Abstract
Background
It is uncertain whether evidence supports routinely estimating a postmenopausal woman's risk of breast cancer and intervening to reduce risk.
Methods
We systematically reviewed prospective studies about models and sex hormone levels to assess breast cancer risk and used meta-analysis with random effects models to summarize the predictive accuracy of breast density. We also reviewed prospective studies of the effects of exercise, weight management, healthy diet, moderate alcohol consumption, and fruit and vegetable intake on breast cancer risk, and used random effects models for a meta-analyses of tamoxifen and raloxifene for primary prevention of breast cancer. All studies reviewed were published before June 2008, and all statistical tests were two-sided.
Results
Risk models that are based on demographic characteristics and medical history had modest discriminatory accuracy for estimating breast cancer risk (c-statistics range = 0.58–0.63). Breast density was strongly associated with breast cancer (relative risk [RR] = 4.03, 95% confidence interval [CI] = 3.10 to 5.26, for Breast Imaging Reporting and Data System category IV vs category I; RR = 4.20, 95% CI = 3.61 to 4.89, for >75% vs <5% of dense area), and adding breast density to models improved discriminatory accuracy (c-statistics range = 0.63–0.66). Estradiol was also associated with breast cancer (RR range = 2.0–2.9, comparing the highest vs lowest quintile of estradiol, P < .01). Most studies found that exercise, weight reduction, low-fat diet, and reduced alcohol intake were associated with a decreased risk of breast cancer. Tamoxifen and raloxifene reduced the risk of estrogen receptor–positive invasive breast cancer and invasive breast cancer overall.
Conclusions
Evidence from this study supports screening for breast cancer risk in all postmenopausal women by use of risk factors and breast density and considering chemoprevention for those found to be at high risk. Several lifestyle changes with the potential to prevent breast cancer should be recommended regardless of risk.
CONTEXT AND CAVEATS
Prior knowledge
Whether evidence supports routinely estimating a postmenopausal woman's risk of breast cancer and intervening to reduce risk is not clear.
Study design
A combination of systematic review and meta-analysis was used to analyze published data from prospective studies on risk assessment models and breast cancer risk and sex hormone levels, breast density, exercise, weight management, diet, tamoxifen, and raloxifene.
Contribution
Results of this analysis support screening for breast cancer risk in all postmenopausal women by use of risk factors and breast density, considering chemoprevention for women found to be at high risk, and encouraging lifestyle changes that may decrease the risk of breast cancer regardless of risk.
Implications
Systems need to be developed to assess and report the results of various tests, including risk factor analyses, breast density, and appropriate referral for genetic counseling, to name just a few.
Limitations
The studies reviewed had diverse designs, diverse populations with different degrees of risk, and diverse methods of analyzing and expressing data, which precluded reporting the results about benefits and harms as absolute rates. Studies on lifestyle changes to reduce risk are generally observational and rely on recall.
From the Editors
In 2007, an estimated 178 480 women in the United States were diagnosed with invasive breast cancer (1). Although mammographic screening results in decreased mortality from breast cancer (2), it does not reduce the number of women who develop the disease and who suffer its physical and emotional consequences.
The US Preventive Services Task Force has suggested (3,4) that clinicians discuss chemoprevention with women at high risk for breast cancer and at low risk of adverse effects, and the American Society of Clinical Oncology Breast Cancer Technology Assessment Working Group concluded that tamoxifen could be offered to women with a 5-year breast cancer risk of 1.66% or more in the absence of contraindications (5). Since these guidelines were issued in 2002, additional trials of chemoprevention have been completed and more accurate ways to assess breast cancer risk have been developed. However, there has not been a systematic attempt to identify women at high risk for breast cancer. Thus, very few high-risk women—almost certainly fewer than 1 in 10—have discussed their breast cancer risk with a physician or considered risk-reducing therapies (6). Efforts to routinely screen women for their risk of breast cancer and recommend interventions to those at high risk should be based on evidence that interventions are effective and that there are reasonably accurate and feasible methods for assessing risk. Therefore, to determine whether evidence supports combined screening for breast cancer risk, we have systematically reviewed evidence about methods for estimating a woman's risk and interventions to reduce her risk of breast cancer.
For subjects who have been studied in systematic reviews or meta-analyses, we reviewed and summarized articles that have been published since the systematic reviews were conducted, including articles published up to June 2008. We searched databases and then reviewed the abstracts of the articles that we found. We selected articles on the basis of the rigor of their design. For example, we limited reviews of chemoprevention to randomized blinded trials and reviews of modifiable risk factors for breast cancer to large prospective studies or randomized trials. Retrospective case–control studies of these issues may be prone to biases in selection of case patients and control subjects and in recall of exposures.
We did not attempt to quantify the relative benefits and costs or cost-effectiveness of prevention of breast cancer because such efforts are underway by other groups. Because our goal was not to quantify benefits and harms, we did not numerically quantify or weight the quality of individual studies.
We studied risk assessment methods that are feasible for screening unselected populations and therefore did not include testing for mutations in BRCA or other genes in women with family histories of breast cancer and did not include a review of risk-reducing surgery for women carrying high-risk genetic variants. We included interventions that are feasible for clinical use and lifestyle modifications that may reduce risk.
Studies and Methods
Studies About Assessing Risk of Breast Cancer
There has been, to our knowledge, no previous systematic review of breast cancer risk models. Therefore, to survey methods of breast cancer risk assessment, we searched the MEDLINE and EMBASE databases, Cochrane clinical trials database, Cochrane reviews database, and the Database of Abstracts of Reviews of Effects from January 1, 1966, through May 31, 2007, with the Medical Subject Heading (MeSH) terms “statistical models,” “risk factors,” or “risk assessment,” and the free text words “Gail model” or “Claus model” cross-referenced with the MeSH terms “breast neoplasm” and “female.” We manually searched the bibliographies of key articles for additional references. We reviewed abstracts for potential relevance and included prospective studies that included at least 100 patients with breast cancer. We found prospective studies of the Gail score (7–14) and several other risk models (Table 1) (15–25). We added three large prospective studies of risk factors for breast cancer published between a systematic review and June 2008 (Table 1) (23–25). We did not systematically review models that are primarily intended to identify women with genetic mutations, such as BRCAPRO (26), or the Tyrer–Cuzick model (27), which has been tested in only a small study (28).
Table 1.
Prospective studies of models assessing the risk of breast cancer: estimates of their calibration and discrimination*
| Model, first author, year (reference) | No. of subjects | Age, y; % race or nationality | Risk factors included in the final model | No. of patients with breast cancer | Calibration, E/O (95% CI) | Discrimination, c-statistic (95% CI) |
| Models with only risk factors | ||||||
| Gail (original) model | ||||||
| Gail, 1989 (7) | 5998 | Median = 55–60; 100% white | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 2852 | NR | NR |
| Spiegelman, 1994 (8) | 115 172 | Median = 45–49; 98% white | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 2396 | 1.33 (1.28 to 1.39) | NR |
| Costantino, 1999 (9) | 5969 | Median = 50–59; 100% white | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 204 | 0.84 (0.73 to 0.97) | NR |
| Gail model 2 (BCPT) | ||||||
| Costantino, 1999 (9) | 5969, with Gail risk >1.66% | Median = 50–59; 100% white | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 204 | 1.03 (0.88 to 1.21) | NR |
| Rockhill, 2001 (10) | 82 109 | Median = 55–59; 100% white | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 1354 | 0.94 (0.89 to 0.99) | 0.58 (0.56 to 0.60) |
| Tice, 2005 (11) | 6904 | Median = 43; 71% white, 11% black, 11% Asian, 5% Hispanic, 2% other | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 400 | NR | 0.62 (NR) |
| Novotny, 2006 (12) | 14 566† | Median = 57; 100% Czech | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 2299 | NR; estimated >2 from data in text | NR |
| Decarli, 2006 (13) | 10 381 | Median = 50–59; 100% Italian | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 194 | 0.93 (0.81 to 1.08) | 0.59 (0.55 to 0.63) |
| Chlebowski, 2007 (23) | 147 916 | Mean = 63; 83% white, 9% black, 3% Asian, 4% Hispanic, <1% American Indian | Age, race, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 3236 | 0.79 (NR, P < .001) | 0.58 (0.56 to 0.60) |
| Claus model | ||||||
| Claus, 1990–1991 (14–16) | 9418 | Range = 20–54; 100% white | Age, first-degree relatives with BC, second- degree relatives with BC, age at each BC diagnosis | 4730 | NR | NR |
| Rosner and Colditz model 1 | ||||||
| Rosner, 1996 (17) | 89 132 | Age, NR; race, NR but ∼98% white‡ | Age, age at menarche, age at birth of first child, age at menopause, parity | 2249 | NR | NR |
| Rockhill, 2003 (18) | 45 210 | Mean = 58; race, NR but ∼98% white‡ | Age, age at menarche, age at birth of first child, age at menopause, parity | 757 | 1.00 (0.93 to 1.07) | 0.57 (0.55 to 0.59) |
| Rosner and Colditz model 2 | ||||||
| Colditz, 2000 (19) | 58 520 | Range = 30–55; race, NR but ∼98% white‡ | Age, age at menarche, age at birth of first child, age at menopause, parity, age at oophorectomy, current HT, years ET, years EPT, height, BMI, benign breast disease | 1761 | NR | NR |
| Rockhill, 2003 (18) | 45 210 | Mean = 58; race, NR but ∼98% white‡ | Age, age at menarche, age at birth of first child, age at menopause, parity, age at oophorectomy, current HT, years ET, years EPT, height, BMI, benign breast disease | 757 | 1.01 (0.94 to 1.09) | 0.63 (0.62 to 0.66) |
| Ueda model | ||||||
| Ueda, 2003 (20) | 806 | Age, NR; 100% Japanese | Age, age at menarche, age at birth of first child, age at menopause, first-degree relatives, BMI | 376 | NR | NR |
| CARE model | ||||||
| Gail, 2007 (21) | 3254 | Age, NR; 100% black | Age, first-degree relatives with BC, age at menarche, age at birth of first child, breast biopsy, atypical hyperplasia | 1607 | 1.08 (0.97 to 1.20) | 0.56 (0.54 to 0.58) |
| WHI model | ||||||
| Chlebowski, 2007 (23) | 147 092 postmenopausal women; cases limited to estrogen receptor–positive BC | Mean = 63; 83% white, 9% black, 3% Asian, 4% Hispanic, <1% American Indian | Age, first-degree relatives with BC, breast biopsy | 2412 | NR | 0.58 (0.56 to 0.60) |
| Risk factors plus breast density | ||||||
| Barlow, 2006§ (24) | ||||||
| Premenopausal | 141 991 | Median = 45–49; 86% white, 6% black, 6% Asian, 1% American Indian, 1% other | Age, first-degree relatives with BC, breast biopsies, BI-RADS breast density | 432 | 1.00 (NR) | 0.63 (0.62 to 0.64) |
| Postmenopausal | 410 355 | Median = 60–64; 87% white, 6% black, 5% Asian, 1% American Indian, 1% other | Age, race, Hispanic, first-degree relatives with BC, breast biopsies, age at birth of first child, oophorectomy, current HT, BMI, BI-RADS breast density | 2303 | 1.01 (NR) | 0.62 (0.62 to 0.63) |
| Chen model | ||||||
| Chen, 2006 (22) | 2891 | Age, NR; 100% white | Age, race, age at birth of first child, first-degree relatives with BC, breast biopsies, atypical hyperplasia, weight, % breast density | 1235 | NR | 0.64 (NR) |
| Tice model | ||||||
| Tice, 2008 (25) | 629 229 | Mean = 53.4; 71% white, 7% black, 3% Asian, 8% Hispanic, 11% other | Age, race, first-degree relatives with BC, breast biopsies, BI-RADS breast density | 8784 | 1.01 (0.99 to 1.03) | 0.66 (0.65 to 0.66) |
E/O = ratio of the number of cancers expected according to the model to the actual number observed in the study. Perfect calibration of the model would give an expected-to-observed ratio of 1.0. Less than 1 means that the model underestimates cancer incidence; if greater than 1, the model overestimates cancer incidence. NR = not reported; BC = breast cancer; HT = hormone therapy; ET = estrogen therapy; EPT = estrogen and progestin therapy; BMI = body mass index; BI-RADS = Breast Imaging Reporting and Data System; WHI = Women's Health Initiative; CARE = model based on the Women's Contraceptive and Reproductive Experiences Study; BCPT = Breast Cancer Prevention Trial; CI = confidence interval.
Average risk in control subjects was greater than average risk in case patients.
The race distribution was not reported in the article, but in the methods section in Cuzick et al. (8), the authors report that approximately 98% of women in the Nurses’ Health Study are white.
Only statistics from the validation study are included. Studies with fewer than 100 cancers for validation sample are not included.
We report the calibration and discrimination of models when these were available in the articles reviewed. Calibration refers to how accurately a model predicts the observed rate of breast cancer and is measured by the ratio of the expected to observed rate. For example, if a model predicts a 5-year rate of breast cancer of 3% and a rate of 3.2% is observed in a population, then the model has an expected-to-observed ratio of 0.94, indicating excellent calibration.
Discrimination refers to how well the model differentiates between women who develop cancer and women who remain free of cancer. It is measured by the c-statistic, which represents the area under the receiver operating characteristics curve. A c-statistic of 0.6, typical of many risk models, means that the chance that a randomly selected (future) woman with breast cancer has a higher estimated relative risk (RR) than a randomly selected control woman is 60%. A value of 0.5 means that the model performs no better than chance.
The systematic review and meta-analysis by McCormack et al. (29) analyzed studies about the association between breast density and risk of breast cancer that were published up to November 30, 2005. To update that review, we surveyed MEDLINE and EMBASE databases from January 1, 2004, through January 1, 2008, by use of the terms “breast density” or “mammographic density” that were cross-referenced with the MeSH term “breast neoplasm” and the free text term “breast cancer.” We found five studies that were not included in the meta-analysis by McCormack et al. (22,24,30–32). Results from the additional studies (22,24,30–32) were combined with those of McCormack et al. by use of a random effects model (STATA version 9.2). We separately note findings from an additional recent study (38) that was published after completion of our meta-analysis that were reported in different categories than we used in our meta-analysis. A prospective study was not included because it studied women who already had ductal carcinoma in situ (33).
The association between sex hormone levels and risk of breast cancer was analyzed in a meta-analysis (34) in 2002 that pooled primary data from nine large observational studies. We updated this 2002 meta-analysis by searching the MEDLINE and EMBASE databases from January 1, 2000, through January 1, 2008, with abstract and MeSH terms “estradiol” or “testosterone” or “gonadal steroid hormone” that were cross-referenced with “breast neoplasm” and free text terms “estradiol,” “testosterone,” or “sex hormone” that were cross-referenced with “breast cancer.” We reviewed abstracts for prospective cohort studies in general populations, including updated results from cohorts included in the 2002 pooled analysis (34). We found six additional prospective studies of postmenopausal women (32,35–39), one study that was limited to in situ breast cancer (40), and two studies of premenopausal women (41,42). Two separate studies were conducted within the Nurses’ Health Study (32,36). We limited the analysis to total estradiol and total testosterone levels because they were assessed in all of the studies about sex hormones and breast cancer risk. Results were adjusted for age, although some studies also adjusted for other covariates. Diverse categorization of hormone levels precluded meta-analysis. Furthermore, some studies also updated results that included patients who were already part of the 2002 pooled analysis (36,37). We did not include two studies (43,44) that were conducted in the placebo groups of randomized trials because the populations were highly selected: one trial was limited to women with osteoporosis and found an association with risk of breast cancer and the other was limited to women at high risk for breast cancer by the Gail model which did not find an association.
We found only one prospective study (32) that assessed a combination of breast density and the level of either estradiol or testosterone for prediction of breast cancer. We included its data on breast density and sex hormone results separately in our analysis and also described the results of the combined analysis.
Studies of Chemoprevention
Evidence of the effectiveness of chemoprevention with the antiestrogens, tamoxifen and raloxifene, was summarized in a 2003 meta-analysis (45). We updated that meta-analysis (by searching MEDLINE and Cochrane clinical trials database from January 1, 2002, through June 2008) with MeSH terms and free text terms “tamoxifen” and “raloxifene” that were cross-referenced with the MeSH terms “breast neoplasm” or “breast cancer” in the abstract. We reviewed the abstracts for placebo-controlled trials with breast cancer outcomes. We found two additional trials of raloxifene (46,47). The trials were combined and analyzed by use of a random effects model (STATA version 9.2). We estimated their effects on the risk of invasive breast cancer (in all cancers and in estrogen receptor–positive disease). We did not include studies that included follow-up after chemoprevention was discontinued (48,49).
Studies of Nonpharmacological Interventions: Modifiable Risk Factors for Breast Cancer
From the many potential modifiable risk factors for breast cancer, we focused our review on exercise, diet, weight, and alcohol intake. We searched the MEDLINE database by use of the terms “exercise, physical activity, or sedentary lifestyle”; “BMI, weight, or weight change”; “diet, nutrition, vegetable, or fruit”; and “alcohol, ethanol, or alcohol consumption.” All terms were cross-referenced with the text term “breast cancer.” Two coauthors (S. Bauer and S. R. Cummings) then reviewed the abstracts and selected randomized trials, systematic reviews, meta-analyses, and prospective cohort studies. We found meta-analyses or systematic reviews of observational studies of the associations between recreational physical activity (50), fruit and vegetable consumption (51,52), or alcohol intake (53) and the risk of breast cancer. In each instance, our review and summary of articles extended from the most recent year covered by the systematic reviews (2006 for physical activity, 2002 for fruit and vegetable intake, and 2005 for alcohol intake) through January 2008. Furthermore, because the meta-analyses and systematic reviews included data from thousands of patients with breast cancer, we reviewed subsequent prospective cohort studies that included at least 200 patients with breast cancer. Because of the potential for recall bias and bias in the selection of control subjects in retrospective case–control studies, we selected prospective cohort studies. Randomized trials with breast cancer endpoints provide the most rigorous evidence about the effect of modifying risk factors. For low-fat diets, we found a very large trial of low-fat diets for new breast cancer (54) and one for breast cancer recurrence (55); therefore, we did not review observational studies of the association between dietary fat and risk of breast cancer.
Statistical Methods
We used random effects models for the meta-analysis of breast density and risk of breast cancer and for the meta-analysis of the effects of tamoxifen and raloxifene on the risk of breast cancer. The summary relative risks and relative risk reductions are accompanied by 95% confidence intervals (CIs). All statistical tests were two-sided.
Results
Risk Factor Models for Estimation of Breast Cancer Risk
Risk models that are based on demographic characteristics and medical histories have modest discriminatory accuracy for estimating breast cancer risk (c-statistics range = 0.58–0.63, Table 1). The Gail model (56) is the most widely used and studied method for assessing the risk of breast cancer. This model estimates risk from a woman's age, race, number of first-degree relatives with breast cancer, history of atypical hyperplasia, number of breast biopsy examinations, number of live births, and age at the birth of her first child. We found eight reports involving a total of 12 935 patients with breast cancer (Table 1) (7–13,23), and these studies generally found that the Gail model was well calibrated. However, it had limited discriminatory accuracy (range of the area under the receiver operating characteristics curve as assessed by the c-statistic = 0.58–0.62). A prospective validation study (10) with 1354 participants diagnosed with breast cancer found that the relative risk of women in the highest decile was only 2.8-fold higher than those in the lowest decile. Chlebowski et al. (23) observed that the Gail model was predictive of estrogen receptor–positive breast cancer (c-statistic = 0.58) but not of estrogen receptor–negative cancer (c-statistic = 0.50).
A few risk factor models other than the Gail model have been developed. Chlebowski et al. (23) used data from the Women's Health Initiative to develop and validate a simple model that included age, family history, and breast biopsy examinations for prediction of estrogen receptor–positive breast cancer; this model and the Gail model had the same c-statistic (ie, 0.58). Gail et al. (21) used risk factors and data from the Women's Contraceptive and Reproductive Experiences (CARE) Study to develop a model for breast cancer in African American women. Although the CARE model was well calibrated, it had limited discrimination (c-statistic = 0.56) for risk of breast cancer.
Breast Density and Estimation of Breast Cancer Risk
Because they are dense, the cells and connective tissue of breast tissue absorb x-rays and appear white on mammograms; the fat of breast tissue is radiolucent and appears black on mammograms. Approximately 60%–70% of the density of breast tissue is heritable (57). The density of breast tissue has been correlated with its content of collagen, stromal tissue, and, to a lesser degree, breast epithelium (58,59).
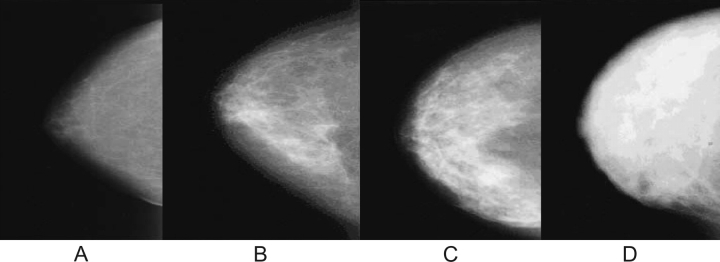
Qualitative approaches to assessing breast density rely on subjective ratings of a radiologist (60). The first system, developed by Wolfe (61,62), classifies breast density as completely fatty breast (N1), prominent ducts occupying less than 25% (P1) or 25%–75% (P2) of the breast image, or no visible ducts with diffuse and extensive nodular density (Dy). Another system, the Breast Imaging Reporting and Data System (BI-RADS) of the American College of Radiology (63), also classifies density in the four categories: almost entirely fat with less than 25% of the area dense tissue (BI-RADS I), scattered fibroglandular densities with 25%–50% dense tissue (BI-RADS II), heterogeneously dense with 51%–75% dense tissue (BI-RADS III), and extremely dense with greater than 75% dense tissue (BI-RADS IV) (Figure 1). Other systems categorize the proportion of the two-dimensional image that is dense in 6 (57) or 21 (64) subjectively estimated categories. Breast density can also be estimated quantitatively by having a trained reader manually outline the portion of a digitized image whose density exceeds a specified threshold (Figure 2).
Figure 1.
Breast density patterns. A) BI-RADS I = fatty breast (<25% dense). B) BI-RADS II = scattered densities (25%–50% dense). C) BI-RADS III = heterogeneously dense (51%–75% dense). D) BI-RADS IV = extremely dense (>75% dense). BI-RADS = Breast Imaging Reporting Data System.
Figure 2.
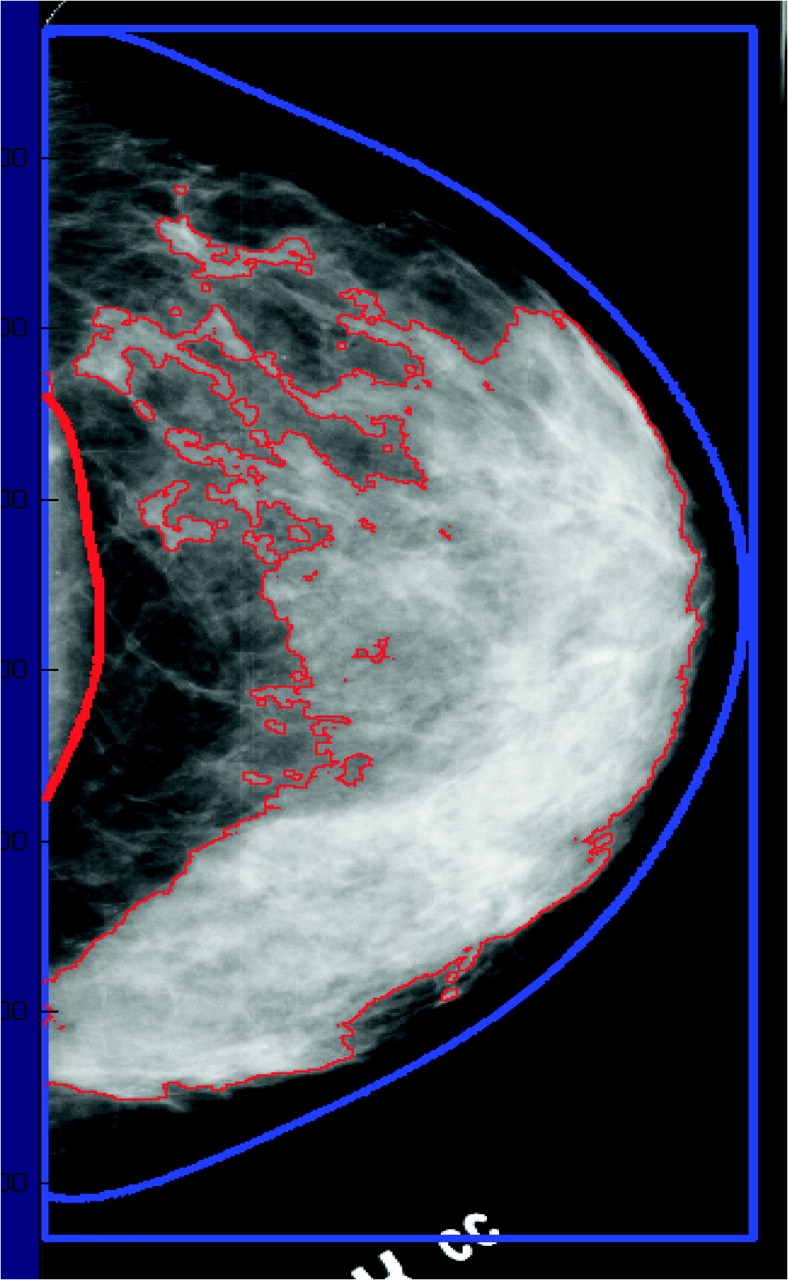
Illustration of the quantitative estimation of breast density from a digitized image of a mammogram. The image of the breast is outlined, and the areas that exceed any certain threshold value of density are also outlined. Percent density is calculated as [(dense area%total area) x 100]. Dense tissue in this breast area of this mammogram accounts for 48% of its area.
A previous meta-analysis (29) in 2006 found consistent association between higher breast density and a greater risk of invasive breast cancer, with four- to fivefold increased breast cancer risk for women in the highest category of breast density compared with those in the lowest. We found five subsequent prospective studies (22,24,30–32) of breast density and subsequent risk of breast cancer, including one that assessed breast density by use of BI-RADS ratings (24) and four that measured percent density (Table 2) (22,30–32). In total, these studies included 28 521 patients with breast cancer. Authors of the studies by Chen et al. (22) and Vachon et al. (31) reanalyzed their published data to fit categories in the meta-analysis. In addition, Tamimi et al. (32) reported data in quartiles, rather than in quintiles as used in our meta-analysis; from a study with 272 patients, they reported that women in the highest quartile of percent density (≥28%) had a 3.8-fold greater risk of breast cancer than those in the lowest quartile (≤5.4%).
Table 2.
Updated meta-analysis of the association between breast density and the incidence of invasive breast cancer in general populations*
| Method of breast density measurement | Category | RR (95% CI) |
| Wolfe grade† | N1 (fatty) | 1 (reference) |
| P1 | 1.76 (1.41 to 2.19) | |
| P2 | 3.05 (2.54 to 3.66) | |
| Dy (most dense) | 3.98 (2.53 to 6.27) | |
| BI-RADS | 1 (fatty) | 1 (reference) |
| 2 (scattered densities) | 2.03 (1.61 to 2.56) | |
| 3 (heterogeneously dense) | 2.95 (2.32 to 3.73) | |
| 4 (extremely dense) | 4.03 (3.10 to 5.26) | |
| % Of breast area that is dense | <5 | 1 (reference) |
| 5–24 | 1.74 (1.50 to 2.03) | |
| 25–49 | 2.15 (1.87 to 2.48) | |
| 50–74 | 2.92 (2.55 to 3.34) | |
| ≥75 | 4.20 (3.61 to 4.89) |
The meta-analysis by McCormack et al. (29) was included in this meta-analysis. All studies were adjusted for age; studies that further adjust for body mass index or weight observed somewhat stronger associations. CI = confidence interval; BI-RADS = Breast Imaging Reporting and Data System; RR = relative risk.
Wolfe grades: N1 = normal fatty breast, P1 = prominent ducts occupy less than 25% of the breast image; P2 = prominent ducts occupy 25%–75%; Dy = dysplastic breast with sheets of dense parenchyma.
Our meta-analysis found that breast density was strongly associated with breast cancer (RR = 4.03, 95% CI = 3.10 to 5.26, for BI-RADS category IV [extremely dense] vs category I [fatty]; RR = 4.20, 95% CI = 3.61 to 4.89, for >75% vs <5% dense area). Although the gradient of risk was similar for all three methods, Wolfe, BI-RADS, and percent density, different cut points in the categories of the qualitative and quantitative methods precluded direct comparisons of predictive value.
One study (24) found that breast density was associated with breast cancer for both pre- and postmenopausal women, and another study (65) found that breast density was associated with estrogen receptor–positive and estrogen receptor–negative breast cancers. One study (66), which was not included in our meta-analysis, found that high breast density was also associated with increased risk of in situ breast cancer, and another study (33) in patients with ductal carcinoma in situ observed an association between breast density that was assessed by BI-RADS in the contralateral, but not ipsilateral, breast and risk of invasive breast cancer.
Combining Breast Density and Risk Factors to Estimate Breast Cancer Risk
From studies involving 12 754 patients with breast cancer, we found that adding breast density improved discriminatory accuracy of models that are based on risk factors (c-statistics range = 0.62–0.66) (Table 1). Chen et al. (22) added measurements of percent breast density to the Breast Cancer Detection Demonstration Project data set, which was originally used to develop the Gail model, and found improved estimates of absolute risk of breast cancer for women with high breast density. Barlow et al. (24) used data from a cohort of more than 1 million women who had undergone screening mammograms to develop and validate models for predicting breast cancer in pre- and postmenopausal women. The model for postmenopausal women included breast density (by BI-RADS grade), age, race, ethnicity, family history of breast cancer in a first-degree relative, previous breast procedure, body mass index, natural menopause, hormone therapy, and previous false-positive mammogram. This model, including breast density, had somewhat greater predictive accuracy (c-statistic = 0.62, 95% CI = 0.62 to 0.63) than the model without it (c-statistic = 0.605, 95% CI = 0.60 to 0.61). Tice et al. (7) used a subset of that cohort to develop and to validate a simple model for predicting breast cancer that was based on age, race, family history of breast cancer, and history of breast biopsy examinations. The Tice model had better discrimination with breast density (c-statistic = 0.66, 95% CI = 0.65 to 0.66) than the Gail model with clinical risk factors alone (c-statistic = 0.61, 95% CI = 0.60 to 0.62). Tice et al. also showed that including BI-RADS ratings of breast density with the risk factors reclassified 34% of women into categories of higher or lower risk that more accurately reflected the observed 5-year incidence of breast cancer of those women (25).
Endogenous Hormone Levels and Estimation of Breast Cancer Risk
A study (34) that combined data from nine prospective cohort studies of breast cancer found that women in the highest quintile of estradiol or testosterone had a higher relative risk of breast cancer (for estradiol, 2.00-fold, 95% CI = 1.47- to 2.71-fold; for testosterone, 2.22-fold, 95% CI = 1.59- to 3.10-fold) than those in the lowest quintile. We found six subsequent prospective studies (32,35–39), all of which found statistically significant associations between estradiol or testosterone levels and the risk of breast cancer in postmenopausal women (Table 3). In total, these studies included 2581 patients with breast cancer. Two prospective studies found association between endogenous estradiol and testosterone and risk of estrogen receptor–positive cancer but not estrogen receptor–negative cancer (37,38). Prospective studies in premenopausal women have also reported associations between testosterone and follicular-phase free estradiol levels and the risk of breast cancer (41).
Table 3.
Association of endogenous sex hormone levels and risk of breast cancer in postmenopausal women in prospective, nested case–control studies*
| Study or author, year (reference), hormone | No. of cases and controls | RR (95% CI) |
P† | ||||
| Category | |||||||
| 1 | 2 | 3 | 4 | 5 | |||
| EHBCCG, 2002 (34) | 663 cases and 1765 controls | ||||||
| Estradiol | 1 (reference) | 1.42 (1.04 to 1.95) | 1.21 (0.89 to 1.66) | 1.80 (1.33 to 2.43) | 2.00 (1.47 to 2.71) | <.001 | |
| Testosterone | 1 (reference) | 1.34 (0.96 to 1.87) | 1.61 (1.16 to 2.24) | 1.59 (1.13 to 2.23) | 2.22 (1.59 to 3.10) | <.001 | |
| Zeleniuch-Jacquotte, 2004 (37) | 293 cases and 563 controls | ||||||
| Estradiol | 1 (reference) | 1.56 (0.95 to 2.56) | 1.14 (0.69 to 1.89) | 1.63 (0.98 to 2.71) | 2.33 (1.40 to 3.88) | .004 | |
| Testosterone | 1 (reference) | 1.63 (0.99 to 2.68) | 1.51 (0.92 to 2.48) | 1.84 (1.11 to 3.02) | 2.15 (1.29 to 3.59) | .005 | |
| Kaaks, 2005 (39) | 677 cases and 1309 controls | ||||||
| Estradiol | 1 (reference) | 1.09 (0.78 to 1.53) | 1.44 (1.04 to 2.00) | 1.71 (1.22 to 2.41) | 2.28 (1.61 to 3.23) | <.001 | |
| Testosterone | 1 (reference) | 1.14 (0.82 to 1.58) | 1.33 (0.96 to 1.84) | 1.56 (1.12 to 2.27) | 1.85 (1.33 to 2.57) | <.001 | |
| Cummings, 2005 (38) | 196 cases and 378 controls | ||||||
| Estradiol | 1 (reference) | 0.8 (0.4 to 1.8) | 1.4 (0.8 to 2.5) | 1.8 (0.9 to 3.5) | 2.9 (1.6 to 5.1) | <.001 | |
| Testosterone | 1 (reference) | 2.6 (1.2 to 5.4) | 2.1 (1.0 to 4.4) | 3.8 (1.9 to 7.8) | 5.1 (2.5 to 10.3) | <.001 | |
| Missmer, 2004 (36) | 322 cases and 637 controls | ||||||
| Estradiol | 1 (reference) | 1.4 (0.9 to 2.1) | 1.3 (0.8 to 2.0) | 2.0 (1.3 to 3.0) | — | <.001 | |
| Testosterone | 1 (reference) | 0.7 (0.4 to 1.2) | 1.4 (0.9 to 2.1) | 1.4 (0.9 to 2.2) | — | .003 | |
| Tamimi, 2007 (32) | 253 cases and 570 controls | ||||||
| Estradiol | 1 (reference) | 1.1 (0.6 to 1.7) | 1.2 (0.8 to 2.0) | 2.4 (1.4 to 3.9) | — | <.001 | |
| Testosterone | 1 (reference) | 0.8 (0.5 to 1.3) | 1.4 (0.9 to 2.2) | 1.8 (1.2 to 2.9) | — | <.001 | |
| Manjer, 2003 (35) | 173 cases and 438 controls | ||||||
| Testosterone | 1 (reference) | 1.08 (0.62 to 1.87) | 1.03 (0.59 to 1.80) | 1.48 (0.88 to 2.34) | — | .40 | |
| Estradiol‡ | 1 (reference) | 1.67 (1.03 to 2.72) | — | — | — | .04 | |
Data were separated into quintiles or quartiles or were dichotomized. EHBCCG = Endogenous Hormones and Breast Cancer Risk Collaborative Group; — = data were separated into quartiles or dichotomized and so the column was not needed; RR = relative risk; CI = confidence interval.
From two-sided test for trend.
The referent category was ≤2.5 pmol/L, and the comparison was >2.5 pmol/L.
Risk Estimation Models That Combine Breast Density and Sex Hormone Levels
In a study with 272 patients with breast cancer, Tamimi et al. (32) found that percent mammographic density and either estradiol or testosterone levels were independently associated with risk of breast cancer (for women in the highest tertiles of both breast density and estradiol, compared with those in the lowest tertiles of both measurements, RR = 4.1, 95% CI = 1.7 to 9.8). The gradient of risk seemed somewhat steeper (RR = 6.0, 95% CI = 2.6 to 14.0) for tertiles of breast density and testosterone. The associations appeared somewhat stronger among women who had never used postmenopausal hormone therapy.
Chemoprevention and the Modification of Breast Cancer Risk
Chemoprevention with the antiestrogens, tamoxifen or raloxifene, is effective at reducing the risk of breast cancer (Figure 3). Our meta-analysis included trials of tamoxifen with 35 525 participants and 545 diagnoses of breast cancer (Table 4). The analysis showed that tamoxifen treatment reduced the relative risk of invasive breast cancer by 33% (RR reduction = 0.67; 95% CI = 0.52 to 0.86) during 5 years of chemoprevention. Trials of raloxifene with a total of 17 806 participants and 171 diagnoses of breast cancer showed that treatment for 4–8 years reduced the relative risk of estrogen receptor–positive breast cancer by 59% (RR reduction = 0.41, 95% CI = 0.27 to 0.62). Because these interventions reduce the risk of estrogen receptor–positive breast cancer but not that of estrogen receptor–negative breast cancer, the reductions in relative risk of estrogen receptor–positive breast cancer are somewhat stronger (Figure 3, B). However, the results for tamoxifen and raloxifene cannot be compared with each other because trials of raloxifene included older exclusively postmenopausal women who were selected because they had osteoporosis or a high risk of heart disease and trials of tamoxifen included premenopausal women who were often selected for a high risk of breast cancer (Table 4).
Figure 3.
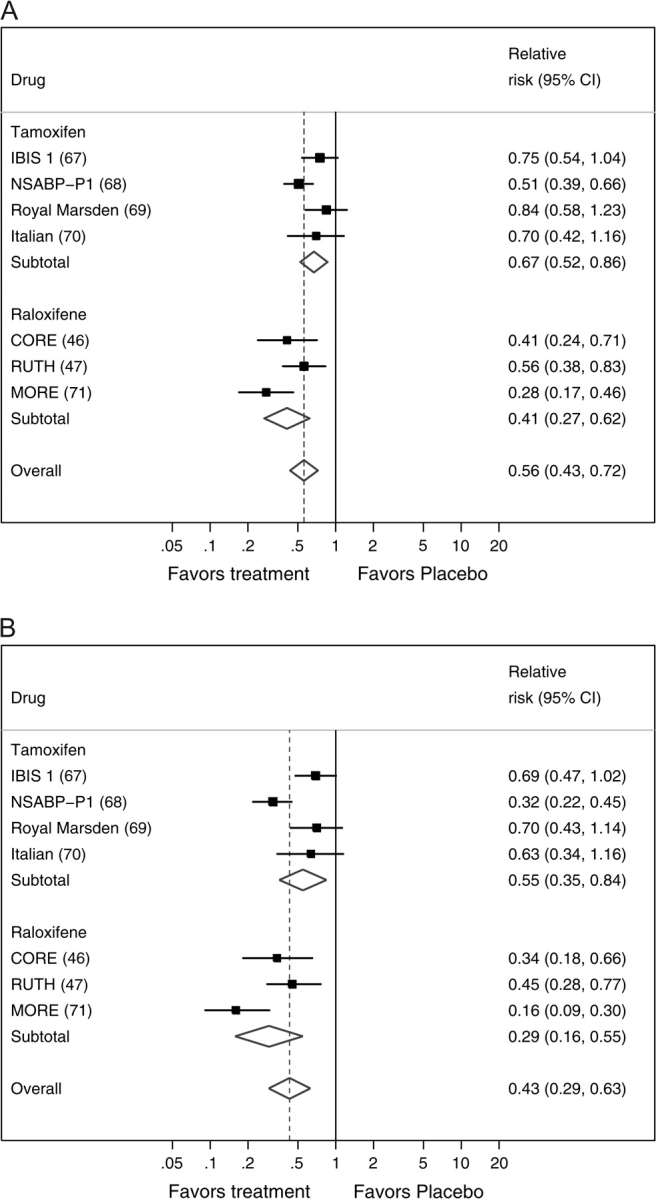
Forest plots of the risk of breast cancer from placebo-controlled trials of tamoxifen or raloxifene, with pooled estimates overall and for each treatment separately. A) All invasive breast cancer. B) Estrogen receptor–positive invasive breast cancer. The solid squares are centered on the point estimate from each study, and the horizontal line through each square represents the 95% CI for the study estimate. The size of each square represents the weight of the study in the meta-analysis. The center of each diamond represents the summary estimate of the effect size, and the horizontal tips represent the 95% CI. The solid vertical line corresponds to no effect, and the dashed vertical line corresponds to the summary estimate. CI = confidence interval.
Table 4.
Selection criteria and design of placebo-controlled randomized trials of tamoxifen and raloxifene for reduction in risk of breast cancer*
| Drug, trial, author, year (reference) | Entry criteria | No. of women analyzed; median or mean age; % of women who were older than 50 y or postmenopausal | Median duration of therapy |
| Tamoxifen | |||
| IBIS-1, Cuzick, 2002 (67) | Age 35–70 y; risk factors indicating an increased risk of breast cancer depending on age (twofold increase at 45–70 y, fourfold increase at 40–44 y, and a 10-fold increase at 30–39 y) | 7144; 50.8 y (mean); 49% postmenopausal | 50 mo |
| NSABP-P1, Fisher, 1998 (68) | Age ≥60 y or equal to 35–59 y with ≥1.66% predicted risk of breast cancer or history of lobular carcinoma in situ | 13 388; 61% age 50 y or older | 50 mo |
| Royal Marsden, Powles, 1998 (69) | Age 30–70 y with a history of breast cancer in one or more first-degree relatives | 2471; 47 y (median); 34% postmenopausal | 70 mo |
| Italian, Veronesi, 1998 (70) | Age 35–70 y with hysterectomy | 5378; 51 y (median) | 30.5 mo |
| Raloxifene | |||
| MORE, Cauley, 2001 (71) | Postmenopausal and age 80 y or younger with osteoporosis | 7705 (5219 on raloxifene and 2576 on placebo); 66.5 y (mean) | 47.4 mo |
| CORE, Martino, 2004 (46) | Postmenopausal women enrolled in MORE who agreed to continue in CORE for 4 more years | 4011 (2725 on raloxifene and 1286 on placebo); 65.8 y (mean) | 4 y |
| RUTH, Barrett-Connor, 2006 (47) | Postmenopausal and age 55 y or older with or at high risk of coronary heart disease | 10 101; 67.5 y (mean) | 60.6 mo |
IBIS-1 = International Breast Cancer Intervention Study-1. NSABP-P1 = National Surgical Adjuvant Breast and Bowel Project P-1 Study; MORE = Multiple Outcomes of Raloxifene Evaluation trial; CORE = Continuing Outcomes of Raloxifene Evaluation; RUTH = Raloxifene Use for the Heart trial.
Lifestyle Changes and the Modification of Breast Cancer Risk
A recent systematic review of 35 studies (50) found substantial heterogeneity in the methods and results from studies of physical activity and breast cancer risk. The authors concluded that there was evidence for an association between leisure time activity and risk of breast cancer from case–control studies, but there was inconsistent evidence about the association for total activity and for premenopausal breast cancer. We found three subsequent prospective cohort studies with 6564 breast cancer patients (72–74), and all three concluded that increased total activity and recreational physical activity were associated with a decreased risk of breast cancer in postmenopausal women (Table 5).
Table 5.
Studies of lifestyle changes and risk of breast cancer in this analysis*
| Lifestyle component, first author, year (reference) | Risk factor(s) | Type of study; No. of breast cancer patients | Results |
| Physical exercise | |||
| Monninkhof, 2007 (50) | Total and leisure time activity | Systematic review; 6 cohort studies and 29 case–control studies | Total activity: overall association between total activity and breast cancer was too small and inconclusive |
| Leisure time activity: prospective studies found inconsistent evidence; higher quality case–control studies found strong evidence for postmenopausal breast cancer but inconclusive evidence for premenopausal breast cancer | |||
| Dallal, 2007 (72) | Strenuous long-term exercise | Prospective cohort; 593 combined pre- and postmenopausal | Comparison of >5 vs <0.5 h/wk/y: RR = 0.80 (95% CI = 0.69 to 0.94) |
| Lahmann, 2007 (73) | Current total physical activity | Prospective cohort; 869 premenopausal and 2554 postmenopausal | Comparison of highest vs lowest quartile of household activity: in premenopausal women, RR = 0.71 (95% CI = 0.55 to 0.90); in postmenopausal women, RR = 0.81 (95% CI = 0.70 to 0.93) |
| Bardia, 2006 (74) | Current recreational physical activity | Prospective cohort; 2548 postmenopausal | Comparison of high vs low activity levels: RR = 0.86 (95% CI = 0.78 to 0.96) |
| Alcohol consumption | |||
| Key, 2006 (53) | Alcohol consumption | Meta-analysis; 75 728 pre- and postmenopausal cases | Comparison of drinkers vs nondrinkers†: 22% (95% CI = 9% to 37%) higher risk among drinkers |
| Tjonneland, 2007 (52) | Current alcohol consumption | Prospective cohort; 4285 postmenopausal | Alcohol intake comparison: per 10 g/d, RR = 1.03 (95% CI = 1.01 to 1.05); >19 g/d vs no intake (recent consumption), RR = 1.13 (95% CI = 1.01 to 1.25) |
| Morch, 2007 (76) | Current alcohol consumption and binge drinking | Prospective cohort; 101 premenopausal and 348 postmenopausal | Comparison: 22–27 vs 1–3 drinks per week: RR = 2.30 (95% CI = 1.56 to 3.39); 10–15 vs 1–3 drinks per weekend: RR = 1.49 (95% CI = 1.04 to 2.13) |
| Zhang, 2007 (77) | Current alcohol consumption | Prospective cohort; 1484‡ | Alcohol intake comparison of 0 vs >30 g/d: RR for invasive cancer = 1.43 (95% CI = 1.02 to 2.02) |
| Visvanathan, 2007 (78) | Current alcohol consumption | Nested case–control; 271 premenopausal and 50 postmenopausal | Comparison of nondrinkers vs drinkers of any amount of alcohol: OR = 1.40 (95% CI = 0.97 to 2.03) |
| Stolzenberg-Solomon, 2006 (79) | Current alcohol consumption | Prospective cohort; 691 postmenopausal | Comparison of highest vs lowest quintile: RR = 1.37 (95% CI = 1.08 to 1.76) |
| Suzuki, 2005 (80) | Current alcohol consumption | Prospective cohort; 1188 postmenopausal | Comparison of drinkers of ≥10 g of alcohol per day vs nondrinkers: RR for ER+ cancer = 1.35 (95% CI = 1.02 to 1.80) |
| Horn-Ross, 2004 (81) | Current alcohol consumption | Prospective cohort; 17422 | Comparison of drinkers of ≥20 g of alcohol per day vs nondrinkers: RR = 1.28 (95% CI = 1.06 to 1.54) |
| Weight change | |||
| Ahn, 2007 (82) | Total adult weight change and by age intervals | Prospective cohort; 2111 postmenopausal (1740 invasive) cases | Among nonusers of postmenopausal hormones: comparison of no change vs gained 10–19 kg, RR = 1.27 (95% CI = 0.90 to 1.87); no change vs gained 30–39 kg, RR = 1.87 (95% CI = 1.29 to 2.72); no change vs gained ≥50 kg, RR = 2.15 (95% CI = 1.35 to 3.42) |
| Among nonusers of postmenopausal hormones: no statistically significant associations | |||
| Eliassen, 2006 (83) | Total adult weight gain | Prospective cohort; 4393 postmenopausal | Comparison of no weight gain vs gained >10 kg since age 18 y, RR = 1.18 (95% CI = 1.03 to 1.35); no weight gain vs gained >25 kg since age 18 y, RR = 1.45 (95% CI = 1.27 to 1.66) |
| Harvie, 2005 (84) | Total adult weight change by age intervals | Prospective cohort; 1987 postmenopausal | Comparison of increased weight vs maintained or lost weight until age 30 y or lost weight from age 30 y to menopause, RR = 0.36 (95% CI = 0.22 to 0.60); increased weight vs maintained or lost weight from age 30 y to menopause or lost weight after menopause, RR = 0.48 (95% CI = 0.22 to 0.65) |
| Lahmann, 2005 (85) | Total adult weight gain | Prospective cohort; 264 premenopausal and 1094 postmenopausal | Comparison of stable weight (±2 kg) vs gained 15–20 kg since age 18 y (in postmenopausal women not currently using hormone replacement therapy): RR = 1.50 (95% CI = 1.06 to 2.13) |
| Feigelson, 2004 (86) | Total adult weight gain | Prospective cohort; 1934 postmenopausal | Comparison of stable weight (±5 pounds) vs gained 21–30 pounds since age 18 y: RR = 1.4 (95% CI = 1.1 to 1.8) |
| Radimer, 2004 (87) | Total adult weight gain | Prospective cohort; 2873 postmenopausal | Comparison of stable weight vs gained 20–25 kg since age 25 y, RR = 2.6 (95% CI = 1.4 to 5.1); stable weight vs gained 15–20 kg since age 25 y, RR = 1.8 (95% CI = 1.0 to 3.5) |
| Huang, 1997 (88) | Total and current adult weight gain | Prospective cohort; 1000 premenopausal and 1517 postmenopausal | Comparison of stable weight vs gained >20 kg since age 18 y (in postmenopausal women whom have never used hormone replacement therapy): RR = 1.99 (95% CI = 1.43 to 2.76) |
| van den Brandt, 1997 (89) | Total adult weight gain | Prospective cohort; 626 postmenopausal | Comparison of no weight gain vs gained >25 kg since age 20 y: RR = 1.57 (95% CI = 0.99 to 2.47)§ |
| Barnes-Josiah, 1995 (90) | Total adult weight gain | Prospective cohort; 769 postmenopausal | Comparison of no weight gain vs gained >19.1 kg since age 18 y (from starting BMI <20.5), RR = 1.92 (95% CI = 1.45 to 2.53); no weight gain vs gained >19.1 kg since age 18 y (from BMI >20.5), RR = 1.59 (95% CI = 1.19 to 2.12) |
| Fruit and vegetable consumption | |||
| Smith-Warner, 2001 (51) | Total fruit and vegetable consumption | Pooled analysis; 7377 combined pre- and postmenopausal | Comparison of highest vs lowest decile intakes: fruits, RR = 0.93 (95% CI = 0.86 to 1.00); vegetables, RR = 0.96 (95% CI = 0.89 to 1.04); total fruit and vegetables, RR = 0.93 (95% CI = 0.86 to 1.00) |
| Riboli, 2003 (52) | Fruit and vegetable consumption | Meta-analysis; 8712 combined pre- and postmenopausal | Comparison per increased daily intakes of 100 g/d (cohort studies only)‖: fruits, RR = 0.99 (95% CI = 0.98 to 1.00); vegetables, RR = 1.00 (95% CI = 0.97 to 1.02) |
| Cade, 2007 (91) | Total fiber consumption (fruit and vegetable sources) | Prospective cohort; 257 premenopausal and 350 postmenopausal | Comparison of highest vs lowest quartile of fruit fiber intake: among premenopausal women, RR = 0.81 (95% CI = 0.44 to 1.49), and among postmenopausal women, RR = 1.10 (95% CI = 0.66 to 1.84); highest vs lowest quartile of vegetable fiber intake: among premenopausal women, RR = 1.26 (95% CI = 0.73 to 2.18), and among postmenopausal women, RR = 1.20 (95% CI = 0.74 to 1.94) |
| van Gils, 2005 (92) | Total fruit and vegetable consumption | Prospective cohort; 3659 combined pre- and postmenopausal | Comparison of highest vs lowest quintiles of intake: fruits, RR = 1.09 (95% CI = 0.94 to 1.25); vegetables, RR = 0.98 (95% CI = 0.84 to 1.14); fruit and vegetable juices, RR = 1.05 (95% CI = 0.92 to 1.20) |
| Sieri, 2004 (93) | Salad vegetable intake | Prospective cohort; 207 combined pre- and postmenopausal | Comparison of highest vs lowest tertile of salad vegetable intake: RR = 066 (95% CI = 0.47 to 0.95) |
| Olsen, 2003 (94) | Current fruit, vegetable, and juice consumption | Prospective cohort; 425 postmenopausal | Comparison per total intake increment of 100 g/d: RR = 1.02 (95% CI = 0.98 to 1.06) |
RR = relative risk; CI = confidence interval; OR = odds ratio; ER+ = estrogen receptor positive; BMI = body mass index.
Definition of nondrinker varied by study.
Menopausal status was not specified.
Authors also note that “weight change was not associated significantly with breast cancer risk … the trends in relative risk also was inconsistent.”
All studies included fruits (RR = 0.99, 95% CI = 0.98 to 1.00) and vegetables (RR = 0.96, 95% CI = 0.94 to 0.98).
A recent meta-analysis of observational studies (53) reported that postmenopausal women who drank alcohol had a 22% (95% CI = 9% to 37%) higher relative risk of breast cancer than those who do not drink alcohol (Table 5). The analysis estimated that every additional 10 g of ethanol consumed per day (approximately one drink) was associated with a 10% (95% CI = 5% to 15%) increase in relative risk. We found seven subsequent large prospective studies (75–81) that assessed the association between alcohol intake and risk of breast cancer; these seven studies plus the meta-analysis included 85 898 patients with breast cancer. All seven studies subsequent to the meta-analysis also found statistically significant associations between increased alcohol intake and increased risk of breast cancer (Table 5). The associations were all modest and consistent with those reported by Key et al. (53) [six (76–81) of the seven studies estimated that the relative risk of breast cancer was 1.28- to 1.5-fold greater among those who typically consumed approximately two drinks per day compared with those who did not drink alcohol or who drank less than three drinks per week].
We found nine prospective cohort studies (83–90) with a total of 18 508 patients with breast cancer that addressed the association between change in weight and subsequent risk of breast cancer, and all nine reported that increased weight from younger to older ages was associated with a statistically significant increased risk of breast cancer for pre- and postmenopausal women. The categories of ages and weights varied from study to study, precluding a quantitative summary of the association (Table 5). One study found that the association between change in weight and breast cancer, however, was not statistically significant among women who used postmenopausal hormone therapy (82).
We found six prospective studies (51,52,91–94) of fruit and vegetable intake that included 20 987 women with breast cancer. All but one (93) of these studies reported no statistically significant association between increased fruit and vegetable intake and risk of breast cancer (Table 5).
We found two large randomized trials of low-fat diets for prevention of breast cancer (54,55). Results from the Women's Health Initiative (54) indicate that a low-fat diet might reduce the relative risk of breast cancer by approximately 9% (95% CI = −1% to +17%); however, the estimated reduction was not statistically significant. Another randomized trial in women with early-stage breast cancer (55) reported that a reduction in the amount of dietary fat of 18–19 g was associated with a decreased risk of breast cancer recurrence (hazard ratio = 0.76; 95% CI = 0.60 to 0.98) (55).
Discussion
These systematic reviews found that a combination of risk factors with breast density was the best approach to estimating a woman's risk of breast cancer. We also found that both tamoxifen and raloxifene reduced the risk of invasive breast cancer. Thus, the evidence supports systematic assessment of women's risk of breast cancer and the recommendation that women at high risk consider chemoprevention to reduce that risk. An additional finding of our review was that most studies suggest that exercise, weight reduction, low-fat diet, and reduced alcohol intake may reduce a woman's risk of breast cancer, supporting that recommendations for lifestyle changes should be part of programs for primary prevention of breast cancer.
Guidelines (3–5) published in 2002 and focused on selecting patients for chemoprevention of breast cancer with tamoxifen recommended that physicians consider prescribing tamoxifen for women who have a high risk for breast cancer and who are at low risk of adverse effects. There have been several developments in prevention of breast cancer since then, including new risk models using assessment of breast density and approval by the Food and Drug Administration of raloxifene for the prevention of breast cancer. The guidelines did not consider sex hormone levels for assessing risk or lifestyle changes for prevention of breast cancer.
Our meta-analysis found that breast density, as determined by either qualitative BI-RADS or quantitative methods, is a strong risk factor for breast cancer. The combination of breast density by either method with risk factors provides the best estimates of breast cancer risk.
Although estradiol and testosterone levels are associated with the risk of developing estrogen receptor–positive breast cancer (36,38), these measurements are not yet ready for routine clinical use because commonly used methods are expensive and only moderately correlated with each other (95). There is a need for a standardized and inexpensive method for measuring sex hormone levels with established value for improving estimates of the risk of breast cancer based on assessments of risk factors and breast density.
The US Food and Drug Administration has approved tamoxifen and raloxifene for prevention of breast cancer in high-risk women. Although our meta-analysis suggested that raloxifene may have a somewhat greater benefit than tamoxifen, the Study of Tamoxifen and Raloxifene, a randomized trial that directly compared these two agents, found that the two drugs had essentially identical effects on the risk of invasive breast cancer (96). Therefore, the choice of agent depends on consideration of the risk profile for potential adverse effects of an individual patient (46,47,68,96–99). The level of risk for breast cancer at which chemoprevention should be considered depends on the balance of benefits and costs of therapy. Analyses to define that level of risk are underway.
Our systematic reviews support recommending exercise, weight management, and reducing alcohol intake to lessen breast cancer risk in postmenopausal women. In contrast, we found that increased intake of fruits and vegetables was not associated with a decreased risk of breast cancer. The results of our reviews of studies published up to 2008 agree with a systematic review by Michels et al. (100) of studies published up to 2005. They found associations between body mass index, weight gain, or alcohol intake and increased risk of postmenopausal breast cancer and no association between fruit and vegetable intake and risk of invasive breast cancer. They also found no association between dietary intake of antioxidant vitamins A, C, and E and carotenoids, and they observed inconsistent or no associations with blood levels of antioxidant vitamins.
Our review and the studies on which it is based have limitations. Most of the evidence that we found about assessing and reducing risk of breast cancer involved postmenopausal women. There is less evidence that combinations of risk factors and breast density also identify high-risk premenopausal women who may consider ways to reduce their risk. Models that combine breast density and risk factors for breast cancer still have modest predictive accuracy for breast cancer (c-statistics range = 0.63 to 0.67); thus, there is a need for new markers that are strongly associated with risk of breast cancer. Although breast density is a strong risk factor for breast cancer, BI-RADS grading that could be widely used has only modest reproducibility and more reproducible quantitative approaches are not yet validated or feasible for clinical use; thus, our estimate of increased predictive accuracy may not be applicable to clinical practice at the current time. Absolute rates of benefits and harms provide clinically meaningful estimates of the value of risk markers and treatments to reduce risk. However, the studies in our reviews had diverse designs, populations with different degrees of risk, and methods of analyzing and expressing data that precluded reporting the results about benefits and harms as absolute rates.
A further limitation is that evidence about lifestyle changes to reduce breast cancer risk is generally based on observational studies. Components of food intake are complex and difficult to ascertain by questionnaire, and self-report has limited accuracy that tends to attenuate associations. There may be interactions or associations between intake of nutrients, such as dietary fat, and total energy intakes or between intakes and personal characteristics, such as body fat, estrogen levels, intake of alcohol, or use of medications. This complexity underscores the uncertainties inherent in observational studies of diet and risk of breast cancer and the necessity of large randomized trials to quantify the effects of specific dietary changes on the risk of breast cancer. The strength of associations between lifestyle changes and risk of breast cancer is modest. Nevertheless, because these lifestyle changes are safe, they can be recommended to all women regardless of breast cancer risk.
Several practical issues must be addressed before systematic assessment for risk of breast cancer is implemented widely. Physicians and patients will need to be educated about breast cancer risk and ways to reduce risk. Systems to routinely assess risk factors and breast density, and to report the patient's risk of breast cancer to her and to her physician, must be developed. Risk estimates would need to be communicated to women in ways that minimize inappropriate worry and support well-informed decisions (101,102). Assessing risk would also identify women who have a strong family history of breast cancer that may warrant genetic testing, so reports will need to encourage appropriate referral for genetic counseling (103). Estimation of breast cancer risk may also be useful for deciding whether to refer a woman for additional assessment with magnetic resonance imaging (104).
In conclusion, evidence from these reviews supports systematic assessment of postmenopausal women for breast cancer risk with risk factors and assessment of breast density. Chemoprevention should be considered for those at high risk; however, cost–benefit analyses are needed to provide specific recommendations about who should be offered chemoprevention. Several lifestyle changes can be recommended to postmenopausal women, regardless of their estimated risk category.
Funding
Supported in part by the Daniel and Phyllis Da Costa Fund at the California Pacific Medical Center Foundation (S.R.C.) and a Breast Cancer Surveillance Consortium cooperative agreement CA63740 (K.K.). Dr S. R. Cummings has received research support and consulting fees from Eli Lilly. Dr V. Vogel has received consulting fees and honoraria from Eli Lilly, AstraZeneca, Pfizer, and Novartis. Drs J. Shepherd, K. Kerlikowske, and S. R. Cummings share a patent on a phantom device used for breast densitometry. Dr J. Cuzick is a statistical consultant for AstraZeneca and has served as an advisory board member for Eli Lilly.
Footnotes
Jamie Low, Liezl Concepcion, and Chantelle Thomas assisted with the referencing, preparation, and submission of the manuscript. There are no original data. Authors had access to all the results of reviews and meta-analyses, take responsibility for the accuracy of the analyses, and had authority over manuscript preparation and approved the decision to submit the manuscript for publication.
The funding sources for this work, the National Cancer Institute and the Daniel and Phyllis Da Costa Fund, had no role in developing or approving the manuscript. Dr J. A. Tice; Mr S. Bauer; and Drs W. S. Browner, R. Smith-Bindman, and C. Vachon have no potential financial conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 pt 1):347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Chemoprevention of breast cancer: recommendations and rationale. Ann Intern Med. 2002;137(1):56–58. doi: 10.7326/0003-4819-137-1-200207020-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(1):59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Col N, Winer EP, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol. 2002;20(15):3328–3343. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan CP, Haas JS, Perez-Stable EJ, et al. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15(1):162–166. doi: 10.1158/1055-9965.EPI-04-0758. [DOI] [PubMed] [Google Scholar]
- 7.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelman D, Colditz GA, Hunter D, Hertzmark E. Validation of the Gail et al. model for predicting individual breast cancer risk. J Natl Cancer Inst. 1994;86(8):600–607. doi: 10.1093/jnci/86.8.600. [DOI] [PubMed] [Google Scholar]
- 9.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 10.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–366. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 11.Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94(2):115–122. doi: 10.1007/s10549-005-5152-4. [DOI] [PubMed] [Google Scholar]
- 12.Novotny J, Pecen L, Petruzelka L, et al. Breast cancer risk assessment in the Czech female population—an adjustment of the original Gail model. Breast Cancer Res Treat. 2006;95(1):29–35. doi: 10.1007/s10549-005-9027-5. [DOI] [PubMed] [Google Scholar]
- 13.Decarli A, Calza S, Masala G, Specchia C, Palli D, Gail MH. Gail model for prediction of absolute risk of invasive breast cancer: independent evaluation in the Florence-European Prospective Investigation into Cancer and Nutrition cohort. J Natl Cancer Inst. 2006;98(23):1686–1693. doi: 10.1093/jnci/djj463. [DOI] [PubMed] [Google Scholar]
- 14.Claus EB, Risch NJ, Thompson WD. Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol. 1990;131(6):961–972. doi: 10.1093/oxfordjournals.aje.a115616. [DOI] [PubMed] [Google Scholar]
- 15.Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- 16.Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993;28(2):115–120. doi: 10.1007/BF00666424. [DOI] [PubMed] [Google Scholar]
- 17.Rosner B, Colditz GA. Nurses’ Health Study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst. 1996;88(6):359–364. doi: 10.1093/jnci/88.6.359. [DOI] [PubMed] [Google Scholar]
- 18.Rockhill B, Byrne C, Rosner B, Louie MM, Colditz G. Breast cancer risk prediction with a log-incidence model: evaluation of accuracy. J Clin Epidemiol. 2003;56(9):856–861. doi: 10.1016/s0895-4356(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Tsukuma H, Tanaka H, Ajiki W, Oshima A. Estimation of individualized probabilities of developing breast cancer for Japanese women. Breast Cancer. 2003;10(1):54–62. doi: 10.1007/BF02967626. [DOI] [PubMed] [Google Scholar]
- 21.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98(17):1215–1226. doi: 10.1093/jnci/djj332. [DOI] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Anderson GL, Lane DS, et al. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst. 2007;99(22):1695–1705. doi: 10.1093/jnci/djm224. [DOI] [PubMed] [Google Scholar]
- 24.Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98(17):1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 25.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmigiani G, Chen S, Iversen ES, Jr, et al. Validity of models for predicting BRCA1 and BRCA2 mutations. Ann Intern Med. 2007;147(7):441–450. doi: 10.7326/0003-4819-147-7-200710020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 28.Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40(11):807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormack VA, Dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 30.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 31.Vachon CM, Brandt KR, Ghosh K, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(1):43–49. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 32.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 33.Hwang ES, Miglioretti DL, Ballard-Barbash R, Weaver DL, Kerlikowske K. Association between breast density and subsequent breast cancer following treatment for ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2587–2593. doi: 10.1158/1055-9965.EPI-07-0458. [DOI] [PubMed] [Google Scholar]
- 34.Key T, Appleby P, Barnes I, Reeves G. Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 35.Manjer J, Johansson R, Berglund G, et al. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden) Cancer Causes Control. 2003;14(7):599–607. doi: 10.1023/a:1025671317220. [DOI] [PubMed] [Google Scholar]
- 36.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE, rogen Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 37.Zeleniuch-Jacquotte A, Shore RE, Koenig KL, et al. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 2004;90(1):153–159. doi: 10.1038/sj.bjc.6601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings SR, Lee JS, Lui LY, Stone K, Ljung BM, Cauleys JA. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1047–1051. doi: 10.1158/1055-9965.EPI-04-0375. [DOI] [PubMed] [Google Scholar]
- 39.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 40.Zeleniuch-Jacquotte A, Gu Y, Shore RE, et al. Postmenopausal levels of sex hormones and risk of breast carcinoma in situ: results of a prospective study. Int J Cancer. 2005;114(2):323–327. doi: 10.1002/ijc.20694. [DOI] [PubMed] [Google Scholar]
- 41.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97(10):755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 42.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 43.Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1) J Natl Cancer Inst. 2006;98(2):110–115. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- 44.Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287(2):216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 45.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 46.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 47.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 48.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 49.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 50.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 51.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285(6):769–776. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
- 52.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 suppl):559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 53.Key J, Hodgson S, Omar RZ, et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17(6):759–770. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 54.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 55.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 56.Gail MH, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86(8):573–575. doi: 10.1093/jnci/86.8.573. [DOI] [PubMed] [Google Scholar]
- 57.Boyd NF, Dite GS, Stone J, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347(12):886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 58.Guo YP, Martin LJ, Hanna W, et al. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10(3):243–248. [PubMed] [Google Scholar]
- 59.Li T, Sun L, Miller N, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 60.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 61.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37(5):2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Wolfe JN, Saftlas AF, Salane M. Mammographic parenchymal patterns and quantitative evaluation of mammographic densities: a case-control study. AJR Am J Roentgenol. 1987;148(6):1087–1092. doi: 10.2214/ajr.148.6.1087. [DOI] [PubMed] [Google Scholar]
- 63.D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR BI-RADS-Mammography. 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 64.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 65.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090–2095. [PubMed] [Google Scholar]
- 66.Gill JK, Maskarinec G, Pagano I, Kolonel LN. The association of mammographic density with ductal carcinoma in situ of the breast: the multiethnic cohort. Breast Cancer Res. 2006;8(3):R30. doi: 10.1186/bcr1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 68.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 69.Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352(9122):98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 70.Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet. 1998;352(9122):93–97. doi: 10.1016/s0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 71.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65(2):125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 72.Dallal CM, Sullivan-Halley J, Ross RK, et al. Long-term recreational physical activity and risk of invasive and in situ breast cancer: the California Teachers Study. Arch Intern Med. 2007;167(4):408–415. doi: 10.1001/archinte.167.4.408. [DOI] [PubMed] [Google Scholar]
- 73.Lahmann PH, Friedenreich C, Schuit AJ, et al. Physical activity and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16(1):36–42. doi: 10.1158/1055-9965.EPI-06-0582. [DOI] [PubMed] [Google Scholar]
- 74.Bardia A, Hartmann LC, Vachon CM, et al. Recreational physical activity and risk of postmenopausal breast cancer based on hormone receptor status. Arch Intern Med. 2006;166(22):2478–2483. doi: 10.1001/archinte.166.22.2478. [DOI] [PubMed] [Google Scholar]
- 75.Tjonneland A, Christensen J, Olsen A, et al. Alcohol intake and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2007;18(4):361–373. doi: 10.1007/s10552-006-0112-9. [DOI] [PubMed] [Google Scholar]
- 76.Morch LS, Johansen D, Thygesen LC, et al. Alcohol drinking, consumption patterns and breast cancer among Danish nurses: a cohort study. Eur J Public Health. 2007;17(6):624–629. doi: 10.1093/eurpub/ckm036. [DOI] [PubMed] [Google Scholar]
- 77.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women's Health Study. Am J Epidemiol. 2007;165(6):667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 78.Visvanathan K, Crum RM, Strickland PT, et al. Alcohol dehydrogenase genetic polymorphisms, low-to-moderate alcohol consumption, and risk of breast cancer. Alcohol Clin Exp Res. 2007;31(3):467–476. doi: 10.1111/j.1530-0277.2006.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol usepostmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006;83(4):895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki R, Ye W, Rylander-Rudqvist T, Saji S, Colditz GA, Wolk A. Alcohol and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status: a prospective cohort study. J Natl Cancer Inst. 2005;97(21):1601–1608. doi: 10.1093/jnci/dji341. [DOI] [PubMed] [Google Scholar]
- 81.Horn-Ross PL, Canchola AJ, West DW, et al. Patterns of alcohol consumption and breast cancer risk in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(3):405–411. [PubMed] [Google Scholar]
- 82.Ahn J, Schatzkin A, Lacey JV, Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 83.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 84.Harvie M, Howell A, Vierkant RA, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2005;14(3):656–661. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 85.Lahmann PH, Schulz M, Hoffmann K, et al. Long-term weight change and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) Br J Cancer. 2005;93(5):582–589. doi: 10.1038/sj.bjc.6602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2004;13(2):220–224. doi: 10.1158/1055-9965.epi-03-0301. [DOI] [PubMed] [Google Scholar]
- 87.Radimer KL, Ballard-Barbash R, Miller JS, et al. Weight change and the risk of late-onset breast cancer in the original Framingham cohort. Nutr Cancer. 2004;49(1):7–13. doi: 10.1207/s15327914nc4901_2. [DOI] [PubMed] [Google Scholar]
- 88.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 89.van den Brandt PA, Dirx MJ, Ronckers CM, van den Hoogen P, Goldbohm RA. Height, weight, weight change, and postmenopausal breast cancer risk: the Netherlands Cohort Study. Cancer Causes Control. 8(1):39–47. doi: 10.1023/a:1018479020716. [DOI] [PubMed] [Google Scholar]
- 90.Barnes-Josiah D, Potter JD, Sellers TA, Himes JH. Early body size and subsequent weight gain as predictors of breast cancer incidence (Iowa, United States) Cancer Causes Control. 1995;6(2):112–118. doi: 10.1007/BF00052771. [DOI] [PubMed] [Google Scholar]
- 91.Cade JE, Burley VJ, Greenwood DC. Dietary fibre and risk of breast cancer in the UK Women's Cohort Study. Int J Epidemiol. 2007;36(2):431–438. doi: 10.1093/ije/dyl295. [DOI] [PubMed] [Google Scholar]
- 92.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293(2):183–193. doi: 10.1001/jama.293.2.183. [DOI] [PubMed] [Google Scholar]
- 93.Sieri S, Krogh V, Pala V, et al. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(4):567–572. [PubMed] [Google Scholar]
- 94.Olsen A, Tjonneland A, Thomsen BL, et al. Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr. 2003;133(7):2342–2347. doi: 10.1093/jn/133.7.2342. [DOI] [PubMed] [Google Scholar]
- 95.Lee JS, Ettinger B, Stanczyk FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91(10):3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 96.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 97.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 98.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 99.Veronesi U, Maisonneuve P, Sacchini V, Rotmensz N, Boyle P. Tamoxifen for breast cancer among hysterectomised women. Lancet. 2002;359(9312):1122–1124. doi: 10.1016/S0140-6736(02)08159-X. [DOI] [PubMed] [Google Scholar]
- 100.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109(12 suppl):2712–2749. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 101.Haas JS, Kaplan CP, Des Jarlais G, Gildengoin V, Perez-Stable EJ, Kerlikowske K. Perceived risk of breast cancer among women at average and increased risk. J Womens Health (Larchmt) 2005;14(9):845–851. doi: 10.1089/jwh.2005.14.845. [DOI] [PubMed] [Google Scholar]
- 102.Bottorff JL, Ratner PA, Johnson JL, et al. Women's responses to information on mammographic breast density. Can J Nurs Res. 2007;39(1):38–57. [PubMed] [Google Scholar]
- 103.Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 104.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]