levels and lower frailty status.
Keywords: dehydroepiandosterone, frailty, ageing, elderly
Introduction
Dehydroepiandosterone (DHEA) and its sulphate ester, DHEAS, are prominent adrenal steroid hormones in humans [1]. DHEA influences peripheral tissues either indirectly via a conversion to androgens, estrogens or both, or directly as a steroid hormone [2]. DHEA shows a characteristic secretion pattern throughout life, with serum levels declining with increasing age. Serum DHEA levels peak in young adults and gradually decline throughout life, so that individuals 70–80 years old have circulating DHEA levels that are 10–20% of their original young adult levels [1]. This age-associated decrease has been termed ‘adrenopause’ and differs from other adrenocortical hormones, which do not demonstrate clear age-related changes [1]. DHEA levels also vary with gender, with higher levels in men than in women [1].
Little is known about the physiological role of DHEA, but human epidemiological studies have suggested that its concentrations may represent a biomarker of successful ageing [3]. Studies show that low serum DHEA levels are associated with increased rates of morbidity and mortality. Several studies have shown an association between reduced serum DHEA(S) levels and depression [4, 5], bone mineral density in women [4], diabetes [6] and other age-related illnesses such as cardiovascular mortality [7] and neurological dysfunction [8].
The geriatric syndrome of frailty is defined as a loss of reserve and is characterized by weight loss, fatigue, weakness and vulnerability to adverse events, which predict increased morbidity and mortality [9, 10]. At present, the biological basis for frailty remains unclear. It has been hypothesized that loss of lean muscle mass and bone mass, decline in protein synthesis, sarcopenia and immune dysfunction influence the onset of frailty [11, 12]. Serum levels of DHEA decline with age, and this decline is paralleled by a decrease in muscle and bone mass [1]. Our study sought to evaluate the relationship between frailty and DHEA levels. Using data from a population of elderly adults, we tested the hypothesis that declining serum DHEA is associated with the frailty phenotype.
Methods
Study population
Healthy adults aged 60 years or older residing in the community or assisted living were recruited to participate in the study. The individuals were screened for potential participation in one of three studies—to assess testosterone effects on bone and frailty in men (TEST), to assess dehydroepiandrosterone and exercise effects on bone and frailty in women (DHEAEX) and to assess osteoporosis evaluation in individuals residing in assisted living (n = 37) and community-living age-matched controls (n = 77) (ALF). The data are baseline assessments, prior to any interventions.
Exclusion criteria for men and women in TEST or DHEAEX were (i) diseases or medications known to affect bone or muscle metabolism (i.e. Paget's disease, osteomalacia, hyperparathyroidism: current use of corticosteroids, calcitonin, heparin, phenytoin, phenobarbital, methotrexane, bisphosphonates, selective oestrogen-receptor modulator or PTH); (ii) use of oestrogen, DHEA or androgen in the preceding year; (iii) metastatic or advanced cancer (other than skin cancer); (iv) history of breast cancer or prostate cancer; (v) active cardiac ischaemia by history of angina or myocardial infarction in the preceding 6 months; (vi) elevation of PSA; (vii) history of sleep apnoea and (viii) polycythaemia. There were no exclusion criteria for the ALF study.
All study participants provided written informed consent. The institutional review board at the University of Connecticut Health Center approved the study.
Frailty measurements
The frailty phenotype was that by Fried et al. [9] included self-reported weight loss of >4.5 kg in the preceding year, grip strength, sense of exhaustion, walking speed and level of physical activity reported in kcal/week. Frailty was defined as three or more of the five characteristics, intermediate frail as one or two criteria and non-frail as no criteria. Fried et al.'s definition of frailty was used in this study because of the previous validation studies revealing its association with poor health outcomes.
Hormone assays
Serum levels of DHEAS were measured in the General Clinical Research Center at University of CT Health Center. DHEAS was measured by an immunoassay (Diagnostic Products Corporation, Immulite 1000) with an intra-assay CV of <5.2%.
Statistical analysis
Descriptive statistics (mean ± standard deviation or count/proportion) were calculated for each study variable. The statistical significance of differences in the values of these statistics between the three groups of subjects that were pooled to create this study's sample was evaluated via the Wilcoxon rank-sum test, the Pearson χ2 test or Fisher's exact test, as appropriate.
A contingency table was created to summarise variation in the frequency of frailty categories (0 = Non-Frail, 1 = Intermediate Frail, 2 = Intermediate Frail and 3–5 = Frail) relative to DHEAS quartiles (15.0–27.3, 27.4–46.3, 46.4–78.1 and 78.3–323.0 μg/dL). The Pearson χ2 test was applied to assess the statistical significance of the relationship between frailty categories and DHEAS levels.
The potential that either an observed association or lack of association between frailty and DHEAS could be due to confounding by other subject characteristics was evaluated. The Pearson χ2 test was applied to investigate relationships between frailty and these other characteristics [study (ALF, DHEA/EX, TEST), gender, age (60–69, 70–79, 80+) and body mass index (<25, 25–30, 30+)].
Multivariable ordinal logistic regression was used to establish the relationship between frailty and DHEAS while controlling for the potential confounding effects of other subject characteristics. Frailty was the dependent variable in the modelling process. DHEAS was the primary independent variable; it was represented on a continuous scale after a logarithmic transformation (base 10) to account for extreme skewness. Categorical covariates included study and gender. Continuous covariates included age and body mass index. Initially, the model assessed the ‘main’ effect of DHEAS while controlling for the other covariates. We then augmented the model to consider the possibility of interaction between DHEAS and the other covariates. Consistency of the data with the proportional odds assumption that underlies ordinal logistic regression was evaluated using the method of Brant [13].
Analyses were performed using the SPSS (version 15.0, SPSS, Inc., Chicago, IL, USA) and Stata (version 9.2, StataCorp, College Station, TX, USA) software packages. A 5% threshold was used for declaring a statistical significance in all tests.
Results
The full study sample (n = 898) was predominantly male (65.8%) with a mean age of 74.6 years (SD = ±7.7) (Table 1). Most subjects had zero (34.9%) or one (44.4%) frailty component. The mean DHEAS level was 59.2 ± 44.6 μg/dL. Not surprisingly, significant differences were present in subject characteristics when subdivided into respective studies (Table 1). Mean age (P < 0.0001) and weight loss (P = 0.045) were moderately lower in the TEST sample compared to those in the DHEA/EX sample. As expected, the men in TEST had a mean level of DHEAS (71.5 μg/dL) substantially larger than that for the women in DHEA/EX (38.8 μg/dL) (P < 0.0001).
Table 1.
Characteristics of study subjects (n/% or mean ± SD)
| Results for individual studies | ||||
|---|---|---|---|---|
| Overall | TEST | DHEAEX | ALF | |
| Gender | ||||
| Male | 591/65.8% | 564/100% | N/A | 27/27.6% |
| Female | 307/34.2% | N/A | 236/100% | 71/72.4% |
| Age (years) | 74.6 ± 7.7 | 72.9 ± 7.9 | 75.5 ± 6.0 | 82.3 ± 5.5 |
| BMI (kg/m2) | 27.1 ± 4.4 | 27.5 ± 3.8 | 27.6 ± 5.6 | 24.7 ± 3.3 |
| DHEAS (μg/dL) | 59.2 ± 44.6 | 71.5 ± 49.1 | 38.8 ± 25.3 | 37.8 ± 22.0 |
| Frailty assessment components | ||||
| 8-foot walking speed (s) | 2.6 ± 1.2 | 2.4 ± 0.7 | 2.6 ± 1.1 | 3.8 ± 2.4 |
| PASE (kcal) | 1315 ± 1173 | 1565 ± 1309 | 1036 ± 753 | 545 ± 510 |
| Handgrip strength (kg) | 25.6 ± 11.2 | 31.2 ± 9.2 | 17.7 ± 6.4 | 12.6 ± 7.9 |
| Weight loss | 50/5.6% | 29/5.1% | 21/8.9% | 0/0.0% |
| Exhaustion | 49/5.5% | 31/5.5% | 12/5.1% | 6/6.1% |
| Number of frailty components | ||||
| 0 (non-frail) | 313/34.9% | 230/40.8% | 78/33.1% | 5/5.1% |
| 1 (intermediate) | 399/44.4% | 244/43.3% | 125/53.0% | 30/30.6% |
| 2 (intermediate) | 135/15.0% | 70/12.4% | 25/10.6% | 40/40.8% |
| 3 (frail) | 40/4.5% | 16/2.8% | 5/2.1% | 19/19.4% |
| 4 (frail) | 11/1.2% | 4/0.7% | 3/1.3% | 4/4.1% |
| 5 (frail) | 0/0.0% | 0/0.0% | 0/0.0% | 0/0.0% |
TEST, individuals recruited for a study of testosterone replacement; DHEAEX, individuals recruited for a study of DHEA replacement/exercise; ALF, individual recruited for a study from assisted living and age and gender matched community living adults; DHEAS dehydroepiandrosterone sulphate; PASE, physical activity scale in the elderly; N/A, not applicable.
The characteristics of individuals recruited to the ALF study differed from those of the combined TEST and DHEA/EX samples (Table 1). The ALF participants were substantially older (82.4 years) (P < 0.0001). Consistent with their older ages, the ALF subjects had smaller mean BMI, slower 8-foot walking speed, lower physical activity levels and reduced handgrip strength (P < 0.0001 for each assessment). However, none of the ALF subjects reported weight loss, as compared to 5.5% and 5.1%, respectively, in the TEST and DHEA/EX studies (P = 0.01). In contrast to subjects in the other two studies, most ALF subjects (94.9%) had one or more frailty component, with 23.5% having three or four components and being classified as ‘frail’ (P < 0.0001). The mean DHEAS level in the ALF participants (37.8 μg/dL) was significantly lower than that for the combined TEST and DHEAS/EX samples (P < 0.0001), but was similar to that among women in the DHEA/EX study (38.8 μg/dL).
A cross-tabulation of frailty categories by DHEAS quartiles (Table 2(a)) suggested a significant association between these variables (P < 0.0001). For example, 48% of subjects in the highest quartile of DHEAS values had zero frailty components. This percentage consistently decreased (48% vs. 39% vs. 28% vs. 25%) across the three lower quartiles. Conversely, the percentage of subjects classified as ‘frail’ (three to five frailty components) increased from the highest quartile to the lowest (3% vs. 5% vs. 5% vs. 9%). The distribution of frailty category differed relative to a number of other factors, including ‘study’ (TEST vs. DHEA/EX vs. ALF), gender and age (Table 2(b)). Frailty was more common in the ALF study, among women and in those over 80 years.
Table 2.
Relationship between DHEAS and frailty and frailty with other study predictors
| Frailty categories (count/%) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3–5 | Total | P-value | |
| Non-frail | Intermediate | Intermediate | Frail | |||
| (a) Frailty categories versus DHEAS quartiles | ||||||
| DHEAS quartiles (μg/dL) | <0.0001 | |||||
| 15.0–27.3 | 56/25% | 109/49% | 40/18% | 19/9% | 224/100% | |
| 27.4–46.3 | 64/28% | 108/48% | 41/18% | 12/5% | 225/100% | |
| 46.4–78.1 | 87/39% | 96/43% | 31/14% | 12/5% | 226/100% | |
| 78.3–323.0 | 106/48% | 86/39% | 23/10% | 8/3% | 223/100% | |
| (b) Frailty categories versus other study predictors | ||||||
| Study predictors | <0.0001 | |||||
| ALF | 5/5.1% | 30/30.6% | 40/40.8% | 23/23.5% | 98/100.0% | |
| DHEAEX | 78/33.1% | 125/53.0% | 25/10.6% | 8/3.4% | 236/100.0% | |
| TEST | 230/40.8% | 244/43.3% | 70/12.4% | 20/3.5% | 564/100.0% | |
| Gender | 0.0005 | |||||
| Females | 233/39.4% | 251/42.5% | 79/13.4% | 28/9.1% | 307/100.0% | |
| Males | 80/26.1% | 148/48.2% | 56/18.2% | 23/3.9% | 591/100.0% | |
| Age (years) | <0.0001 | |||||
| 60–69 | 137/55.9% | 82/33.5% | 18/7.3% | 8/3.3% | 245/100.0% | |
| 70–79 | 143/36.4% | 193/49.1% | 44/11.2% | 13/3.3% | 393/100.0% | |
| 80+ | 33/12.7% | 124/47.7% | 73/28.1% | 30/11.5% | 260/100.0% | |
| BMI (kg/m2) | 0.32 | |||||
| <25 | 106/35.5% | 130/43.5% | 46/15.4% | 17/5.7% | 299/100.0% | |
| 25–30 | 154/37.7% | 170/41.7% | 60/14.7% | 24/5.9% | 408/100.0% | |
| 30+ | 53/27.7% | 99/51.8% | 29/15.2% | 10/5.2% | 191/100.0% | |
Ordinal logistic regression was used to model the relationship between frailty and log-transformed DHEAS, adjusting for study, age, gender and BMI. The modelling process revealed that the magnitude of the association between frailty and DHEAS was dependent on BMI. In addition, BMI alone (per kg/m2 at a DHEAS of 46.3 μg/dL; OR = 1.08; 95% CI 1.05,1.12, P < 0.001), age (per year; OR = 1.10; 95% CI 1.08, 1.12; P < 0.0001) and study (with TEST as reference, DHEA/EX OR = 0.85; 95% CI 0.35, 2.06; P = 0.72 and ALF OR = 5.25; 95% CI 2.42, 11.4; P < 0.0001) were significant contributors to the modelling while gender (male as reference; female OR = 0.99; 95% CI 0.43,2.28; P = 0.98) was not. A significant DHEAS–BMI interaction (OR = 1.17; 95% CI 1.05, 1.30; P = 0.003) suggested that, in general, the relationship between higher levels of frailty decreased relative to higher levels of DHEAS, but that the magnitude of the decrease was larger at lower BMI values and smaller at higher BMI values. For example, if using this model, the relationship between being more frail decreased by a factor of 0.41 (95% CI 0.23, 0.74; P = 0.003) for every one unit increase in the logarithmic value of DHEAS when BMI was 24.2 (the lowest BMI quartile cut-off) or less. In contrast, being more frail decrease only by a factor of 0.94 (95% CI 0.55, 1.59; P = 0.81) for every one unit increase in the logarithmic value of DHEAS when BMI was 29.4 (the highest BMI quartile cut-off) or more. Having an intermediate BMI (26.7 kg/m2; 50th percentile) resulted in an intermediate change (0.61;95% CI 0.38, 0.99; P = 0.047).
When the ‘raw’ data were simultaneously cross-classified by frailty category, DHEAS quartile and BMI level, the modifying effect of BMI on the association between frailty and DHEAS was evident (Figure 1). However, this graphical representation of the association suggested that there might be a threshold such that the inverse relationship between frailty and DHEAS held for BMI values below the threshold and was weaker or absent for BMI values above the threshold. Further modelling (with adjustment for the other covariates) supported this conjecture. The inverse relationship between DHEAS and higher levels of frailty was strongest when BMI was <29 kg/m2 (OR = 0.60, 95% CI = (0.36, 0.97, P = 0.04) and substantially weaker (OR = 0.82, 95% CI = (0.50, 1.37, P = 0.45) when BMI exceeded 29 kg/m2.
Figure 1.
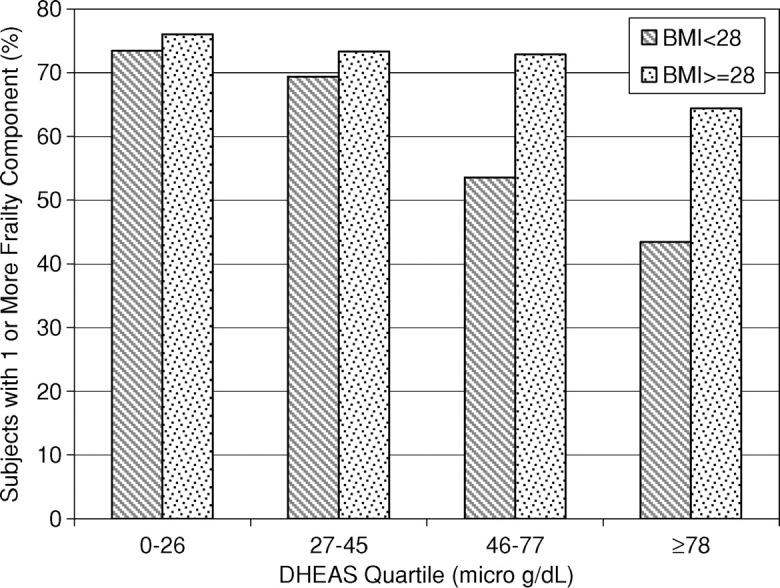
The Association between DHEAS and BMI in those with some frailty characteristics. The portion of subjects with frailty decreases with higher DHEAS levels in those with a BMI of <28 kg/m2, but this association is attenuated in those whose BMI ≥ 28 kg/m2.
Discussion
In this cross-sectional study of 932 older men and women, we found age, DHEAS and a DHEAS/ BMI interaction associated with higher frailty categorization. We combined subjects from three study populations so that a representative mix of healthy and frail adults were featured. Our study subjects were similar to other populations in regard to the percentage of non-frail, intermediate frail and frail categories [9].
Our study found a negative correlation between DHEAS and frailty levels. We confirmed previous findings by Leng et al. [14], but with a larger sample size. Others found higher DHEAS levels correlated with better self-reported physical function [15] or better assessed physical function [16] or measured VO2max [17]. These studies all support the relationship between DHEAS and frailty. In contrast, a recent cross-sectional study of 60 heart failure patients failed to find an association between frailty and DHEAS [18] and a randomized, controlled trail of older men, treated with 50 mg DHEA/day, detected no changes in upper and lower extremity strength or physical performance [19].
Several previous studies examined the relationship between DHEA and BMI with conflicting findings. Barrett-Connor and Ferrara [20], in a study of post-menopausal women, found DHEAS levels to be directly associated with central obesity. Conversely, DHEA levels are inversely related with abdominal obesity or BMI > 30 kg/m2 in middle age and elderly men [21, 22]. Abbasi et al. [17] found DHEAS to be positively correlated to BMI in women 60–80 years old, but not in men.
While the studies on the relationship between obesity and DHEA levels have been conflicting, frailty has been linked to higher BMI in a syndrome known as ‘sarcopenic obesity’ [23, 24]. The association between frailty and a higher BMI may explain the attenuation of the association between DHEA and frailty at higher BMI. The mechanism may be direct. Sarcopenic obesity is associated with a decrease in strength and a decrease in mobility, two factors associated with frailty [25, 26]. The mechanism may also be indirect, as obesity is related to several biochemical markers associated with frailty, including elevated levels of inflammatory markers such as IL-6 and C-reactive protein and lower antioxidant capacity [27, 28]. Blaum et al. [29] evaluated the association between obesity and frailty in elderly women and found that overweight (BMI 25–29.99 kg/m2) individuals were more likely to be classified as intermediate frail and obese individuals (BMI > 30 kg/m2) were more likely to be frail. We did not find a direct association between BMI and frailty, but did find an interaction between BMI and DHEAS that was associated with frailty status. DHEAS exhibited a protective effect against frailty in those with normal or overweight BMI, but the effect was lost in those with BMI > 30 kg/m2 and may be explained by the biochemical markers associated with frailty, lower antioxidant capacity or the increased disabilities related to sarcopenic obesity. To our knowledge, no other study has evaluated BMI and DHEAS for an association with frailty.
We found that age had a significant association with frailty; for every increase in 5 years, the association corresponded to an increase in frailty characteristics. This finding is supported by other work. Frailty and age are associated with incidence increasing from 3.2% of individuals aged 65–70 to 25.7% of individuals aged 85–89 [9].
We found no gender interactions in the association between frailty and DHEAS. Differences in DHEAS levels between sexes have been described by others [1]. Further, other studies have found an association between DHEAS levels and physical performance in men, but not women [17, 30]. While we found an association between frailty and DHEAS levels, the association did not differ by gender.
Our study has limitations. This study is cross-sectional and does not address whether interventions to improve DHEAS levels or modification in BMI will impact frailty status. The individuals involved in this analysis were recruited for three other clinical trails and the differences in those recruited were significant. While the men and women recruited for interventions of testosterone or DHEA supplementation were similar (∼3–3.5% meeting frail criteria), individuals recruited from assisted living or age- and gender-matched community controls were older and more likely to be frail (25%). The differences in the recruitment populations are evident in the logistic regression. The goal of combining the studies was to have a sample with a full range of frailty factors, and ‘study’ was included in the analysis to control for the bias that may have been introduced.
Conclusion
This study found an association between frailty and DHEAS levels, similar to studies that have found associations between DHEA and physical function. Whether the association is due to similar conditions resulting in lower DHEA levels and more susceptibility to frailty or whether lower DHEA levels have an impact on increasing frailty cannot be addressed by this cross-sectional analysis. We did not find whether gender altered the association but did find that obesity or BMI> 30 kg/m2 attenuated the association between higher DHEA levels and lower frailty status.
Key points
The role of DHEA in frailty is uncertain, although low DHEA levels have been associated with increased rates of morbidity and mortality.
In this cross-sectional analysis of a population of 898 older adult men and women, higher DHEAS levels were associated with fewer frailty characteristics.
A body mass index >30 kg/m2 attenuated the association found between DHEAS levels and frailty.
Further research will need to be done to ascertain whether the associations are due to similar conditions or whether lower DHEAS levels impact increasing frailty.
Acknowledgments
This work has been supported by the R01-AG18887, NNG04GK63G, General Clinical Research Center (MO1-RR06192), and a grant from the International Society for Clinical Densitometry. In addition, we wish to thank Alison Kleppinger for her assistance with data management.
Conflicts of interest
All authors report no conflict of interest. Ms Voznesensky was involved in study concept and design, acquisition of subjects and preparation of manuscript. Dr Walsh and Ms Dauser were involved in data analysis, interpretation of data and preparation of manuscript. Ms Brindisi was involved in acquisition of subjects and data, analysis of data and preparation of manuscript. Dr Kenny was involved with study concept and design, acquisition of subjects and data, analysis and interpretation of data and manuscript preparation.
References
- 1.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–5. doi: 10.1210/jcem-59-3-551. 1996; 81(9): 3147–51. [DOI] [PubMed] [Google Scholar]
- 2.Perrini S, Laviola L, Natalicchio A, Giorgino F. Associated hormonal declines in aging: DHEAS. J Endocrinol Invest. 2005;28(3 Suppl):85–93. [PubMed] [Google Scholar]
- 3.Thomas G, Frenoy N, Legrain S, Sebag-Lanoe R, Baulieu EE, Debuire B. Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab. 1994;79(5):1273–6. doi: 10.1210/jcem.79.5.7962319. [DOI] [PubMed] [Google Scholar]
- 4.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997;12(11):1833–43. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 5.Ravaglia G, Forti P, Maioli F, et al. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab. 1996;81(3):1173–78. doi: 10.1210/jcem.81.3.8772596. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117(10):807–11. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 7.LaCroix AZ, Yano K, Reed DM. Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation. 1992;86(5):1529–35. doi: 10.1161/01.cir.86.5.1529. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Edelstein SL. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: the Rancho Bernardo Study. J Am Geriatr Soc. 1994;42(4):420–23. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 Suppl):1–29. [PubMed] [Google Scholar]
- 11.Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128(2 Suppl):351S–5S. doi: 10.1093/jn/128.2.351S. [DOI] [PubMed] [Google Scholar]
- 12.Greenlund LJ, Nair KS. Sarcopenia–consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124(3):287–99. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 13.Brant R. proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46:1171–78. [PubMed] [Google Scholar]
- 14.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 15.Berkman LF, Seeman TE, Albert M, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46(10):1129–40. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 16.Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6(4):277–84. [PubMed] [Google Scholar]
- 17.Abbasi A, Duthie EH, Sheldahl L, et al. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc. 1998;46(3):263–73. doi: 10.1111/j.1532-5415.1998.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 18.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56(3):454–61. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 19.Muller M, Van Den Beld AW, Van Der Schouw YT, Grobbee DE, Lamberts SW. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. J Clin Endocrinol Metab. 2006;91(10):3988–91. doi: 10.1210/jc.2005-2433. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1996;81(1):59–64. doi: 10.1210/jcem.81.1.8550794. [DOI] [PubMed] [Google Scholar]
- 21.Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord. 1993;17(11):643–9. [PubMed] [Google Scholar]
- 22.Hsieh CC SL, Lipworth L, Lagiou P, Mantzoros CS, Trichopoulos D. Predictors of sex hormone levels among the elderly: a study in Greece. J Clin Epidemiol. 1998;51(10):837–41. doi: 10.1016/s0895-4356(98)00069-9. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 24.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 25.Himes CL. Obesity, disease, and functional limitation in later life. Demography. 2000;37(1):73–82. [PubMed] [Google Scholar]
- 26.Lee JS, Kritchevsky SB, Tylavsky F, et al. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2005;60(8):1007–12. doi: 10.1093/gerona/60.8.1007. [DOI] [PubMed] [Google Scholar]
- 27.Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: The ATTICA study. Nutr Metab Cardiovasc Dis. 2007;63:155–61. doi: 10.1016/j.numecd.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the women's health and aging studies. J Am Geriatr Soc. 2005;53(6):927–34. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 30.Schaap LA, Pluijm SM, Smit JH, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63(2):152–60. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]


