Abstract
A number of previous studies have reported an inverse association between maternal smoking and preeclampsia. Additionally, some have suggested that smokers who develop preeclampsia have worse maternal and fetal outcomes than nonsmokers who develop preeclampsia. The authors examined the relation of smoking to preeclampsia among 674,250 singleton pregnancies in New York City between 1995 and 2003. Although smoking was associated with a reduced risk of preeclampsia overall (adjusted odds ratio = 0.88, 95% confidence interval: 0.82, 0.94), no association was found for preeclampsia superimposed on chronic hypertension (adjusted odds ratio = 1.04, 95% confidence interval: 0.90, 1.21). Furthermore, the apparent protection conferred by maternal smoking was restricted to women aged ≤30 years. Contrary to previous reports, the authors found evidence of a negative interaction between smoking and preeclampsia with respect to preterm delivery and birth weight; smokers who developed preeclampsia had a lower risk of preterm delivery, and a lower adjusted mean difference in birth weight, than would have been expected based on the independent effects of smoking and preeclampsia. These data suggest that smoking is only protective against preeclampsia without pregestational hypertension, and even then principally among younger women. Additionally, smokers who develop these disorders have no increased risk of adverse birth outcomes relative to nonsmokers who develop the same conditions.
Keywords: birth weight, pre-eclampsia, preterm birth, smoking
Preeclampsia is one of the most common complications of pregnancy, with incidence rates in the United States of 2%–7% among healthy, primiparous women (1). It is defined by the presence of elevated maternal blood pressure (systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg in a woman who was previously normotensive prior to 20 weeks’ gestation) in combination with protein in the urine after 20 weeks’ gestation (1). The only cure for preeclampsia is delivery; consequently, preeclampsia remains one of the most common complications resulting in a medically indicated preterm delivery (2).
The etiology of preeclampsia is still unknown; however, it is of placental origin, sometimes preceded by or concurrent with a secondary maternal systemic illness such as chronic hypertension (3), which can complicate diagnosis. Maternal smoking increases the risk of several major pregnancy complications including intrauterine growth restriction (4), placental abruption (5), low birth weight (6–10), and preterm delivery (4). Paradoxically, the risk of preeclampsia is decreased by an estimated 30% among smokers (11). This risk has been found in numerous populations (11) and is particularly perplexing in light of the fact that smoking is causally linked to increased risk of some of the major risk factors for preeclampsia including the occurrence of type 2 diabetes (12). Furthermore, it has been reported that, among smokers who develop preeclampsia, fetal and maternal outcomes are significantly worse than among preeclamptic nonsmokers (13–15), although not all previous investigations have found synergistic effects (16–18). By linking birth records for over 650,000 pregnancies in New York City with hospital discharge data, we were able to examine these associations with greater precision and scope than has been possible previously.
MATERIALS AND METHODS
Data from the New York City Department of Health and Mental Hygiene on livebirths during the period 1995–2003 were linked to the hospital discharge data from the Statewide Planning and Research Cooperative System. Starting with the 1,173,053 births from vital records for the period 1995–2003, 1,084,882 (92.5%) hospital discharge records were successfully linked to births, with 88,171 lost because of missing personal information utilized in the matching algorithm. Of 1,133,020 singleton births from vital records for the years 1995–2003, 1,067,356 (94.2%) were successfully linked to a hospital discharge record (19). These data include all births that occurred between 1995 and 2003; therefore, data for some women may appear more than once if they experienced multiple livebirths within this period. For the purpose of this analysis, only ethnic groups with a prevalence of maternal smoking greater than 1% were included (non-Hispanic White, African American, non-Hispanic Caribbean, Puerto Rican, and Dominican), resulting in a total of 674,250 singleton births.
Discharge diagnosis codes were used to identify preeclampsia cases, and both discharge diagnosis codes and information from the birth record were used to identify pregestational chronic hypertension. These methods were shown by Lydon-Rochelle et al. (20) to result in the most sensitive classification of cases compared with medical record review when using linked birth certificate and hospital discharge data.
Preeclampsia was defined as International Classification of Diseases, Ninth Revision (ICD-9) codes 642.4–642.7. Pregestational chronic hypertension was defined as present based on ICD-9 codes 401–405, 642.0–642.2, and 642.9 on hospital discharge records or having “chronic hypertension” indicated on the birth certificate. If a woman had a diagnosis code for both pregnancy-induced hypertension (ICD-9 code 642.3) and preeclampsia, she was classified as having preeclampsia. Women with a diagnosis of pregnancy-induced hypertension (ICD-9 code 642.3) alone were not excluded but were not examined specifically as a case population. Because preeclampsia superimposed on preexisting chronic hypertension may result from a different etiologic pathway, we conducted all analyses among 3 separate case groups: preeclampsia/eclampsia (n = 25,937) (hereafter referred to as “total preeclampsia”), preeclampsia/eclampsia excluding those with preexisting chronic hypertension (ICD-9 code 642.7; n = 22,340) (hereafter referred to as “isolated preeclampsia”), and preeclampsia superimposed on chronic hypertension (ICD-9 code 642.7 alone; n = 2,646) (hereafter referred to as “hypertension with preeclampsia” (HPE)). In addition, women with discharge diagnosis codes indicating preeclampsia without chronic hypertension (ICD-9 codes 642.4-6) who were also defined as pregestational hypertension cases as outlined above were reclassified as HPE (n = 1,040), yielding a total sample size of 3,597 for this subgroup. Although it would also have been of interest to examine pregnancy-induced hypertension as a separate disease entity, it was considered beyond the scope of the present analysis.
Information on mother's demographic characteristics (age, ethnic ancestry, education), smoking, prepregnancy weight, nativity, and parity was obtained from the birth data. Maternal smoking was recorded as ever smoked during pregnancy (yes/no) and amount smoked per day (none, <1/2 pack, 1/2–1 pack, and >1 pack). No dose response was evident when the association between maternal smoking and preeclampsia was examined by intensity (total preeclampsia-adjusted odds ratios (aORs) for <1/2 pack = 0.85, 1/2–1 pack = 0.89, >1 pack = 0.86; isolated preeclampsia aOR for <1/2 pack = 0.81, 1/2–1 pack = 0.87, >1 pack = 0.97; HPE aOR for <1/2 pack = 1.06, 1/2–1 pack = 0.98, >1 pack = 0.33; all relative to nonsmokers). Therefore, all analyses treated smoking as a 2-level variable (ever vs. never). Ethnicity was determined by self-reported ethnic ancestry (19). Presence of gestational diabetes was determined by an algorithm that used information from both the hospital discharge and birth data (19). We were unable to calculate body mass index because mother's height was not available. Birth weight and the clinical estimate of gestational age were also obtained from the birth certificate. Birth weights of less than 100 g were excluded. Likewise, gestational ages of less than 22 or more than 44 weeks were excluded.
Multivariable logistic regression in PROC LOGISTIC (SAS version 9.1.3 software; SAS Institute, Inc., Cary, North Carolina) was used to obtain adjusted odds ratios and 95% confidence intervals for the relation between smoking and preeclampsia. Potential confounders included maternal age (≤20, 21–30, 31–40, ≥41 years), maternal education (<12, 12, >12 years), foreign-born status (yes/no), parity (0, 1, ≥2), gestational diabetes (yes/no), self-reported prepregnancy maternal weight in quartiles (≤124, 125–140, 141–165, ≥166 pounds (1 pound = 0.454 kg)), and year of delivery. Trimester at initiation of prenatal care was also available and was examined as a potential confounder in the models. For a large number of women (10%), information on this variable was missing, so we dropped it from further consideration after determining that it did not confound the estimate of prenatal smoking on preeclampsia. Otherwise, because of the large study size, we included all variables in the multivariable models rather than restricting them to those covariates that acted as confounders.
Deviations from multiplicativity were assessed by including a preeclampsia-by-maternal-age product term, in which maternal age was treated as a class variable (3 df chi-square). Logistic regression was also used to examine the relation of preeclampsia and smoking to preterm birth (<37 weeks). Deviations from multiplicativity were assessed by including a preeclampsia-by-smoking product term. Deviations from additivity were also assessed (21). Generalized linear models, using PROC GENMOD (SAS Institute, Inc.), were developed to examine the associations among preeclampsia, smoking, and birth weight while adjusting for gestational age at delivery and the covariates outlined above. Deviations from additivity, assessed by including a preeclampsia-by-smoking product term, were assessed for total preeclampsia, isolated preeclampsia, and HPE.
RESULTS
The risk of total preeclampsia in this population was 3.3%, and the risk of HPE was much lower at 0.5%. Overall, the rate of smoking in this population was 4.55%. Among all preeclamptics, isolated preeclampsia, and HPE, the rates were 3.91%, 3.66%, and 5.48%, respectively. In general, the patterns of association between the majority of covariates and total preeclampsia, isolated preeclampsia, and HPE were similar, with more pronounced associations for the HPE subgroup compared with isolated or total preeclampsia (Table 1). Non-Hispanic whites had the lowest risks of total preeclampsia, isolated preeclampsia, and HPE. Risks of total and isolated preeclampsia were elevated among the oldest group of women but also among the youngest (Table 1, Figure 1). Women with less educational attainment had higher risks of isolated preeclampsia and HPE relative to women with more than a high school education. Risk of isolated preeclampsia and HPE increased with increasing maternal prepregnancy weight, albeit to a much stronger degree for HPE.
Table 1.
Multivariate-adjusted Odds Ratios for Maternal Characteristics in Relation to Risk of Preeclampsia in 674,250 Singleton Pregnancies in New York City Between 1995 and 2003
| Maternal Characteristic | Total Preeclampsia Cases (N = 25,937) | Isolated Preeclampsia (n = 22,340) | Chronic Hypertension With Preeclampsia (n = 3,597) | ||||||
| Crude Risk | aORa | 95% CI | Crude Risk | aORa | 95% CI | Crude Risk | aORa | 95% CI | |
| Race/ethnicity | |||||||||
| White | 2.23 | 1.00 | 2.01 | 1.00 | 0.23 | 1.00 | |||
| African American | 5.49 | 2.40 | 2.31, 2.49 | 4.60 | 2.18 | 2.10, 2.27 | 0.98 | 4.11 | 3.72, 4.54 |
| Dominican | 4.76 | 2.32 | 2.21, 2.44 | 4.24 | 2.22 | 2.11, 2.34 | 0.57 | 3.07 | 2.67, 3.54 |
| Non-Hispanic Caribbean | 5.15 | 2.17 | 2.06, 2.28 | 4.18 | 1.95 | 1.85, 2.06 | 1.06 | 3.83 | 3.35, 4.37 |
| Puerto Rican | 4.15 | 1.92 | 1.84, 2.01 | 3.75 | 1.87 | 1.79, 1.96 | 0.43 | 2.17 | 1.90, 2.47 |
| Maternal age, years | |||||||||
| ≤20 | 5.56 | 1.00 | 5.29 | 1.00 | 0.30 | 1.00 | |||
| 21–30 | 3.55 | 0.94 | 0.90, 0.98 | 3.21 | 0.91 | 0.87, 0.94 | 0.36 | 1.62 | 1.39, 1.89 |
| 31–40 | 3.57 | 1.22 | 1.16, 1.27 | 2.85 | 1.04 | 0.99, 1.09 | 0.76 | 4.10 | 3.50, 4.80 |
| ≥41 | 5.47 | 2.03 | 1.88, 2.19 | 3.94 | 1.56 | 1.43, 1.70 | 1.66 | 9.22 | 7.63, 11.15 |
| Maternal education, years | |||||||||
| >12 | 3.50 | 1.00 | 3.03 | 1.00 | 0.50 | 1.00 | |||
| <12 | 4.58 | 1.18 | 1.14, 1.23 | 4.01 | 1.15 | 1.11, 1.20 | 0.62 | 1.40 | 1.27, 1.54 |
| 12 | 3.91 | 1.10 | 1.06, 1.13 | 3.36 | 1.08 | 1.05, 1.12 | 0.59 | 1.21 | 1.12, 1.31 |
| Foreign-born status | 4.15 | 1.02 | 0.98, 1.05 | 3.55 | 1.02 | 0.98, 1.06 | 0.65 | 0.97 | 0.88, 1.08 |
| Parity | |||||||||
| ≥2 | 2.79 | 1.00 | 2.13 | 1.00 | 0.69 | 1.00 | |||
| 1 | 2.66 | 1.11 | 1.07, 1.16 | 2.21 | 1.17 | 1.11, 1.22 | 0.46 | 0.99 | 0.91, 1.09 |
| 0 | 5.27 | 2.56 | 2.47, 2.65 | 4.79 | 2.82 | 2.71, 2.93 | 0.53 | 1.58 | 1.43, 1.75 |
| Maternal smoking | 3.31 | 0.88 | 0.82, 0.94 | 2.68 | 0.84 | 0.78, 0.91 | 0.66 | 1.04 | 0.90, 1.21 |
| Prepregnancy weight, poundsb | |||||||||
| ≤124 | 2.62 | 1.00 | 2.45 | 1.00 | 0.18 | 1.00 | |||
| 125–140 | 3.13 | 1.22 | 1.17, 1.27 | 2.83 | 1.20 | 1.15, 1.25 | 0.32 | 1.60 | 1.39, 1.84 |
| 141–165 | 4.08 | 1.57 | 1.51, 1.63 | 3.55 | 1.51 | 1.45, 1.57 | 0.57 | 2.47 | 2.16, 2.82 |
| ≥166 | 6.01 | 2.27 | 2.18, 2.36 | 4.83 | 2.04 | 1.96, 2.12 | 1.30 | 4.88 | 4.31, 5.33 |
| Gestational diabetes | 6.65 | 1.52 | 1.45, 1.60 | 5.28 | 1.48 | 1.40, 1.56 | 1.52 | 1.66 | 1.50, 1.84 |
| Chronic hypertension | 25.34 | 7.63 | 7.31, 7.96 | N/A | N/A | ||||
Abbreviation: aOR, adjusted odds ratio; CI, confidence interval; N/A, not applicable.
The multivariate model included maternal age, race/ethnicity, maternal education, foreign-born status, parity, maternal smoking, prepregnancy weight, year of birth, and presence of gestational diabetes.
One pound = 0.454 kg.
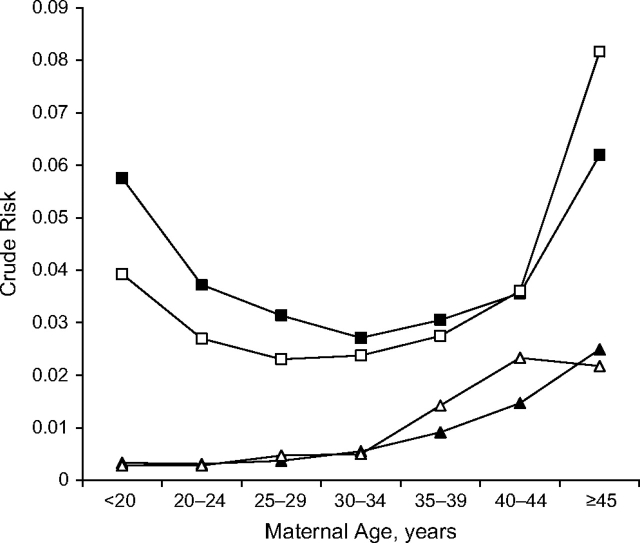
Figure 1.
Maternal age and crude risk of preeclampsia and preeclampsia with chronic hypertension by smoking status in 674,250 singleton pregnancies in New York City between 1995 and 2003. Shown are the unadjusted risks of isolated preeclampsia (squares) and chronic hypertension with preeclampsia (triangles) comparing smokers (white squares and triangles) with nonsmokers (black squares and triangles) and stratified by maternal age in 5-year increments. Risk of isolated preeclampsia was clearly reduced among smokers until approximately age 30 years, when the curves began to overlap and then cross at age 40 years or older. No reduced risk was apparent for chronic hypertension with preeclampsia; in fact, smokers seemed to be at a somewhat higher risk in the older age groups.
Associations between maternal age and parity differed for isolated preeclampsia and HPE. There was no substantial effect of maternal age on isolated preeclampsia until age 40 years; however, as expected given the rise in chronic hypertension with advancing age, risk of HPE increased monotonically by maternal age, with women aged 41 years or older having a 9.22-fold (95% confidence interval (CI): 7.63, 11.15) increased risk. In contrast, nulliparity was a much stronger risk factor for the development of isolated preeclampsia (aOR = 2.82, 95% CI: 2.71, 2.93) than HPE (aOR = 1.58, 95% CI: 1.43, 1.75).
We found a decreased risk of total preeclampsia among women who smoked during pregnancy (aOR = 0.88, 95% CI: 0.82, 0.94) (Table 1). However, whereas maternal smoking was associated with a significantly decreased risk of isolated preeclampsia (aOR = 0.84, 95% CI: 0.78, 0.91), no decreased risk of chronic hypertension with preeclampsia was apparent (aOR = 1.04, 95% CI: 0.90, 1.21) (Table 1). Furthermore, the apparent protection conferred by smoking on preeclampsia appeared to be age dependent (interaction P values = 0.003 for all preeclampsia cases and 0.070 for isolated preeclampsia specifically) (Table 2, Figure 1). Among isolated preeclampsia cases, the protection conferred by maternal smoking appeared to progressively decrease with advancing maternal age. For HPE, the results were imprecise, but there is a gradient across age, with smokers having a lower risk at early ages and a higher risk at advanced ages.
Table 2.
Multivariate-adjusted Interaction Between Maternal Age and Prenatal Smoking on the Risk of Preeclampsia in 674,250 Singleton Pregnancies in New York City Between 1995 and 2003
| Maternal Age, years | Total Preeclampsia Cases (N = 25,937) | Isolated Preeclampsia (n = 22,340) | Chronic Hypertension With Preeclampsia (n = 3,597) | |||||||||
| Smoker | Nonsmoker | aORa | 95% CI | Smoker | Nonsmoker | aORa | 95% CI | Smoker | Nonsmoker | aORa | 95% CI | |
| ≤20 | 158 | 4,108 | 0.78 | 0.66, 0.92 | 149 | 3,897 | 0.78 | 0.66, 0.92 | 9 | 211 | 0.80 | 0.41, 1.56 |
| 21–30 | 381 | 10,824 | 0.79 | 0.71, 0.88 | 329 | 9,761 | 0.78 | 0.70, 0.87 | 52 | 1,063 | 0.92 | 0.70, 1.22 |
| 31–40 | 421 | 8,896 | 1.00 | 0.90, 1.10 | 308 | 7,083 | 0.95 | 0.85, 1.07 | 113 | 1,813 | 1.09 | 0.90, 1.32 |
| ≥41 | 54 | 1,095 | 1.08 | 0.81, 1.43 | 31 | 782 | 0.91 | 0.63, 1.31 | 23 | 313 | 1.37 | 0.88, 2.11 |
| Interaction P valueb | 0.003 | 0.070 | 0.388 | |||||||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
The multivariate model included race/ethnicity, maternal age, maternal smoking, maternal education, parity, prepregnancy weight, year of birth, presence of gestational diabetes, and maternal age-by-smoking product term.
Wald chi-square P value for 3-df test of the maternal age-by-smoking product term.
We examined whether smokers with preeclampsia had a higher risk of preterm delivery (Table 3) or lower birth weight (Table 4) than would have been expected based on the independent risks that each of these factors carries. In our data, smoking alone carried an approximately 76% increased risk of preterm delivery (95% CI: 1.69, 1.82). Preeclampsia alone carried a markedly increased risk of preterm delivery, with adjusted odds ratios of 4.13 (95% CI: 4.00, 4.27) for isolated preeclampsia and 7.94 (95% CI: 7.40, 8.53) for HPE. In both instances, however, the joint effect of both preeclampsia and smoking was less than would have been expected based on the multiplication of their individual risks (aOR = 3.46, 95% CI: 2.94, 4.08 for isolated preeclampsia and aOR = 6.22, 95% CI: 4.67, 8.30 for HPE). This negative interaction on the multiplicative scale was statistically significant for all subgroups (P < 0.0001 for the Wald chi-square test of the interaction term), resulting in a risk of preterm delivery for preeclamptic smokers that is actually slightly less than the risk of preterm delivery conferred by preeclampsia alone. The negative interaction was also statistically below what would have been predicted based on additivity of the smoking and preeclampsia effects (P < 0.05) for all subtypes.
Table 3.
Multivariate-adjusted Interaction Between Maternal Smoking and Preeclampsia on the Risk of Preterm Delivery Before 37 Weeks’ Gestation in 674,250 Singleton Pregnancies in New York City Between 1995 and 2003
| Level of Smoking by Preeclampsia Interaction | Total Preeclampsia Cases | Isolated Preeclampsia | Chronic Hypertension With Preeclampsia | |||
| aORa | 95% CI | aORa | 95% CI | aORa | 95% CI | |
| Nonsmoker and no preeclampsia | 1.00 | 1.00 | 1.00 | |||
| Smoker only | 1.76 | 1.69, 1.82 | 1.75 | 1.69, 1.82 | 1.73 | 1.67, 1.94 |
| Preeclampsia only | 4.59 | 4.30, 4.57 | 4.13 | 4.00, 4.27 | 7.94 | 7.40, 8.53 |
| Smoker and preeclampsiab | 3.99 | 3.46, 4.59 | 3.46 | 2.94, 4.08 | 6.22 | 4.67, 8.30 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
The multivariate model included race/ethnicity, maternal age, maternal smoking, maternal education, parity, preeclampsia subtype, prepregnancy weight, year of birth, presence of gestational diabetes, and smoking-by-preeclampsia-subtype product term.
The 1-df Wald chi-square interaction P values: all preeclampsia (P < 0.001), isolated preeclampsia (P < 0.001), and chronic hypertension with preeclampsia (P < 0.001).
Table 4.
Multivariate-adjusted Interaction Between Maternal Smoking and Preeclampsia on Birth Weight in 674,250 Singleton Pregnancies in New York City Between 1995 and 2003
| Level of Smoking by Preeclampsia Interaction | Total Preeclampsia Cases | Isolated Preeclampsia | Chronic Hypertension With Preeclampsia | |||
| Adjusted Difference, ga | 95% CI | Adjusted Difference, ga | 95% CI | Adjusted Difference, ga | 95% CI | |
| Nonsmoker and no preeclampsia | 0 | 0 | 0 | |||
| Smoker only | −134.64 | −139.91, −129.38 | −135.02 | −140.28, −129.76 | −135.22 | −140.45, −129.99 |
| Preeclampsia only | −177.52 | −183.20, −171.84 | −163.39 | −169.45, −157.33 | −278.06 | −292.91, −263.22 |
| Smoker and preeclampsiab | −261.61 | −296.87, −242.35 | −242.33 | −272.66, −212.00 | −390.43 | −451.45, −329.42 |
Abbreviation: CI, confidence interval.
The multivariate model included race/ethnicity, maternal age, maternal smoking, maternal education, parity, preeclampsia subtype, prepregnancy weight, year of birth, presence of gestational diabetes, gestational age, and smoking-by-preeclampsia-subtype product term.
The 1-df Wald chi-square interaction P values: all preeclampsia (P = 0.0013), isolated preeclampsia (P = 0.0004), and chronic hypertension with preeclampsia (P = 0.477).
Likewise for birth weight, the adjusted difference in birth weight attributable to smoking alone was a loss of approximately 135 g in our data (Table 4). Similarly, preeclampsia alone predicted a birth weight reduction of approximately 163 g and HPE predicted a 278-g reduction in birth weight. However, the adjusted difference in birth weight for preeclamptic smokers was less than what we would have expected based on the sum of the individual adjusted differences (242.33 g < 135.02 g + 163.39 g for isolated preeclampsia; 390.43 g < 135.22 g + 278.06 g for HPE), although slightly more than the adjusted difference due to preeclampsia alone. This negative interaction was significant for total preeclampsia and isolated preeclampsia, but not for HPE (P = 0.477). Similar results were found when examining low birth weight (<2,500 g), term low birth weight (<2,500 g, ≥37 weeks’ gestation), and small-for-gestational-age birth (<5th percentile) (data not shown).
DISCUSSION
The inverse association between smoking and preeclampsia in our study appeared to be weaker than in previous reports (an approximate aOR of 0.7 in previous studies (11) vs. 0.8 in ours) and was not found for HPE. The sources of information on outcome and exposure could reasonably explain this discrepancy. Preeclampsia was defined on the basis of ICD-9 discharge diagnosis codes. Geller et al. (22) examined the accuracy of these codes in assigning preeclampsia in a US population and found that the positive predictive value for severe preeclampsia was 84.8% but only 45.3% for mild preeclampsia. In most instances, the consequences of nondifferential misclassification of a binary disease variable are the same as they are for a binary exposure variable (i.e., toward the null); however, it can depend on the sensitivity and specificity of the ICD-9 codes (23). Nevertheless, the rates of preeclampsia found in this population are similar to those found in other large cohort or data-linkage studies (11, 13, 24, 25).
This analysis included only those ethnic subgroups in which the rate of maternal smoking was more than 1%. Even so, the rate of smoking in our population overall was low (<5%). Although underreporting of smoking during pregnancy is known to be common (26), our population also includes a substantial number of Hispanic and foreign-born women, and both of these groups tend to have lower rates of smoking (27).
Smoking information is collected on the birth certificates of women at the time of delivery. These data are routinely used to monitor trends in maternal smoking in the United States. If underreporting of smoking behavior is nondifferential, we can expect bias to be toward the null because smoking is a binary exposure (28), which may explain the attenuation in risk ratios we observed relative to studies with detailed questionnaire-based information on prenatal smoking. Differential misclassification (in which cases are more likely to underreport smoking) will bias the effect estimate downward so that smoking looks more protective.
Alternatively, or in addition to misclassification of maternal smoking, the attenuation in the relative risk of smoking among isolated preeclamptics may be attributable in part to misclassification of preexisting chronic hypertension (i.e., HPE misclassified as isolated preeclampsia) since there appeared to be no relation between smoking and HPE. Additionally, smoking was dichotomized as ever versus never in our study because there was no dose response evident when examining the more quantitative estimate of smoking available. Therefore, smoking timing, intensity, and frequency may be influencing the magnitude of this estimate by including a large number of light or infrequent smokers in the exposed category. Misclassification of smoking intensity may be responsible for the lack of a dose response using the more quantitative smoking question available on the questionnaire. This misclassification may result from underreporting and variation in smoking behavior over the course of pregnancy.
Additionally, the data sources we utilized lacked information on certain risk factors for preeclampsia, such as prepregnancy height to calculate body mass index, which may result in residual confounding. However, even with these limitations, a clear inverse association between smoking and isolated preeclampsia was observed, qualitatively consistent with previous reports.
The biologic mechanism underlying the repeatedly observed inverse association between smoking and preeclampsia has not been established; however, recent research suggests that carbon monoxide may be the elusive mediator of this paradoxical relation (29). Although carbon monoxide has historically been considered a toxic by-product of smoking, it also has important physiologic functions (29–34), including inhibition of proinflammatory cytokines and chemokines (35), prevention of vascular constriction (36), inhibition of platelet aggregation and plasminogen activation (37), and inhibition of apoptosis (38, 39). Additionally, carbon monoxide has been found to inhibit formation of reactive oxygen species (34) and inhibit apoptosis in the differentiated syncytiotrophoblast layer of the placenta specifically (30). Furthermore, fms-like tyrosine kinase-1 is thought to be critical to the pathogenesis of preeclampsia (40), smoking is known to decrease circulating levels of fms-like tyrosine kinase-1 (40), and carbon monoxide has been found to inhibit fms-like tyrosine kinase-1 production in mice (41).
There are few studies with sufficient power to examine modification of the smoking-preeclampsia relation by maternal age given that both prenatal smoking and pregnancy at the extremes of maternal age are relatively rare events. We found substantial evidence of modification of the smoking-preeclampsia relation by maternal age such that the protection conferred by smoking was limited to women aged approximately 30 years or less. Moreover, although no overall relation between smoking and HPE was detected, there was a suggestion that, at more advanced maternal ages, smoking may actually increase risk of HPE. That being said, there is some uncertainty in ascertaining cause and effect in the HPE analysis. Women who smoke during pregnancy are very likely to have been prepregnancy smokers, which may have been a cause of their pregestational hypertension. Additionally, preeclampsia in the presence of chronic hypertension is very difficult to diagnose, and chronic hypertension may not be accurately recorded in the discharge records in the absence of related pregnancy complications. If smoking exacerbated underlying chronic hypertension, causing decompensation that is mistakenly diagnosed as preeclampsia, then the relation between smoking and preeclampsia superimposed on chronic hypertension would at least be biased toward the null and possibly begin to look as if smoking increased the risk of HPE. In short, the results with respect to HPE are best conceptualized as cross-sectional and would be improved by more reliable clinical information.
The literature has suggested that, although smoking may decrease the risk of preeclampsia overall, smokers who develop preeclampsia have a higher risk of adverse maternal and fetal outcomes (13–15). However, not all previous investigations have found evidence of this association (16–18), and few have appeared to adequately account statistically for the independent and joint effects of smoking and preeclampsia on adverse birth outcomes. As a consequence, a belief has arisen that preeclampsia and smoking somehow interact biologically to produce a net harmful effect on birth outcomes via some biologic interplay involving fetoplacental circulation. Our results are counter to this view.
In our study, of a total of 25,937 preeclampsia cases, 1,014 smoked; therefore, we had sufficient power to disentangle the independent effects of preeclampsia and smoking from their joint effects on pregnancy outcome. Our results were unexpected, yet consistent across indicators of fetal growth and were estimated with exceptional precision. Rather than smokers who develop preeclampsia having an excess risk of adverse birth outcomes, we found that their risks were actually lower than would have been expected based on the independent effects of smoking and preeclampsia. Specifically, preeclampsia cases who smoked were at slightly less risk of a preterm delivery than preeclampsia cases who did not smoke, although their risk of preterm delivery was still substantially elevated relative to smokers who did not develop preeclampsia and relative to nonsmokers without preeclampsia, respectively (the reference group).
The same holds true for birth weight. Although the difference in birth weight was greater for smokers who developed preeclampsia than for smokers only, or preeclampsia cases only, it was smaller than the sum of their independent effects, meaning that there was some net negative interaction between these factors. However, unlike preterm delivery, it did appear that smokers who developed preeclampsia had a slightly higher difference in mean birth weight than preeclampsia cases who did not smoke. Note that we detected no interaction between smoking and chronic hypertension on birth weight, meaning that the differences in birth weight between preeclampsia cases who smoked and preeclampsia cases who did not smoke were indistinguishable. These data directly counter the notion that smokers who develop preeclampsia have worse fetal and maternal outcomes than nonsmokers who develop preeclampsia, and they further suggest that, at least among some members of this population, some causal antagonism may be present among smoking and preeclampsia with respect to measures of fetal growth.
However, there is potentially a problem of competing risks when considering the associations between and among smoking, preeclampsia, and preterm delivery. Smoking is related to both pregnancy outcomes, albeit in opposite directions, and preeclampsia often results in a medically indicated preterm delivery. Additionally, preterm delivery may truncate the period at risk of preeclampsia. As has been suggested in other contexts (42–45), the appropriate risk set to disentangle these effects is the population of women pregnant at the time of preeclampsia diagnosis. However, our data source lacks critical information on gestational age at preeclampsia diagnosis that would be required to address these issues analytically. Moreover, imputing date of diagnosis based on date of delivery is not straightforward given that watchful waiting, particularly among the less severe cases or those that occur quite early, may result in a large difference between gestational age at delivery and gestational age at diagnosis. However, we conducted a sensitivity analysis by restricting our population to births that occurred after 32 weeks’ gestation and found that the overall relative risk of smoking and preeclampsia was unchanged (relative risks = 0.88–0.90). We were unable to account reliably for disease severity with the existing data, and it is possible that smokers with preeclampsia tend to have a less severe form of the disease and are therefore more likely to be treated expectantly and less likely to deliver early. Future investigations would benefit by specific information on gestational age at diagnosis and clinical severity data so that these issues can be disentangled.
In conclusion, we examined the relation among smoking, preeclampsia, and measures of fetal growth in a linked data resource containing over 650,000 singleton pregnancies resulting in a livebirth in New York City between 1995 and 2003. The large size of this data set permitted detailed examination of potential modifiers of these associations and provided evidence against the hypothesis that smokers who develop preeclampsia have worse birth outcomes than nonsmokers who develop preeclampsia. Furthermore, the protection from preeclampsia conferred by maternal smoking appears to be limited to cases without pregestational hypertension and also to younger age groups. It is possible that these associations reflect an underlying heterogeneity in preeclampsia as a disease entity, such that older preeclampsia cases, even those without pregestational hypertension, may have a higher prevalence of comorbidities (e.g., underlying vascular, renal, or autoimmune diseases), which mitigates the degree to which smoking can be causally protective. However, clinical studies would be required to provide a biologic basis for these findings.
Acknowledgments
Author affiliations: Department of Community and Preventive Medicine, Mount Sinai School of Medicine, New York, New York (Stephanie M. Engel, Cheryl R. Stein, David A. Savitz); and Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Teresa M. Janevic).
Funded by grants from the National Institute of Child Health and Human Development (HD050739-01) and National Institute of Environmental Health Sciences and Environmental Protection Agency Children’s Center (ES09584,R827039) (S. M. E.).
Conflict of interest: none declared.
Glossary
Abbreviations
- aOR
adjusted odds ratio
- CI
confidence interval
- HPE
hypertension with preeclampsia
- ICD-9
International Classification of Diseases, Ninth Revision
References
- 1.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24(suppl A):S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud AO, Bujold E, Sorokin Y, et al. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192(6):1856–1862. doi: 10.1016/j.ajog.2004.12.057. discussion 1862–1863. [DOI] [PubMed] [Google Scholar]
- 5.Ananth CV, Cnattingius S. Influence of maternal smoking on placental abruption in successive pregnancies: a population-based prospective cohort study in Sweden. Am J Epidemiol. 2007;166(3):289–295. doi: 10.1093/aje/kwm073. [DOI] [PubMed] [Google Scholar]
- 6.Macmahon B, Alpert M, Salber EJ. Infant weight and parental smoking habits. Am J Epidemiol. 1965;82(3):247–261. doi: 10.1093/oxfordjournals.aje.a120547. [DOI] [PubMed] [Google Scholar]
- 7.Butler NR, Goldstein H, Ross EM. Cigarette smoking in pregnancy: its influence on birth weight and perinatal mortality. Br Med J. 1972;2(5806):127–130. doi: 10.1136/bmj.2.5806.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horta BL, Victora CG, Menezes AM, et al. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–151. doi: 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 9.Meyer MB, Jonas BS, Tonascia JA. Perinatal events associated with maternal smoking during pregnancy. Am J Epidemiol. 1976;103(5):464–476. doi: 10.1093/oxfordjournals.aje.a112248. [DOI] [PubMed] [Google Scholar]
- 10.Sprauve ME, Lindsay MK, Drews-Botsch CD, et al. Racial patterns in the effects of tobacco use on fetal growth. Am J Obstet Gynecol. 1999;181(1):S22–S27. doi: 10.1016/s0002-9378(99)70468-0. [DOI] [PubMed] [Google Scholar]
- 11.Conde-Agudelo A, Althabe F, Belizan JM, et al. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026–1035. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Ajani UA, Liu S, et al. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109(7):538–542. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 13.Cnattingius S, Mills JL, Yuen J, et al. The paradoxical effect of smoking in preeclamptic pregnancies: smoking reduces the incidence but increases the rates of perinatal mortality, abruptio placentae, and intrauterine growth restriction. Am J Obstet Gynecol. 1997;177(1):156–161. doi: 10.1016/s0002-9378(97)70455-1. [DOI] [PubMed] [Google Scholar]
- 14.Newman MG, Lindsay MK, Graves W. Cigarette smoking and pre-eclampsia: their association and effects on clinical outcomes. J Matern Fetal Med. 2001;10(3):166–170. doi: 10.1080/714904321. [DOI] [PubMed] [Google Scholar]
- 15.Xiao R, Sorensen TK, Williams MA, et al. Influence of pre-eclampsia on fetal growth. J Matern Fetal Neonatal Med. 2003;13(3):157–162. doi: 10.1080/jmf.13.3.157.162. [DOI] [PubMed] [Google Scholar]
- 16.Marcoux S, Brisson J, Fabia J. The effect of cigarette smoking on the risk of preeclampsia and gestational hypertension. Am J Epidemiol. 1989;130(5):950–957. doi: 10.1093/oxfordjournals.aje.a115427. [DOI] [PubMed] [Google Scholar]
- 17.Odegard RA, Vatten LJ, Nilsen ST, et al. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–955. [PubMed] [Google Scholar]
- 18.Beste LA, England LJ, Schisterman EF, et al. Pregnancy outcomes in smokers who develop pre-eclampsia. Paediatr Perinat Epidemiol. 2005;19(1):12–18. doi: 10.1111/j.1365-3016.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 19.Savitz DA, Janevic TM, Engel SM, et al. Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG. 2008;115(8):969–978. doi: 10.1111/j.1471-0528.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 20.Lydon-Rochelle MT, Holt VL, Cárdenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–134. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Geller SE, Ahmed S, Brown ML, et al. International Classification of Diseases-9th revision coding for preeclampsia: how accurate is it? Am J Obstet Gynecol. 2004;190(6):1629–1633. doi: 10.1016/j.ajog.2004.03.061. discussion 1633–1634. [DOI] [PubMed] [Google Scholar]
- 23.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 24.Bodnar LM, Catov JM, Klebanoff MA, et al. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2):234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 25.Catov JM, Ness RB, Kip KE, et al. Risk of early or severe preeclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36(2):412–419. doi: 10.1093/ije/dyl271. [DOI] [PubMed] [Google Scholar]
- 26.Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Rep. 2001;116(4):327–335. doi: 10.1016/S0033-3549(04)50054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perreira KM, Cortes KE. Race/ethnicity and nativity differences in alcohol and tobacco use during pregnancy. Am J Public Health. 2006;96(9):1629–1636. doi: 10.2105/AJPH.2004.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dosemeci M, Wacholder S, Lubin JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol. 1990;132(4):746–748. doi: 10.1093/oxfordjournals.aje.a115716. [DOI] [PubMed] [Google Scholar]
- 29.Bainbridge SA, Sidle EH, Smith GN. Direct placental effects of cigarette smoke protect women from pre-eclampsia: the specific roles of carbon monoxide and antioxidant systems in the placenta. Med Hypotheses. 2005;64(1):17–27. doi: 10.1016/j.mehy.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Bainbridge SA, Belkacemi L, Dickinson M, et al. Carbon monoxide inhibits hypoxia/reoxygenation-induced apoptosis and secondary necrosis in syncytiotrophoblast. Am J Pathol. 2006;169(3):774–783. doi: 10.2353/ajpath.2006.060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilban M, Bach FH, Otterbein SL, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24(5):601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Chin BY, Jiang G, Wegiel B, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104(12):5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10(3):650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Wang Y, Kim HP, et al. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282(3):1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 35.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Kaide JI, Rodriguez-Mulero F, et al. Vasoregulatory function of the heme-heme oxygenase-carbon monoxide system. Am J Hypertens. 2001;14(6 pt 2):62S–67S. doi: 10.1016/s0895-7061(01)02071-4. [DOI] [PubMed] [Google Scholar]
- 37.Fujita T, Toda K, Karimova A, et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7(5):598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 38.Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XM, Chapman GB, Peyton KJ, et al. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2002;55(2):396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 40.Powers RW, Roberts JM, Cooper KM, et al. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193(1):185–191. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 41.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 42.Joseph KS. The fetuses-at-risk approach: clarification of semantic and conceptual misapprehension. BMC Pregnancy Childbirth. 2008;8:11. doi: 10.1186/1471-2393-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph KS. Incidence-based measures of birth, growth restriction, and death can free perinatal epidemiology from erroneous concepts of risk. J Clin Epidemiol. 2004;57(9):889–897. doi: 10.1016/j.jclinepi.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Joseph KS. Theory of obstetrics: the fetuses-at-risk approach as a causal paradigm. J Obstet Gynaecol Can. 2004;26(11):953–960. doi: 10.1016/s1701-2163(16)30414-5. [DOI] [PubMed] [Google Scholar]
- 45.Joseph KS, Allen AC, Lutfi S, et al. Does the risk of cerebral palsy increase or decrease with increasing gestational age? BMC Pregnancy Childbirth. 2003;3(1):8. doi: 10.1186/1471-2393-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]