Abstract
Serum phosphorus levels in the general population have been reported to be associated with cardiovascular morbidity and mortality and increased carotid intima-media thickness. The authors examined gender heterogeneity in the association of phosphorus with all-cause mortality and incident coronary artery disease using data from the Atherosclerosis Risk in Communities Study (1987–2001). Baseline phosphorus levels were higher in women and were associated differently among men and women with traditional atherosclerosis risk factors such as age, low density lipoprotein cholesterol, diabetes mellitus, and hypertension. In a multivariable-adjusted model, men in the highest quintile of serum phosphorus level (>3.8 mg/dL) had an increased mortality rate (hazard ratio = 1.45, 95% confidence interval: 1.12, 1.88), while women did not (hazard ratio = 1.18, 95% confidence interval: 0.89, 1.57). The multivariable likelihood ratio test of effect modification by gender was significant at α = 0.1 (P = 0.085) for all-cause mortality. Although the associations of phosphorus with coronary artery disease also appeared to differ substantially by gender, the multivariable test for effect modification suggested that the difference was consistent with random variation (P = 0.195). These results suggest the need for further investigation into gender differences in the contribution of mineral metabolism to cardiovascular disease in the general population.
Keywords: cardiovascular diseases, coronary artery disease, mortality, phosphorus, risk, sex factors
Current evidence indicates that elevated phosphorus levels are associated with increased mortality among patients with chronic kidney disease (1) and dialysis patients (2–5), probably through an increase in cardiovascular disease rates. Interestingly, 2 recent publications demonstrated that serum phosphorus levels within what is considered the normal range are associated with an increased risk of cardiovascular disease among persons without overt kidney disease (6, 7). It was also previously reported that serum phosphorus levels are linked with subclinical atherosclerosis in the general population, with the suggestion that this association may be limited to men (8) and that phosphorus levels differ substantially by gender (6, 7). However, the potential gender differences in the association of phosphorus with these outcomes have not been addressed (6, 7). Accordingly, in this analysis we examined the presence of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease (CAD) and all-cause mortality among subjects from the general population enrolled in the Atherosclerosis Risk in Communities (ARIC) Study.
MATERIALS AND METHODS
Study subjects
Subjects aged 45–64 years from the general population were recruited between 1987 and 1989 from 4 sites in the United States: Jackson, Mississippi; Forsyth County, North Carolina; Minneapolis, Minnesota; and Washington County, Maryland. Detailed descriptions of the methods and primary aims of the ARIC Study have been published previously (9). In total, 15,732 subjects were enrolled in the ARIC Study, provided consent for data-sharing, and had follow-up information. Of these subjects, 150 were excluded from the current analysis because phosphorus levels were missing, and 1,010 were excluded because of self-reported history of stroke or CAD (defined as self-reported myocardial infarction, coronary bypass surgery or angioplasty, or silent myocardial infarction detected at the first ARIC clinic visit by electrocardiography). Finally, we excluded 574 additional subjects: 392 with overt moderate-to-severe chronic kidney disease, as indicated by an estimated glomerular filtration rate less than 60 mL/minute/1.73 m2, and 182 with implausible estimated glomerular filtration rate values above 150 mL/minute/1.73 m2. This left 13,998 subjects available for analysis (56.6% women, 24.7% blacks).
Classification of diabetes mellitus was based on having either an 8-hour fasting plasma glucose concentration greater than 126 mg/dL or a nonfasting plasma glucose concentration greater than 200 mg/dL, or reporting having diabetes or taking diabetes medication. Hypertension was defined as diastolic blood pressure greater than or equal to 90 mm Hg, systolic blood pressure greater than or equal to 140 mm Hg, or self-reported use of medication for hypertension in the 2 weeks prior to the baseline interview. Current smoking status at baseline was defined according to self-report. Medication use was assessed for the 2-week period prior to the baseline clinic visit. At the time of the first clinic visit, subjects were asked to bring all prescription and nonprescription medications used during this period to the clinic, and medications were coded according to drug category.
Laboratory methods
Measurement of serum phosphorus level was performed at the baseline visit with the subject in a fasting state. During this visit, approximately 60 mL of blood was drawn for clinical chemistry, hematology, hemostasis, and lipid testing. For clinical chemistry analysis, including determination of phosphorus and creatinine levels, samples were incubated at room temperature for 30 minutes, centrifuged at 4°C, and then frozen at −70°C and sent on dry ice to a central clinical chemistry laboratory at the University of Minnesota, Minneapolis, Minnesota. Inorganic phosphate level was assessed using the DART phosphorus reagent (Coulter Diagnostics, Hialeah, Florida), which is a modification of the Daly and Ertingshausen method (10, 11).
At each study site, the blood tubes were either kept at room temperature for hematologic analysis or immediately stored on ice for hemostasis and lipid testing. After centrifugation and within 90 minutes of collection, all blood samples were frozen at −70°C for later processing. Hemostasis and lipid laboratory tests were conducted at centralized laboratories. Hematologic tests were performed at local laboratories. Further details on sample collection and laboratory procedures are available online (http://www.cscc.unc.edu/aric/pubuse/).
Renal function assessment
We calculated the estimated glomerular filtration rate using the abbreviated modification of diet in renal disease equation (12), as recommended in the guidelines of the National Kidney Foundation. In accordance with these guidelines, we calibrated serum creatinine values by subtracting 0.24 from each participant's measured value (13). Although there is an ongoing debate as to whether the Cockcroft-Gault equation or the modification of diet in renal disease equation should be used for estimation of glomerular filtration rate, each one of these methods offers advantages and limitations, and they often provide very close estimations. We simply decided to employ the most frequently used formula.
Study outcomes
For the present study, we utilized 2 outcomes: 1) incident CAD, defined as definite or probable fatal or nonfatal myocardial infarction or death due to CAD, and 2) all-cause mortality. CAD events in cohort members were identified through annual follow-up interviews of subjects and surveys of area hospital discharge lists for the names of study participants. When potential events were detected through follow-up interviews or when discharge summaries featured diagnosis codes indicative of possible cardiovascular disease, hospital records were abstracted and analyzed by trained ARIC Study personnel. Subsequent classification of definite or probable myocardial infarction was based upon a combination of chest pain, elevation in cardiac enzyme levels, and electrocardiographic changes. Classification of definite or probable CAD events in subjects with chest pain required either a positive electrocardiographic finding or abnormal cardiac enzyme levels. Classification of CAD events in subjects without chest pain required both a positive electrocardiographic finding and the presence of abnormal cardiac enzyme levels. Deaths among cohort members were identified through review of state health department lists of deaths corresponding to each study site, as well as systematic review of death certificates, annual follow-up interviews, obituary notices, and hospital records, interviews with one or more next of kin, physician questionnaires, and coroner or autopsy reports.
Subjects were followed through the end of 2001. Further information on follow-up procedures is publicly available elsewhere (14).
Statistical analyses
All analyses were completed using SAS 9.1 (SAS Institute Inc., Cary, North Carolina). We first compared serum phosphorus levels according to gender using a t test. Next we determined whether gender and other covariables were independent determinants of serum phosphorus level by fitting a linear model with phosphorus as the dependent variable and age, sex, black race, body mass index, diabetes, hypertension, total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, current smoking, estimated glomerular filtration rate, serum fibrinogen, postmenopausal status, and current use of estrogen replacement therapy as the independent variables. We performed backwards elimination to remove nonsignificant independent variables until all variables remaining in the model had P values less than 0.05.
For subsequent analysis, serum phosphorus level was categorized according to quintiles of the entire study population and expressed as a class variable to avoid imposing a linear relation. Although phosphorus levels differed significantly by gender, we used the same cutpoints for men and women to facilitate translation of results to the clinical world, where laboratory reference ranges for serum phosphorus levels do not differ by gender. To explore potential gender differences in the relation of baseline variables with phosphorus, we calculated mean levels of baseline covariables for men and women separately, according to quintile of serum phosphorus. Because phosphorus was strongly associated with age among men and women, we adjusted for age the means, frequencies, and P values for all other covariables. This was done by estimating least-square means using age-adjusted linear or logistic models in which phosphorus-quintile dummy variables and age were included as the independent variables and the covariable being assessed served as the dependent variable.
To gauge the association between serum phosphorus level and incident CAD and all-cause mortality, we first plotted age-adjusted CAD incidence and all-cause mortality curves for men and women according to quintile of phosphorus level, using survival output from Cox proportional hazards models stratified by phosphorus quintile. We used likelihood ratio tests in nonstratified Cox models to calculate overall P values for survival differences according to serum phosphorus observed in survival curves.
We then calculated crude incidence density rates for men and women according to serum phosphorus quintile and fitted the data to age- and multivariable-adjusted Cox proportional hazards models, testing for effect modification by gender, estrogen replacement therapy, and menopausal status using likelihood ratio tests. Multivariable models included adjustment for age, black race, current smoking, total cholesterol, HDL cholesterol, diabetes, hypertension, menopausal status, baseline use of estrogen replacement therapy, and estimated glomerular filtration rate. We tested all variables to ensure that they satisfied the proportional hazards assumption. Missing values for covariables included in multivariable models were imputed using multiple imputation methods (15). Fewer than 1% of the values for all covariables used in the multivariable analyses were missing and thus imputed.
RESULTS
Serum phosphorus levels were mostly within the normal range of 2.5–4.5 mg/dL, with fewer than 3% of the patients falling outside of this range. The mean serum phosphorus level was significantly higher among women (3.56 mg/dL; standard deviation, 0.46) than among men (3.25 mg/dL; standard deviation, 0.45) (P < 0.0001) (Figure 1). In multivariable analysis of the independent determinants of serum phosphorus level, male gender remained independently associated (P < 0.0001) with lower serum phosphorus levels (0.23 mg/dL lower than females) (Table 1). Current use of estrogen replacement therapy and hypertension were also associated with lower serum phosphorus levels, while postmenopausal status and current smoking were associated with higher serum phosphorus levels (Table 1). Total cholesterol, HDL cholesterol, and triglycerides were positively associated with serum phosphorus levels, while body mass index was negatively associated. Age, black race, diabetes, estimated glomerular filtration rate, and fibrinogen were not significantly associated with serum phosphorus level at α = 0.05 and were dropped from the model.
Figure 1.
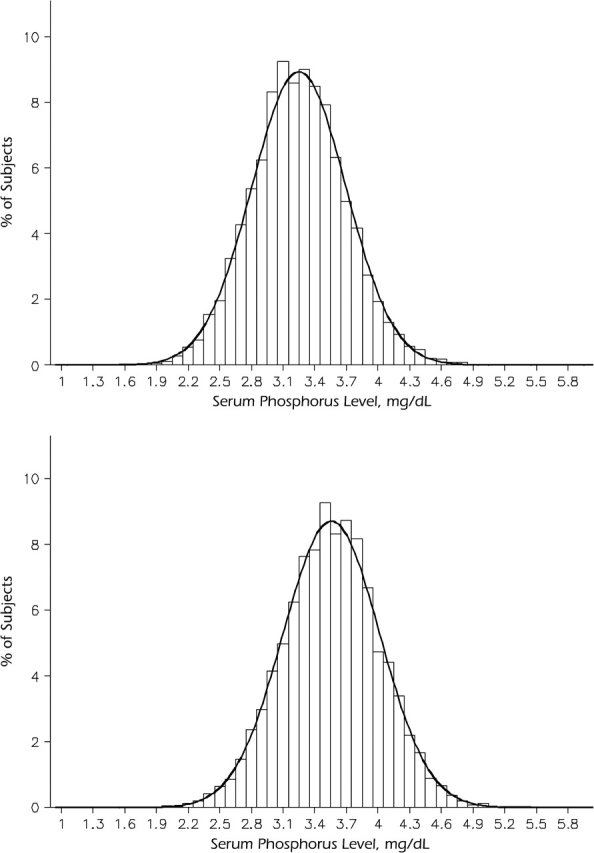
Distribution of serum phosphorus levels among men (top; mean = 3.25 mg/dL; standard deviation, 0.45) and women (bottom; mean = 3.56 mg/dL; standard deviation, 0.46), Atherosclerosis Risk in Communities Study, 1987–2001. T test for gender difference: P < 0.0001.
Table 1.
Independent Determinants of Serum Phosphorus Levela Among Participants in the Atherosclerosis Risk in Communities Study, 1987–2001*
| Independent Variable | βb | Standard Error |
| Male gender (yes vs. no) | −0.24 | 0.012 |
| Hypertension (yes vs. no) | −0.037 | 0.0088 |
| Body mass indexc (1 unit) | −0.0052 | 0.00082 |
| Total cholesterol (1 SD (41.6 mg/dL)) | 0.018 | 0.0043 |
| High density lipoprotein cholesterol (1 SD (17.0 mg/dL)) | 0.05 | 0.0050 |
| Triglycerides (1 SD (87.4 mg/dL)) | 0.04 | 0.0046 |
| Current smoking (yes vs. no) | 0.10 | 0.0092 |
| Postmenopausal (yes vs. no) | 0.12 | 0.012 |
| Current use of estrogen replacement therapy (yes vs. no) | −0.19 | 0.014 |
Abbreviation: SD, standard deviation.
P < 0.0001 for all variables in table.
Age, black race, diabetes mellitus, estimated glomerular filtration rate, and fibrinogen were removed from the model in backwards elimination with nonsignificance at α = 0.05.
Difference in mean serum phosphorus level (mg/dL) associated with a 1-unit increase in the independent variable.
Weight (kg)/height (m)2.
Examination of baseline characteristics according to gender suggested that many variables were associated with serum phosphorus differently among men and women (Table 2). For example, higher serum phosphorus levels were significantly associated with younger age among men (P = 0.02) but older age among women (P < 0.0001) (Table 2). Similarly, significant but opposite-trending associations among men and women were also observed for black race, body mass index, diabetes, and hypertension. Smoking, total cholesterol, and HDL cholesterol were positively associated with phosphorus level among both men and women, but low density lipoprotein cholesterol was associated with phosphorus only among women and triglycerides were associated with phosphorus only among men. Current estrogen use was lower and prevalence of menopause was higher at higher serum phosphorus levels (Table 2).
Table 2.
Baseline Data on Covariates According to Gender and Quintile of Serum Phosphorus Level, Atherosclerosis Risk in Communities Study, 1987–2001
| Variable | Serum Phosphorus Level (mg/dL) |
P Value | ||||
| <3.1 | 3.1–3.2 | 3.3–3.5 | 3.6–3.8 | >3.8 | ||
| No. of participants | ||||||
| Men | 2,007 | 1,092 | 1,555 | 946 | 523 | |
| Women | 1,050 | 883 | 1,943 | 1,984 | 2,012 | |
| Mean age, years | ||||||
| Men | 54.5 | 54.4 | 54.3 | 54.0 | 53.6 | 0.02 |
| Women | 52.8 | 53.0 | 53.5 | 54.0 | 54.4 | <0.0001 |
| Black race, % | ||||||
| Men | 20.4 | 20.7 | 23.3 | 22.7 | 28.8 | 0.0007 |
| Women | 33.4 | 28.7 | 28.7 | 28.3 | 27.3 | 0.01 |
| Mean body mass indexab | ||||||
| Men | 27.5 | 27.3 | 27.2 | 27.6 | 27.7 | 0.05 |
| Women | 29.2 | 28.5 | 27.8 | 27.6 | 26.7 | <0.0001 |
| Diabetes mellitusb, % | ||||||
| Men | 9.8 | 8.9 | 8.7 | 10.7 | 17.7 | <0.0001 |
| Women | 13.0 | 9.9 | 9.9 | 8.8 | 9.4 | 0.008 |
| Hypertensionb, % | ||||||
| Men | 32.4 | 30.6 | 28.1 | 32.4 | 35.3 | 0.01 |
| Women | 40.0 | 32.7 | 33.4 | 31.0 | 30.0 | <0.0001 |
| Current smokingb, % | ||||||
| Men | 24.2 | 26.4 | 27.4 | 30.9 | 33.7 | <0.0001 |
| Women | 15.1 | 18.6 | 22.0 | 26.5 | 33.1 | <0.0001 |
| Mean total cholesterol levelb, mg/dL | ||||||
| Men | 208.0 | 209.4 | 211.5 | 211.8 | 215.0 | 0.001 |
| Women | 213.2 | 212.6 | 217.3 | 217.6 | 222.6 | <0.0001 |
| Mean low density lipoprotein cholesterol levelb, mg/dL | ||||||
| Men | 138.4 | 138.2 | 139.1 | 138.9 | 139.8 | 0.92 |
| Women | 132.7 | 131.7 | 135.9 | 135.8 | 138.9 | <0.0001 |
| Mean high density lipoprotein cholesterol level, mg/dL | ||||||
| Men | 43.4 | 44.9 | 45.8 | 45.6 | 45.7 | <0.0001 |
| Women | 56.3 | 57.3 | 57.7 | 58.1 | 59.0 | 0.0008 |
| Mean total triglyceride levelb, mg/dL | ||||||
| Men | 132.7 | 135.1 | 137.7 | 144.9 | 167.7 | <0.0001 |
| Women | 122.0 | 117.9 | 120.2 | 119.8 | 124.3 | 0.20 |
| Mean estimated glomerular filtration ratebc, mL/minute/1.73 m2 | ||||||
| Men | 91.5 | 92.8 | 92.6 | 93.5 | 92.7 | 0.02 |
| Women | 95.4 | 94.5 | 93.5 | 94.2 | 94.1 | 0.08 |
| Mean fibrinogen levelb, mg/dL | ||||||
| Men | 295.1 | 294.4 | 293.4 | 295.9 | 298.4 | 0.60 |
| Women | 304.3 | 303.0 | 304.2 | 307.9 | 307.8 | 0.11 |
| Current estrogen use (women only)b, % | 25.2 | 25.5 | 20.2 | 18.5 | 13.8 | <0.0001 |
| Postmenopausal (women only)b, % | 71.7 | 69.7 | 73.9 | 77.7 | 81.6 | <0.0001 |
Weight (kg)/height (m)2.
P value and mean or percentage were adjusted for age.
Glomerular filtration rate calculated with the abbreviated modification of diet in renal disease equation (10).
Median follow-up for CAD events was 13.1 years (interquartile range, 12.3–13.9), and 922 subjects (63% men) experienced incident CAD. In gender-specific cumulative incidence plots of CAD, the age-adjusted incidence differed significantly by phosphorus quintile among men (P = 0.005) but not among women (P = 0.37) (Figure 2).
Figure 2.
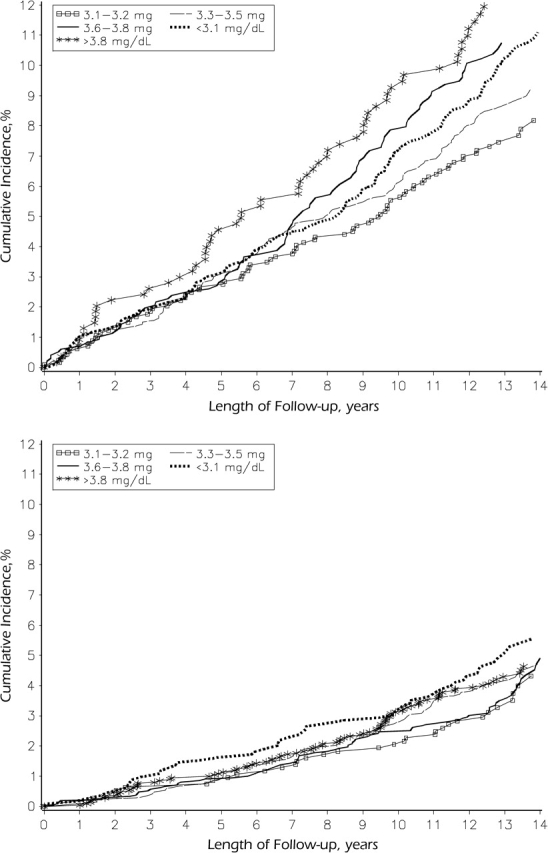
Cumulative incidence of coronary artery disease according to quintile of serum phosphorus level among men (top; for comparison of outcomes in various phosphorus quintiles, P = 0.005) and women (bottom; P = 0.37), Atherosclerosis Risk in Communities Study, 1987–2001.
Median follow-up for all-cause mortality was 13.2 years (interquartile range, 12.4–13.9), and 1,546 subjects (55% men) died. The age-adjusted cumulative incidence of all-cause mortality is shown in Figure 3. Likelihood ratio tests suggested that mortality differed significantly by phosphorus quintile among men (P < 0.0001) but not among women (P = 0.16).
Figure 3.
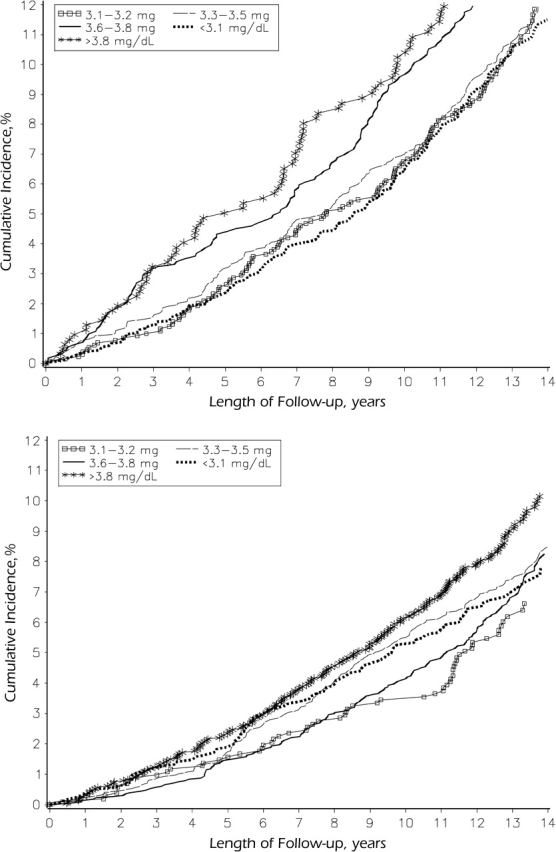
Age-adjusted all-cause mortality according to quintile of serum phosphorus level among men (top; for comparison of outcomes in various phosphorus quintiles, P < 0.0001) and women (bottom; P = 0.16), Atherosclerosis Risk in Communities Study, 1987–2001.
In age-adjusted Cox proportional hazards models, the likelihood ratio tests for effect modification by gender were significant at α = 0.1 for both CAD (P = 0.068) and mortality (P = 0.018) (Table 3). Compared with men with phosphorus levels of 3.1–3.3 mg/dL, the age-adjusted rate of CAD was 43% higher (95% confidence interval (CI): 6, 92) for those with phosphorus levels of 3.6–3.8 mg/dL and 81% higher (95% CI: 30, 250) for those with phosphorus levels greater than 3.8 mg/dL. Elevated phosphorus levels were not associated with CAD among women in this model. Men with phosphorus levels less than 3.1 mg/dL also experienced a 35% (95% CI: 5, 74) increased rate of CAD. A similar increase in CAD rate was noted in women, but it was not statistically significant (Table 3). After multivariable adjustment (Table 3), the rate of CAD remained significantly elevated for men with phosphorus levels greater than 3.8 mg/dL (hazard ratio = 1.45, 95% CI: 1.04, 2.01) or less than 3.1 mg/dL (hazard ratio = 1.30, 95% CI: 1.01, 1.68). Once more, a similar but nonsignificant increase in CAD rate was observed among women with low phosphorus levels. The P value for the multivariable-adjusted likelihood ratio test for effect modification by gender was 0.195 for the CAD outcome.
Table 3.
Incidence Density Rates and Hazard Ratios for Incident Coronary Artery Disease and All-Cause Mortality According to Gender and Quintile of Serum Phosphorus Level, Atherosclerosis Risk in Communities Study, 1987–2001
| Serum Phosphorus Level (mg/dL) |
P Valuea | |||||||||||||||||||
| <3.1 |
3.1–3.3 |
3.4–3.5 |
3.6–3.8 |
>3.8 |
||||||||||||||||
| No. of Cases | Person-Years | Rate or HR | 95% CI | No. of Cases | Person-Years | Rate or HR | No. of Cases | Person-Years | Rate or HR | 95% CI | No. of Cases | Person-Years | Rate or HR | 95% CI | No. of Cases | Person-Years | Rate or HR | 95% CI | ||
| Coronary artery disease | ||||||||||||||||||||
| Crude ID rateb | ||||||||||||||||||||
| Men | 204 | 24,608 | 8.29 | 83 | 13,526 | 6.14 | 134 | 19,020 | 7.05 | 96 | 11,286 | 8.51 | 64 | 6,117 | 10.46 | |||||
| Women | 54 | 13,223 | 4.08 | 33 | 11,344 | 2.91 | 85 | 24,677 | 3.44 | 81 | 25,401 | 3.19 | 88 | 25,394 | 3.46 | |||||
| Age-adjusted HR | ||||||||||||||||||||
| Men | 1.35 | 1.05, 1.74 | 1.0c | 1.16 | 0.88, 1.52 | 1.43 | 1.06, 1.92 | 1.81 | 1.30, 2.50 | 0.068 | ||||||||||
| Women | 1.48 | 0.94, 2.23 | 1.0c | 1.15 | 0.77, 1.72 | 1.03 | 0.69, 1.55 | 1.11 | 0.74, 1.65 | |||||||||||
| Multivariable-adjustedd HR | ||||||||||||||||||||
| Men | 1.30 | 1.01, 1.68 | 1.0c | 1.15 | 0.88, 1.51 | 1.33 | 0.99, 1.78 | 1.45 | 1.04, 2.01 | 0.195 | ||||||||||
| Women | 1.32 | 0.85, 2.03 | 1.0c | 1.07 | 0.71, 1.60 | 0.95 | 0.63, 1.42 | 0.95 | 0.63, 1.41 | |||||||||||
| Mortality | ||||||||||||||||||||
| Crude ID rateb | ||||||||||||||||||||
| Men | 252 | 25,586 | 9.85 | 140 | 13,861 | 10.10 | 203 | 19,638 | 10.34 | 147 | 11,712 | 12.55 | 102 | 6,394 | 15.95 | |||||
| Women | 54 | 13,223 | 4.08 | 33 | 11,344 | 2.91 | 85 | 24,677 | 3.44 | 81 | 25,401 | 3.19 | 88 | 25,394 | 3.46 | |||||
| Age-adjusted HR | ||||||||||||||||||||
| Men | 0.96 | 0.78, 1.19 | 1.0c | 1.03 | 0.83, 1.28 | 1.30 | 1.03, 1.64 | 1.73 | 1.34, 2.24 | 0.018 | ||||||||||
| Women | 1.20 | 0.87, 1.66 | 1.0c | 1.21 | 0.90, 1.61 | 1.09 | 0.82, 1.46 | 1.35 | 1.02, 1.79 | |||||||||||
| Multivariable-adjustedd HR | ||||||||||||||||||||
| Men | 0.97 | 0.79, 1.19 | 1.0c | 1.04 | 0.84, 1.29 | 1.25 | 0.99, 1.57 | 1.45 | 1.12, 1.88 | 0.085 | ||||||||||
| Women | 1.16 | 0.84, 1.60 | 1.0c | 1.12 | 0.84, 1.50 | 1.01 | 0.75, 1.35 | 1.18 | 0.89, 1.57 | |||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; ID, incidence density.
P value for gender effect modification.
Rate per 1,000 person-years.
Referent.
Adjusted for age, black race, current smoking, diabetes mellitus, hypertension, total cholesterol, high density lipoprotein cholesterol, estimated glomerular filtration rate, current use of estrogen replacement therapy, and menopausal status.
The age-adjusted mortality rate was elevated 73% (95% CI: 34, 224) among men with phosphorus levels greater than 3.8 mg/dL and 35% (95% CI: 2, 79) among women with phosphorus levels greater than 3.8 mg/dL (Table 3). With multivariable adjustment, the mortality rate remained 45% (95% CI: 12, 88) higher among men but only 18% (95% CI: 0.89, 57) higher among women in comparison with subjects with phosphorus levels of 3.1–3.3 mg/dL. The P value for the multivariable-adjusted likelihood ratio test for effect modification by gender was 0.085 for mortality as an outcome (Table 3).
Multivariable tests of effect modification by estrogen replacement therapy and menopausal status were not significant at α = 0.1 for either outcome.
DISCUSSION
Our results suggest the possibility of gender heterogeneity in the association of serum phosphorus level with mortality among members of the general population who are free of cardiovascular disease and overt renal failure, with elevated phosphorus levels being associated with increased mortality among men but not among women. We also observed a similar trend toward gender heterogeneity in the association of phosphorus with incident CAD, although the multivariable test for heterogeneity was not significant and was consistent with random variation. Interestingly, our data also suggest that serum phosphorus maintains different associations with age, race, serum cholesterol levels, diabetes, and several other risk factors for atherosclerosis in men and women.
Numerous studies have shown a link between hyperphosphatemia and mortality in patients undergoing hemodialysis (2–4, 16). In 2 recent publications, Block et al. (4) and Kalantar-Zadeh et al. (5) described an increased mortality rate with high phosphorus levels but also with low phosphorus levels in hemodialysis patients. While the increased mortality with low phosphorus levels in hemodialysis patients may be secondary to malnutrition and an associated enhanced inflammatory state (16), for subjects from the general population the potential mechanisms are less clear. In a small series of 255 subjects without renal disease, Kalaitzidis et al. (17) found an association between low serum phosphorus levels and the metabolic syndrome. In that study, significantly lower serum phosphorus concentrations were present among persons with progressively more components of the metabolic syndrome (P < 0.001). Nonetheless, in our study cohort, the prevalence of obesity, hypertension, diabetes, and low HDL cholesterol levels in relation to phosphorus levels differed by gender.
Kestenbaum et al. (1) reported associations between serum phosphorus level and mortality and myocardial infarction in male veterans with chronic kidney disease who were not undergoing dialysis. Each 1-mg/dL (0.323-mmol/L) increase in serum phosphate level was associated with a 23% increase in risk of death (95% CI: 1.12, 1.36) and a 35% increase in risk of acute myocardial infarction (hazard ratio = 1.35, 95% CI: 1.09, 1.66) (1). Finally, 2 recent publications showed an association between cardiovascular events and phosphorus levels in patients without known chronic kidney disease (7, 8). Tonelli et al. (6) demonstrated that in patients admitted to the hospital with acute myocardial infarction and decompensated heart failure, serum phosphorus levels were associated with all-cause mortality and recurrent events. These patients, however, were patients with known CAD and potentially covert renal disease. Dhingra et al. (7) demonstrated an association between phosphorus levels and incident cardiovascular events in approximately 3,000 subjects in the Framingham Offspring Study. What distinguishes our work is the analysis of data from a very large community-based cohort of free-living subjects with over 50% women, the careful assessment of cardiovascular endpoints, and the fact that phosphorus appears to be a marker of risk, independently of estimated glomerular filtration rate, but more so in men than in women. The series by Kestenbaum et al. (1) included 98% men, and neither Dhingra et al. (7) nor Tonelli et al. (6) provided gender-specific data, although both sets of authors indicated that serum phosphorus levels differed between genders. Whether estrogen and testosterone are related to this difference is unclear. In a recent study, Burnett-Bowie et al. (18) reported that the experimental suppression of testosterone levels in healthy volunteers was associated with increasing serum phosphorus concentration. Estrogens are thought to facilitate the incorporation of phosphorus in remodeling bone; therefore, it seems appropriate that with advancing age, serum phosphorus levels in women may increase. However, at this time we are unable to offer a clear rationale for the different associations of phosphorus level with atherosclerosis risk factors and outcomes in men and women.
A number of mechanisms could explain the association of phosphorus with an adverse outcome. Serum phosphorus levels may reflect the type of diet followed by Western populations. In experimental animals fed on a high-lipid diet, serum phosphorus levels rise quickly (19). Clear nutritional records were not available, and we could not include them in these analyses. Nicotinamide, a drug with demonstrated efficacy in raising HDL cholesterol levels and, to a lesser extent, decreasing low density lipoprotein cholesterol levels, effectively prevents phosphate absorption from the gut (20, 21). One may wonder which of these mechanisms affords the favorable cardiovascular effects of this drug (22). Phosphorus has been shown to induce the expression of a sodium-phosphorus cotransporter on the membrane of cultured smooth muscle cells (23, 24). The internalization of large quantities of phosphorus in turn induces the expression of genes encoding for alkaline phosphatase and other mediators of osteoblastic differentiation (23, 24). This is typically followed by calcification of the medium on which the cells are cultured. Similar mechanisms appear to be operative in vivo; in animal experiments, large doses of phosphorus are followed by subsequent calcification of vessels and soft tissues and increased morbidity and mortality (25). Nonetheless, a corresponding human model is yet to be discovered. A polymorphism in the gene for fibroblast growth factor 23, a phosphaturic agent involved in the 1-α hydroxylation of vitamin D, has been linked with the development of atherosclerosis (26). Experimental ablation of the gene encoding for fibroblast growth factor 23 in mice causes accelerated aging of the mice and atherosclerosis in association with hyperphosphatemia (27). On the other hand, overexpression of fibroblast growth factor 23 and Klotho, the gene regulating its synthesis (28), is associated with hypophosphatemia and increased longevity in experimental animal models (29). Furthermore, the Klotho gene appears to have antioxidative activity, and it is therefore potentially an antiatherosclerotic agent (30). Despite all of the above, a full understanding of the mechanisms linking phosphorus to cardiovascular disease is still elusive.
This study had several limitations. Phosphorus levels were measured only once. The glomerular filtration rate was estimated rather than measured and, although we utilized a well-validated method, potential error cannot be excluded. Elevated parathormone levels and decreased vitamin D levels, also associated with adverse outcomes in renal failure, were not measured in the ARIC Study. However, the serum level of these hormones does not start to increase (parathormone) or decrease (vitamin D) until the estimated glomerular filtration rate drops below 60–70 mL/minute/1.73 m2 (31, 32), and we excluded all patients with this degree of renal insufficiency. Although a large number of participants were enlisted in ARIC and the follow-up period was long, power to detect effect modification by gender for the CAD outcome may have been limited by the nonlinear relation observed and the lack of overlap of men and women in the lowest and highest quintiles of serum phosphorus. Because this study was observational, unmeasured confounding is a potential source of bias. On the other hand, the richness of the data in the ARIC Study allowed us to control for many potentially confounding factors. The core measurement of laboratory assays and the carefully adjudicated outcome data represent further important advantages of using the ARIC cohort. Finally, although other potentially important risk factors, such as the presence of the metabolic syndrome and proteinuria, may have been of interest, data on these factors were not collected.
In conclusion, the results of this study suggest that there may be gender differences in the association of higher serum phosphorus levels with increased all-cause mortality in subjects free of known cardiovascular disease and kidney disease. This finding, together with trends in gender heterogeneity in the association of phosphorus with incident CAD and CAD risk factors, adds to the literature on gender specificity in the role of phosphorus as a potential copromoter of atherosclerosis development (8). Whether phosphorus marks the presence of other underlying risk factors or should be considered an additional risk factor in itself is unclear at this time. Nonetheless, our results, along with evidence previously published, should encourage future research addressing the complex and different relations between mineral metabolism and the cardiovascular system in men and women.
Acknowledgments
Author affiliations: Agricultural Research Service, US Department of Agriculture, Stoneville, Mississippi (Stephen J. Onufrak); Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia (Antonio Bellasi, Francesca Cardarelli, Viola Vaccarino, Leslee J. Shaw, Paolo Raggi); and Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, New York (Paul Muntner).
The Atherosclerosis Risk in Communities (ARIC) Study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the ARIC Study Investigators. The current analysis was carried out using a limited-access data set obtained by the NHLBI, and this article does not necessarily reflect the opinions or views of the ARIC study group or the NHLBI.
Conflict of interest: none declared.
Glossary
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- CAD
coronary artery disease
- CI
confidence interval
- HDL
high density lipoprotein
References
- 1.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16(2):520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 3.Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44(5 suppl.2):34–38. doi: 10.1053/j.ajkd.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Sacks F, Pfeffer M, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 7.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 8.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199(2):424–431. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 10.Daly JA, Ertingshausen G. Direct method for determining inorganic phosphate in serum with the “CentrifiChem.”. Clin Chem. 1972;18(3):263–265. [PubMed] [Google Scholar]
- 11.ARIC Coordinating Center. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. Atherosclerosis Risk in Communities (ARIC) Study. Operations manual no. 10: clinical chemistry determinations. Version 1.0. [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 14.ARIC Coordinating Center. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1997. Atherosclerosis Risk in Communities study protocol. Manual 3: surveillance component procedures. Version 4.0. [Google Scholar]
- 15.Barnard J, Meng XL. Applications of multiple imputation in medical studies: from AIDS to NHANES. Stat Methods Med Res. 1999;8(1):17–36. doi: 10.1177/096228029900800103. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Ikizler TA, Block G, et al. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Kalaitzidis R, Tsimihodimos V, Bairaktari E, et al. Disturbances of phosphate metabolism: another feature of metabolic syndrome. Am J Kidney Dis. 2005;45(5):851–858. doi: 10.1053/j.ajkd.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Burnett-Bowie SM, Mendoza N, Leder BZ. Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone. 2007;40(4):913–918. doi: 10.1016/j.bone.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CT, Wells H, Kramsch DM. Suppression of calcific fibrous-fatty plaque formation in rabbits by agents not affecting elevated serum cholesterol levels. The effect of thiophene compounds. Circ Res. 1978;43(1):115–125. doi: 10.1161/01.res.43.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Eto N, Miyata Y, Ohno H, et al. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005;20(7):1378–1384. doi: 10.1093/ndt/gfh781. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Tanaka A, Nakamura T, et al. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65(3):1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 22.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 23.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 25.Stubbs JR, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18(7):2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 26.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20(6):720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuda H, Chikuda H, Suga T, et al. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126(12):1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15(4):437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 29.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280(45):38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 32.Rickers H, Christiansen C, Christensen P, et al. Serum concentrations of vitamin D metabolites in different degrees of impaired renal function. Estimation of renal and extrarenal secretion rate of 24,25-dihydroxyvitamin D. Nephron. 1985;39(3):267–71. doi: 10.1159/000183383. [DOI] [PubMed] [Google Scholar]


