Abstract
Multiple putative free fatty acid (FFA) transduction mechanisms have been identified in the oral cavity. They reportedly differ in their distribution on the tongue and each has a unique range of ligand specificities. This suggests that there should be regional differences in sensory responses to varying FFAs. This was assessed through spatial testing with caproic (C), lauric (L), and stearic (S) FFAs among 35 healthy adults. Stimuli were applied to the fungiform (FU), foliate (FO), and circumvallate (CV) papillae with a cotton-tipped applicator. Oral detection thresholds were measured by an ascending, 3-alternative, forced-choice, sip and spit procedure. Intensity ratings were obtained on the general labeled magnitude scale. Nongustatory cues were minimized by testing with the nares blocked, eyes covered, and by masking tactile cues with the addition of gum acacia and mineral oil to the stimuli vehicle. Thresholds were obtained from nearly all individuals at each site, and the concentration was similar across the 3 FFAs. Absolute intensity ratings differed significantly with C > L > S overall and at the CV and FO papillae. At the FU papillae, the L and S ratings were comparable. Ratings were highest at the FU followed by the CV and then the FO papillae. Slopes of the concentration–intensity rating functions were higher for L compared with C and S at the CV papillae as well as both L and C compared with S at the FO papillae. However, overall, slopes were comparable across sites. These findings strengthen evidence for oral FFA perception in humans by replicating threshold sensitivity findings and documenting monotonic scaling ability for these stimuli. Further, they challenge current views on transduction as sensory responsiveness was observed at tongue sites not predicted to support FFA detection.
Keywords: chemosensory, fat taste, taste threshold, taste transduction
Introduction
Human psychophysical studies can both inform and confirm basic research on chemosensory physiology as well as clinical assessment. One powerful tool is spatial testing (Bartoshuk 1989), which allows isolation of peripheral transduction mechanisms, neural pathways, and central coding processes. For example, recent applications reveal regional differences in taste responsiveness to sweetness (Warnock and Delwiche 2006) and saltiness (Grover and Frank 2008), suggestive of variations in transduction mechanisms for these taste qualities at different sites. The technique was used in the present study to explore regional differences in free fatty acid (FFA) detection in the oral cavity with the aim of identifying likely transduction mechanisms.
Psychophysical studies using whole-mouth stimulation document that humans can detect FFAs in the oral cavity when nongustatory cues are minimized (Nasser et al. 2001; Kamphuis et al. 2003; Chale-Rush et al. 2007a; Mattes 2008). This has been demonstrated for FFAs of common saturation but varying in chain length (at least from C:6 to C:18), as well as varying degree of saturation (i.e., polyunsaturated, monounsaturated, and saturated) with constant chain length. However, attribution of these results to taste remains problematic. FFAs are odorous irritants that contribute tactile sensations, so isolation of a taste component is difficult. In addition, there is a lack of consensus on the existence and nature of transduction mechanisms for the detection of FFAs.
A number of putative receptors for FFAs have been proposed, including delayed rectifying potassium channels (DRK), CD36, and several G-protein–coupled receptors (e.g., GPCR40, GPCR41, GPCR43, and GPCR120), but none alone binds the array of FFAs detected by humans. DRKs are blocked by unsaturated long-chain FFAs (Gilbertson et al. 1997); CD36 and GPCR120 bind FFAs with >14 carbons (Baillie et al. 1996; Ibrahimi et al. 1996; Hirasawa et al. 2005); GPR40 binds medium- and long-chain FFAs (Briscoe et al. 2003; Itoh et al. 2003), whereas GPCR41 and GPCR43 only bind short-chain FFAs (Brown et al. 2003; Le Poul et al. 2003; Xiong et al. 2004). To date, based primarily on mice and rat data, only DRK and GPCR120 have been localized to the apical membrane of cells in fungiform (FU) papillae (Gilbertson et al. 1997; Damak et al. 2007; Matsumura et al. 2007). All but GPCR40 have been identified in foliate (FO) papillae (Fukuwatari et al. 1997; Laugerette et al. 2005; Hansen et al. 2006; Damak et al. 2007; Matsumura et al. 2007), and all are reportedly present in circumvallate (CV) papillae (Fukuwatari et al. 1997; Laugerette et al. 2005; Hansen et al. 2006; Damak et al. 2007; Matsumura et al. 2007). Consequently, current knowledge would lead to the hypothesis that medium-chain FFAs should not be detectable at FO and FU papillae and short-chain FFAs should not be detectable at FU papillae. Further, it is likely that an array of FFAs would be present in the oral cavity when consuming a high-fat food (e.g., Kintner and Day 1965; Brown et al. 1979; Woo and Lindsay 1983; Woo et al. 1984; Molteberg et al. 1995), so if the number of receptors activated influences FFA intensity ratings, it might be predicted that intensity ratings would be rank ordered as CV > FO > FU. However, if there are other unidentified receptor systems or if detection is based on nonspecific mechanisms, such as FFA diffusion across taste cell membranes with subsequent activation of intracellular signaling systems, there may be no regional differences. Evidence for the latter mechanism has been provided for sodium chloride (NaCl) and acids where Na and protons pass through ion channels (DeSimone and Lyall 2006) as well as selected sweet and bitter stimuli capable of diffusing through the taste cell membranes (DeSimone 2000; Peri et al. 2000; Zubare-Samuelov et al. 2005). Threshold and suprathreshold responses to FFAs varying in chain length were obtained from different tongue regions to test these hypotheses.
Materials and methods
Participants
Eligibility criteria included the following: 18–60 years of age, healthy, nonsmoker, and normal orosensory function. Interested participants were required to provide written informed consent and were remunerated for their time and effort. The protocol was approved by the University Institutional Review Board.
General protocol
Prospective participants responding to public announcements about the study completed an extensive screening questionnaire eliciting information about general health, diet, activity, and selected personality traits. Those meeting age, health, and smoking status eligibility criteria were invited to a sensory screening session. This session entailed measurement of threshold sensitivity for sucrose on the anterior dorsal, posterior lateral, and posterior central tongue. Participants with sucrose thresholds falling within published normative ranges at each site were recruited for further evaluation. NaCl and propylthiouracil (PROP) were measured by whole-mouth exposure, and findings were only used for descriptive purposes. Participants were then scheduled for three 30- to 60-min sessions over a 3-week period. During these sessions, detection thresholds and suprathreshold intensity ratings were obtained for stearic acid, lauric acid, and caproic acid at each of the 3 tongue sites.
Sensory stimuli
Taste thresholds for sucrose were assessed using a concentration range from 0.0001 to 1.0 M, with dilutions by 0.25 log units. Intensity ratings were obtained for 5 PROP concentrations 3.2 × 10−5, 1.0 × 10−4, 3.2 × 10−4, 1.0 × 10−3, and 3.2 × 10−3 mol/l and 5 NaCl concentrations 0.01, 0.032, 0.1, 0.32, and 1.0 mol/l NaCl. All stimuli were prepared in deionized water. The FFAs were homogenized in deionized water containing 0.01% ethylenediaminetetraacetic acid, 5% gum acacia, and 5% mineral oil. The FFA stimuli were made daily and stored in tightly sealed light-protected bottles. Concentrations ranged from 0.00028% to 5%, with dilutions differing by 0.25 log units. Because stearic and lauric acids are solids at room temperature, they were presented at 67–69 °C. Caproic acid and the blank were also presented at 67–69 °C for consistency.
Spatial testing procedure
Participants reported to the laboratory for the screening and 3 test sessions after having refrained from food, beverage, and oral care product exposures for at least 2 h prior to arrival. All testing was conducted with participants wearing blindfolds to minimize visual cues and noseclips to minimize olfactory stimulation. For sucrose and FA threshold testing, stimuli were presented in an ascending, 2-alternative, forced-choice, procedure. Three areas of the tongue were assessed: the anterior tongue (FU papillae), posterior lateral tongue (FO papillae), and posterior central tongue (CV papillae). In random order, stimuli were applied via sterile cotton swabs on one side of the tongue and a blank, the vehicle without a FA, on the other side. The participant was then asked to identify which side of the tongue received the stimulus. Participants rinsed with deionized water between samples. This procedure continued until the participant correctly identified the stimulus at a given concentration 3 times. Participants completed testing for a single FFA per session. Following threshold determination, 5 suprathreshold concentrations of the FFA tested on that day were presented to each tongue site. Participants rated their intensity on a general Labeled Magnitude Scale (gLMS) scale presented with Compusense 4.8 software, expectorated, and rinsed with deionized water between samples. The scale descriptors and geometric mean values were strongest imaginable (1.98), very strong (1.70), strong (1.52), moderate (1.21), weak (0.76), barely detectable (0.14), and no sensation (0.00). Duplicate ratings were obtained.
PROP classification
Classification of PROP taster status was determined by participants’ PROP/NaCl intensity ratio. Participants rated the perceived intensity of the 5 concentrations of each stimulus. Stimuli were presented in random order at room temperature. Participants placed 10 ml of the sample in their mouth, rated the perceived intensity on a gLMS scale presented with Compusense 4.8 software, and expectorated. Participants rinsed with deionized water between samples. If the PROP/NaCl ratio was less than 0.8, between 0.8 and 1.2, or greater than 1.2, participants were classified as nontasters, tasters, and supertasters, respectively (Kamphuis and Westerterp-Plantenga 2003).
Statistical analyses
An initial data review revealed that the threshold data were skewed. Thus, the data are presented as medians with the semi-interquartile range (SIQR = [75th–25th% score]/2) as the index of variance. Nonparametric statistics (i.e., Friedman tests) were used for analyses of these data. No stearic acid threshold value was obtained for 2 individuals at the CV site, 1 at the FO site, and 3 at the FU site. For lauric acid, no threshold was measured for 2 people at the CV site and 4 people at the FU site. It was not the same individuals who were generally less sensitive. When no threshold was obtained, the highest concentration tested (5% w/w) was entered as the threshold value. Given the use of nonparametric statistics, this had little impact on the analyses. Testing was also conducted omitting these individuals with no substantive change in findings. Intensity ratings were contrasted by repeated measures analysis of variance (ANOVA) with stimulation site (CV, FO, and FU) and FFA stimulus (stearic, lauric, and caproic) as within factors. Analyses were conducted with the raw data and log-transformed data. The former better reveal concentration ranges where sensation growth may differ according to stimulus of site, whereas the latter provides a measure of intensity gain over the full range of stimuli tested. Associations were evaluated by Pearson correlation coefficients. Documentation of sex, BMI, and PROP taster effects was not an objective of the trial. However, exploration of differences in FA sensitivity between the small subgroups of participants with these characteristics (i.e., 13 males and 22 females; 22 lean, 8 overweight and 5 obese; 7 nontasters and 21 tasters; 7 supertasters) revealed no significant effects or trends; thus, the pooled sample was used in all analyses. Where multiple comparisons were tested, the Bonferroni correction was applied. The criterion for statistical significance was P < 0.05, 2 tailed.
Results
Participants
Data were collected from 35 men (N = 13) and women (N = 22). Their mean age was 23.7 ± 0.6 years, and their mean BMI was 24.5 ± 0.8 kg/m2. Twenty-nine self-classified themselves as white, nonhispanic, 1 as African American, 1 as Asian, and 4 as “other.”
Thresholds
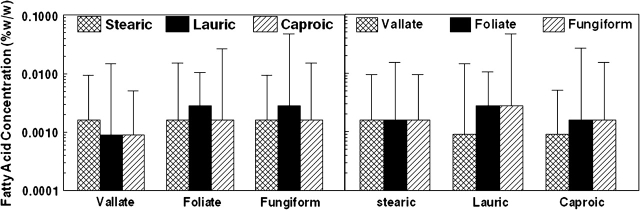
Median (SIQR) threshold concentrations for stearic, lauric, and caproic FAs measured at the CV, FO, and FU sites are presented in Figure 1. The left panel displays data by site of stimulation, whereas the right panel groups thresholds by FFA type. No significant differences were observed across sites or FFAs. The variance in threshold values was extremely large, generally 4 orders of magnitude. For stearic acid at the CV, FO, and FU sites, the ranges were 0.0003–1.6% w/w, 0.00028–2.8% w/w, and 0.0003–1.6% w/w, respectively. The ranges for lauric acid were 0.0028–1.6% w/w, 0.0003–0.9% w/w, and 0.0003–1.6% w/w and for caproic acid they were 0.0003–0.5% w/w, 0.00003–0.9% w/w, and 0.0003–0.9% w/w, respectively. Thresholds for FFAs at the 3 tongue sites were significantly correlated indicating the stability of the values. For stearic and lauric acids, all correlations were P < 0.001. This was also the case for caproic except for 2 comparisons where the significance was P = 0.001 and P = 0.012.
Figure 1.
Median (SIQR) detection threshold values at the CV, FO, and FU papillae sites for stearic (C:18), lauric (C:12), and caproic (C:6) FFAs (left panel) and the median (SIQR) detection thresholds for stearic, lauric, and caproic FFAs at CV, FO, and FU sites (right panel).
Intensity ratings
Correlation coefficients between duplicate ratings for each FFA at each test site were uniformly high (r = 0.42–0.89) and statistically significant (all P < 0.015). Thus, means were computed and used for further analyses. A repeated measures ANOVA with tongue site, fat type, and concentration as within-subject factors revealed significant main effects for fat type [F(2,68) = 142.6, P < 0.001] and concentration [F(4,136) = 132.1, P < 0.001], as well as interactions for tongue site × fat type [F(4,136) = 3.86, P = 0.005], tongue site × concentration [F(8,272) = 2.32, P = 0.02], and fat type × concentration [F(8,272) = 9.16, P < 0.001]. Collapsed over tongue sites and concentration, the absolute intensity ratings of the 3 FFAs differed with caproic (mean [standard error {SE}] = 1.53 [0.03]) > lauric (0.92 [0.45]) > stearic (0.81 [0.06]). Averaged over the 3 tongue sites and 3 FFAs, intensity ratings for all concentrations differed from each other and increased monotonically (0.82 [0.045], 1.01 [0.04], 1.11 [0.04], 1.21 [0.04], and 1.28 [0.04]). The various interactions were explored further as described below.
Intensity ratings by stimulation site
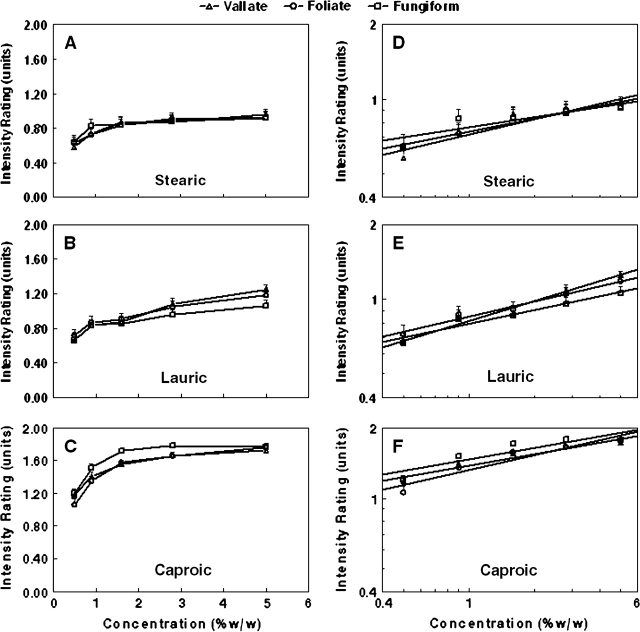
The absolute intensity ratings differed significantly at each of the 3 tongue sites with the highest ratings for stimulation of FU (mean [SE] = 1.099 [0.042]) followed by CV (1.082 [0.043]) and then FO (1.077 [0.043]) papillae. Intensity ratings for the 5 FFA stimulus concentrations obtained at the 3 tongue sites are plotted in Figure 2 grouped by FFA. Plots on the left are raw scores, and plots on the right are the same data plotted on log–log axes to better highlight the change of intensity with concentration.
Figure 2.
Intensity ratings at CV, FO, and FU sites for stearic (C:18), lauric (C:12), and caproic (C:6) FFAs. Plots on the left are raw data, and plots on the right are least-square regression lines plotted on log–log coordinates.
At all 3 stimulation sites, there were significant main effects of fat type (all P < 0.001), concentration (all P < 0.001), and a fat type by concentration interaction (all P < 0.001). With respect to fat type, at all sites, absolute ratings for caproic acid were significantly higher than for the other 2 FFAs (both P < 0.001). Ratings for lauric acid were higher than for stearic acid at the CV (P = 0.033) and FO (P = 0.02) sites but were similar at the FU site. The concentration effect reflected the ability of participants to discriminate between all concentrations at all sites. The interaction effect was attributable to the greater initial rise of intensity with caproic acid compared with lauric and stearic. However, over the full concentration ranges, the slopes of the concentration–intensity rating functions were similar.
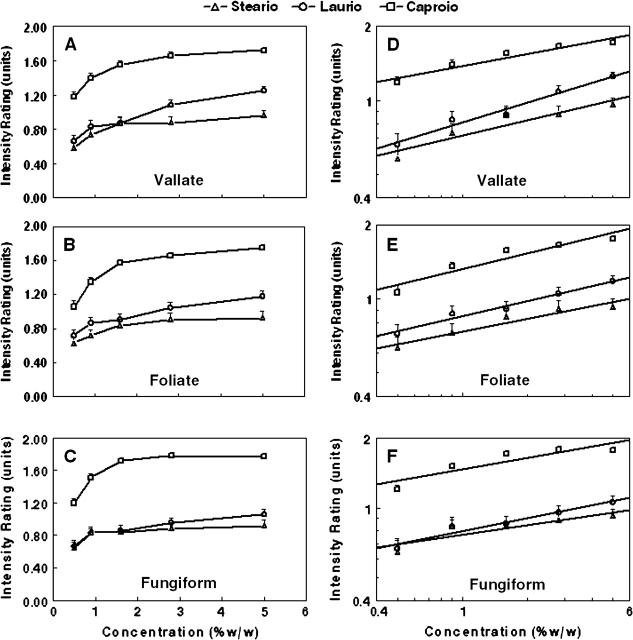
Intensity ratings by FFA
Figure 3 contains the data contrasting the FFA intensity ratings for each FFA at the 3 stimulation sites. The left plots display the raw data, whereas the right plots depict the least-square regression lines on log–log coordinates of the same data to highlight the growth of sensation at each site with increasing concentration. Although there was a significant concentration effect [F(4,136) = 23.06, P < 0.001], there was no main effect of site or a site by concentration interaction for stearic acid. The same results held for lauric acid. However, for caproic acid, there were significant main effects of site [F(4,68) = 7.03, P = 0.002] and concentration [F(4,136) = 116.8, P < 0.001] as well as a significant site by concentration interaction [F(8,272) = 2.8, P = 0.005]. Ratings for caproic acid were significantly higher at the FU site compared with the other 2 sites (both P < 0.001). Ratings at the FO site were also significantly greater than those at the CV site (P = 0.019).
Figure 3.
Intensity ratings for stearic (C:18), lauric (C:12), and caproic (C:6) FFAs at CV, FO, and FU sites. Plots on the left are raw data, and plots on the right are least-square regression lines plotted on log–log coordinates.
Interactions and associations
The ability of participants to monotonically rate the intensity of the 3 FFAs at each stimulation site was assessed by determining the least-square regression line of the log-transformed concentration–intensity judgment data for the 6 combinations and calculating the coefficients of determination to assess the goodness of fit. Table 1 contains the mean slope values, coefficients of determination, and the proportion of coefficients <0.5. A repeated measures ANOVA revealed a significant [F(2,68) = 4.48, P = 0.015] effect of intensity ratings at the CV papillae such that the slopes were significantly lower for caproic acid than lauric acid (P = 0.023) and a trend for stearic acid to be lower than lauric (P < 0.07). Further, the slope of responses for stearic acid was significantly lower than either lauric (P = 0.016) or caproic (P = 0.012) acids at the FO papillae. No significant differences were noted at the FU papillae. There were marked differences in the goodness of fit of the functions. The proportion of coefficients of determination below 50% was only one-third to one-half as common for the caproic acid samples compared with the stearic and lauric acids. Poor fit is interpreted as poor scaling ability. No individual had a coefficient of determination value of <0.5 for all trials.
Table 1.
Indices of suprathreshold intensity ratings for stearic, lauric, and caproic FAs rated at the CV, FO, and FU papillae areas
| Mean (SE) slope | Mean (SE) R2 | Proportion of slopes with R2 < 0.5 | |
| Stearic-CV | 0.22 (0.06) | 0.61 (0.06) | 37.1 |
| Stearic-FO | 0.10 (0.03) | 0.48 (0.06) | 54.3 |
| Stearic-FU | 0.16 (0.05) | 0.56 (0.06) | 47.1 |
| Lauric-CV | 0.37 (0.06) | 0.56 (0.06) | 40.0 |
| Lauric-FO | 0.26 (0.05) | 0.53 (0.05) | 42.9 |
| Lauric-FU | 0.21 (0.06) | 0.55 (0.05) | 37.1 |
| Caproic-CV | 0.18 (0.05) | 0.72 (0.04) | 11.4 |
| Caproic-FO | 0.25 (0.03) | 0.72 (0.04) | 14.3 |
| Caproic-FU | 0.18 (0.02) | 0.69 (0.04) | 20.0 |
Column 1 is the mean of the individual slopes of the least-squared regression lines of the concentration–intensity judgment plots. Column 2 is the coefficient of determination of the functions. (N = 35 except for stearic-FU where N = 34).
With correction for multiple comparisons, slopes of intensity ratings were significantly correlated for stearic acid at the CV and FU papillae (r = −0.54) and FO and FU papillae (r = 0.48); lauric acid at the FO and FU papillae (r = 0.58); caproic acid at the CV and FO (r = 0.61), CV and FU (r = 0.54), and FO and FU (r = 0.60) papillae. Correlations between FFAs at each site were not significant. No planned correlations for threshold values between FFAs or between sites were statistically significant. In addition, no significant correlations were observed between the threshold concentrations and slopes of the intensity functions for any of the FFA at any of the stimulation sites.
Discussion
This study sought to further document the capacity of humans to detect fat in the oral cavity with nongustatory cues minimized and to use a psychophysical approach to highlight potential mechanisms. The present findings build on earlier evidence of oral fat detection using single stimulus concentrations of the sodium salt of linoleic acid (Kamphuis et al. 2003) and conjugated linoleic acid (Nasser et al. 2001) as well as thresholds for linoleic, oleic, stearic, lauric, and caproic acids (Chale-Rush et al. 2007a, 2007b; Mattes 2008). The threshold data confirm with a different sensory testing technique that detection is made by nearly all individuals, albeit over a wide concentration range. Absolute threshold concentrations are comparable to those reported previously using the same vehicle for caproic acid but slightly lower for lauric and stearic acids (Mattes 2009). The latter may be attributable to greater effective concentrations at the taste receptor cell due to reduced salivary dilution of the stimulus with the application procedure compared with a whole-mouth exposure (Smutzer et al. 2008). It may also just fall within the error of measurements given the limited sample sizes of the study populations tested to date and wide variance of thresholds. Threshold concentrations were similar for the 3 tested FFAs, whereas an earlier trial reported a lower threshold for caproic acid compared with lauric and stearic acids (Mattes 2009). Again, this may reflect a more uniform exposure with the direct application procedure. The longer chain FFA would be less soluble in saliva, so possibly transported to taste receptor cells less efficiently with a whole-mouth sip and spit procedure.
New data are presented on suprathreshold ratings for the selected FFA. Generally, the responses demonstrate the ability of participants to monotonically grade intensity with concentration for each FFA. Moreover, based on strong test–retest correlations of the ratings, they are reliable within individuals. However, the slopes of the intensity functions are low, well below 1.0, indicating a compressed function. The use of suprathreshold stimuli complicates attribution of the ratings to a particular sensory mechanism, such as taste, because there is greater potential for the higher FFA concentrations to add odor, irritancy, or textural cues. Testing blindfolded individuals with nares blocked is a well-established effective control for visual and olfactory-based responses. The stimuli were presented mixed with high levels of gum acacia and mineral oil to thicken and add lubricity to the vehicle and mask the contribution of the FFA to these cues. However, the adequacy of this control is not known because fats impart an array of somesthetic cues that extend beyond viscosity and lubricity (e.g., mouth coating, wetability, and heat transfer). Exclusion of irritancy is perhaps most problematic. Threshold studies (Chale-Rush et al. 2007a; Mattes 2009) have revealed no differences in performance when testing is conducted with or without prior capsaicin desensitization. However, extrapolation of findings from threshold studies to suprathreshold ratings is uncertain. Further, there is recent evidence of DRK and G-protein–coupled receptors that bind FAs on trigeminal fibers (Hansen et al. 2006; Yu et al. 2008). Thus, like the threshold data, the suprathreshold ratings are consistent with, but are not definitive evidence for, a taste mechanism.
Findings from the spatial tests raise questions about the contribution of different putative FFA transduction mechanisms. The most striking observation was that thresholds and suprathreshold intensity responses were obtained from tongue regions not believed to support transduction mechanisms for selected stimuli (Mattes 2009). Specifically, thresholds and monotonically increasing intensity ratings were observed with application of caproic acid on FU papillae, which are not believed to harbor GPCR41 or GPCR43, the putative short-chain FFA-binding proteins on taste cells. Similarly, threshold and suprathreshold responses were obtained for lauric acid application to the FO and FU papillae, which are not believed to contain GPCR40, the binding protein for shorter, medium-chain FFAs. Multiple explanations may account for these observations. First, it is possible that current knowledge of the distribution of currently identified FFA-binding proteins is incorrect. There is only preliminary evidence for the localization of these proteins. Second, the ligand specificity of each receptor may not be correct. Systematic testing with an array of FFAs has been conducted with DRK (Gilbertson et al. 1997) and CD36 (Laugerette et al. 2005), but much less is known about GPCRs and FFA transport proteins (FATPs) on taste cells.
Third, uncharacterized receptors may be present at these sites that are capable of transducing these FFA. Many GPCRs remain orphaned (Metpally and Sowdhamini 2005), and the potential for FATPs, present in the gut (Stahl et al. 1999), to serve as FFA transporters in taste cells warrants further investigation. FATP4 is present in lingual and palatal mucosa (Laugerette et al. 2005) and binds saturated and unsaturated FFAs between C10 and C26. FATP5 is another potential candidate as knockouts reduce energy intake on a high-fat diet independently of changes in satiety hormones or digestive efficiency (Hubbard et al. 2006). This suggests that altered taste is a testable contributor. FATP may also trap FFA intracellularly creating a gradient to draw FFAs into cells where they may activate intracellular signaling systems (Mashek and Coleman 2006).
Fourth, there may be a nonspecific mechanism for FFA detection, such as passive diffusion across taste cell membranes and activation of intracellular signaling systems. Detection of lipophilic sweet and bitter compounds has been documented by this mechanism (DeSimone 2000; Peri et al. 2000; Zubare-Samuelov et al. 2005). The amphipathic property of FFAs allows them to rapidly translocate across membranes (Hamilton and Kamp 1999; Hamilton et al. 2001; Kamp and Hamilton 2006). However, based on their diffusion coefficients (Baillie et al. 1996), which vary directly with chain length, there would be some differentiation between FFAs. The present data reveal a rank ordering of intensity ratings directly related to chain length, and studies with murine taste cells reveal a direct association between Ca2+ influx and exposure to FFAs of increasing chain length (i.e., palmitic, linoleic, and docosahexaenoic acid) (Gaillard et al. 2008). Consistent with such a nonspecific mechanism, no differences in thresholds were observed for the FFAs across all stimulation sites and intensity ratings were highly correlated between most sites.
A diffusion mechanism does not preclude receptor-mediated transduction. Indeed, they likely coexist (Baillie et al. 1996). Receptor-mediated transduction may predominate at low stimulus concentrations, but when receptor binding is saturated and FFAs are available, diffusion may be the predominant route of passage across membranes (Ibrahimi and Abumrad 2002). Unbound FFA concentrations in the circulation may be in the nanomolar range (Azzazy et al. 2006), but concentrations 6–8 orders of magnitude higher may occur in the oral cavity while eating (Rukunudin et al. 1998; Wan et al. 1998; Bertran et al. 1999; Che Man et al. 1999; Gopala Krishna et al. 2006; Li et al. 2008).
In summary, the present findings build on the evidence that humans can detect FFAs in the oral cavity when nongustatory cues are minimized. Further, they challenge existing views of FFA transduction mechanisms because detection and reliable monotonic intensity scaling of FFAs not believed to be ligands for putative receptors at various sites were observed. As “taste” mechanisms for sucrose (Dyer et al. 2005; Jang et al. 2007) and amino acids (Bezencon et al. 2007) in the duodenum suggest the presence of a nutrient signaling system continuum in the gastrointestinal tract, the long recognized FFA detection and absorption mechanisms in the intestine provide a basis for hypothesizing the presence of such a system in the oral cavity and, perhaps, how it may function.
Funding
Public Health Service grant # R01 DK45294.
Acknowledgments
The author thanks Maire Ni Nia, Christen Wood, and Judy George with their assistance in the conduct of this study and Jenny Houchins for comments on the manuscript.
References
- Azzazy HM, Pelsers MM, Christenson RH. Unbound free fatty acids and heart type fatty acid-binding protein: diagnostic assays and clinical applications. Clin Chem. 2006;52:19–29. doi: 10.1373/clinchem.2005.056143. [DOI] [PubMed] [Google Scholar]
- Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. Clinical evaluation of the sense of taste. Ear Nose Throat J. 1989;68:331–337. [PubMed] [Google Scholar]
- Bertran E, Blanco M, Coello J, Iturriaga H, Maspoch S, Montoliu I. Determination of olive oil free fatty acid by Fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:611–616. [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Brown HG, Melton SL, Riemann MJ, Backus WR. Effects of energy intake and feed source on chemical changes and flavor or ground beef during frozen storage. J Anim Sci. 1979;48:338–347. [Google Scholar]
- Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 2007a;32:423–431. doi: 10.1093/chemse/bjm007. [DOI] [PubMed] [Google Scholar]
- Chale-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G1206–G1212. doi: 10.1152/ajpgi.00471.2006. [DOI] [PubMed] [Google Scholar]
- Che Man YB, Moh MH, Van de Voort FR. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using Fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:485–490. [Google Scholar]
- Damak S, Le-Coutre J, Bezencon C, Cartoni C. Fat taste receptors and their methods of use. 2007. International application published under the patent cooperation treaty. p. 15. [Google Scholar]
- DeSimone JA. Focus on “rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction”. Am J Physiol Cell Physiol. 2000;278:C13–C16. doi: 10.1152/ajpcell.2000.278.1.C13. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1005–G1010. doi: 10.1152/ajpgi.00235.2006. [DOI] [PubMed] [Google Scholar]
- Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36 mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- Gopala Krishna AG, Hemakumar KH, Khatoon S. Acidity of oryzanol and its contribution to free fatty acids value in vegetable oils. J Am Oil Chem Soc. 2006;83:999–1005. [Google Scholar]
- Grover R, Frank ME. Regional specificity of chlorhexidine effects on taste perception. Chem Senses. 2008;33:311–318. doi: 10.1093/chemse/bjm095. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. doi: 10.1385/JMN:16:2-3:99. discussion. 151–1107. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–2269. doi: 10.2337/diabetes.48.12.2255. [DOI] [PubMed] [Google Scholar]
- Hansen DR, McKenna L, Shah BP, Gilbertson TA. 2006. Expression of fatty acid activated G protein coupled receptors in chemosensory cells. [abstract] Chem Senses. doi:10.1093/chemse/bjj055 2006; 31:A105. [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hubbard B, Doege H, Punreddy S, Wu H, Huang X, Kaushik VK, Mozell RL, Byrnes JJ, Stricker-Krongrad A, Chou CJ, et al. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterol. 2006;130:1259–1269. doi: 10.1053/j.gastro.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Curr Opin Clin Nutr Metab Care. 2002;5:139–145. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Ibrahimi A, Sfeir Z, Magharaie H, Amri E, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci USA. 1996;93:2646–2651. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins LeukotEssent Fatty Acids. 2006;75:149–159. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kamphuis MM, Saris WH, Westerterp-Plantenga MS. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid non tasters. Br J Nutr. 2003;90:199–206. doi: 10.1079/bjn2003858. [DOI] [PubMed] [Google Scholar]
- Kamphuis MM, Westerterp-Plantenga MS. PROP sensitivity affects macronutrient selection. Physiol Behav. 2003;79:167–172. doi: 10.1016/s0031-9384(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Kintner J, Day E. Major free fatty acids in milk. J Dairy Sci. 1965;48:1575–1581. doi: 10.3168/jds.s0022-0302(65)88205-4. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- Li Y, Garcia-Gonzalez DL, van de Voort FR. Determination of free fatty acids in edible oils with the use of a variable filter array IR spectometer. J Am Oil Chem Soc. 2008;85:599–604. [Google Scholar]
- Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–278. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- Mattes RD. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses. 2008;34:145–150. doi: 10.1093/chemse/bjn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metpally RP, Sowdhamini R. Cross genome phylogenetic analysis of human and Drosophila G protein-coupled receptors: application to functional annotation of orphan receptors. BMC Genomics. 2005;6:106. doi: 10.1186/1471-2164-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteberg E, Vogt G, Nilsson A, Frolich W. Effects of storage and heat processing on the content and composition of free fatty acids in oats. Cereal Chem. 1995;72:88–93. [Google Scholar]
- Nasser JA, Kissileff HR, Boozer CN, Chou CJ, Pi-Sunyer FX. PROP taster status and oral fatty acid perception. Eat Behav. 2001;2:237–245. doi: 10.1016/s1471-0153(01)00031-9. [DOI] [PubMed] [Google Scholar]
- Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, Nir S, Naim M. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Physiol. 2000;278:C17–C25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- Rukunudin IH, White PJ, Bern CJ, Bailey TB. A modified method for determining free fatty acids from small soybean oil sample sizes. J Am Oil Chem Soc. 1998;75:563–568. [Google Scholar]
- Smutzer G, Lam S, Hastings L, Desai H, Abarintos RA, Sobel M, Sayed N. A test for measuring gustatory function. Laryngoscope. 2008;118:1411–1416. doi: 10.1097/MLG.0b013e31817709a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Wan PJ, Pakarinen DR, Wakelyn PJ. Concerns for the determination of free fatty acid in cottonseed. J Am Oil Chem Soc. 1998;75:1321–1324. [Google Scholar]
- Warnock A, Delwiche J. Regional variation in sweet suppression. J Sens Stud. 2006;21:348–361. [Google Scholar]
- Woo A, Kollodge S, Lindsay R. Quantification of major free fatty acids in several cheese varieties. J Dairy Sci. 1984;67:874–878. [Google Scholar]
- Woo A, Lindsay R. Stepwise discriminant analysis of free fatty acid profiles for identifying sources of liplytic enzymes in rancid butter. J Dairy Sci. 1983;66:2070–2075. [Google Scholar]
- Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Shah B, Liu P, Hansen DR, Gilbertson TA. Fatty acid-induced changes in intracellular calcium in somatosensory cells; mechanisms underlying the textural perception of fat. Chem Senses. 2008;33:756. [Google Scholar]
- Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Tirosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:C483–C492. doi: 10.1152/ajpcell.00547.2004. [DOI] [PubMed] [Google Scholar]