Abstract
The authors examined the associations of hostility measured in adulthood with subsequent body mass index (BMI; weight (kg)/height (m)2) assessed at 4 time points over a 19-year period (1985–2004) in a United Kingdom cohort study. A total of 6,484 participants (4,494 men and 1,990 women) aged 35–55 years at baseline (1985–1988) completed the Cook-Medley Hostility Scale. BMI was assessed upon medical examination in phases 1 (1985–1988), 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004). Mixed-models analyses of repeated measures showed clear evidence of increasing BMI over follow-up in both sexes. In women, higher levels of hostility were associated with higher BMI at baseline, and this effect remained constant throughout the follow-up period. In men, hostility levels were also strongly associated with BMI at baseline, but results for the interaction between time and hostility also suggested that this association increased over time, with persons in the highest quartile of hostility gaining an excess of 0.016 units (P = 0.023) annually over the follow-up period as compared with persons in the lowest quartile. The authors conclude that the difference in BMI as a function of hostility levels in men is not stable over time.
Keywords: body mass index, health behavior, hostility, psychology
Numerous epidemiologic investigations have found hostility, a measure of general cynicism and interpersonal mistrust, to be associated with an increased risk of hypertension (1–4), subclinical atherosclerosis (5), myocardial infarction (6, 7), coronary heart disease (8–10), and all-cause mortality (6, 8, 11, 12). However, the mechanisms through which hostility affects health remain unclear. Several studies have identified hostility as related to higher alcohol consumption, less physical activity, smoking, and greater body mass index (BMI) and caloric intake (3, 6, 13–16), supporting health behaviors as a possible pathway linking hostility to health (15).
Although the possibility that hostility influences health outcomes via health-related behaviors has gained recognition, statistical adjustments for these variables show only marginal (11, 17) to moderate (6) effects on the hostility-health association. Lack of attenuation of associations upon adjustment for hypothesized mediating factors may indicate at least 2 issues. First, it is possible that health behaviors are not important mediators or that their effect is masked by other mediators of the association between hostility and health (14). Second, measurement imprecision may dilute the effect of health behaviors on the association between hostility and health. Measurement imprecision is possible because health-related behaviors are typically assessed at only 1 point in time, usually baseline (4, 11, 14); the assumption being made is that the effect of hostility on health behaviors remains constant over time. However, this assumption is rarely tested, even though there is some evidence to suggest that health behaviors vary over the course of adult life (18–21).
Our main objective in this study was to examine the association between hostility and BMI trajectories over the adult life course after controlling for potentially confounding factors. We conducted a prospective investigation using data from a large cohort of British civil servants to examine the temporal association of hostility measured in adulthood with BMI assessed at 4 time points over a 19-year period. We focused on BMI because high BMI is an important risk factor for several chronic and organic diseases (22–24) and has been found to be associated with hostility (3, 16). Moreover, BMI may reflect the effects of other health-related behaviors such as physical activity (22), dietary patterns (25, 26), and alcohol consumption (27). In contrast to many previous studies with self-reported measures of health behavior, in the present study BMI was assessed objectively during clinical examinations.
MATERIALS AND METHODS
Data were drawn from the Whitehall II Study, established in 1985 as a longitudinal study to examine the socioeconomic gradient in health and disease among 10,308 British civil servants (6,895 men and 3,413 women) (28). All civil servants aged 35–55 years in 20 London-based government departments were invited to participate by letter, and 73% agreed. Baseline screening (phase 1) took place during 1985–1988 and involved a medical examination and a self-administered questionnaire. Subsequent phases of data collection have alternated between postal questionnaires alone (phases 2 (1989–1990), 4 (1995–1996), 6 (2001), and 8 (2006)) and postal questionnaires accompanied by a medical examination (phases 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004)). The University College London ethics committee approved the study.
Measures
Hostility was assessed using the Cook-Medley Hostility Scale (29) in phase 1 (1985–1988). The internal consistency, test-retest reliability, and construct validity of this scale have been demonstrated (30). Participants completed an abridged 38-item version (Cronbach’s α = 0.83) of the original 50-item instrument. Item savings were necessary because of the length of the original questionnaire; the Minnesota Multiphasic Personality Inventory (29) numbers of the omitted items are 19, 183, 237, 253, 386, 394, 410, 455, 458, 485, 504, and 558. Higher scores on the scale denote greater hostility, but no natural or clinically based thresholds exist for defining “high” levels of hostility (29). Therefore, in order to investigate threshold effects, we categorized hostility scores into 4 groups based on quartiles as in previous studies (31, 32): lowest (0–6), middle lowest (7–10), middle highest (11–15), and highest (>16). The lowest quartile was the reference category.
BMI, calculated by dividing weight in kilograms by height in meters squared, was assessed during the medical examination in phases 1 (1985–1988), 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004). In phase 1, participants were asked to report their height and weight at age 25 years, which allowed us to calculate their BMI at age 25 years. In the longitudinal analyses, continuous measures of BMI were used, but these were categorized into 4 groups (<20, 20–24.9, 25–29.9, or ≥30), as in previous studies (33, 34), for the descriptive analysis.
Sociodemographic measures taken from the phase 1 questionnaire included sex (male vs. female), ethnicity (white vs. other), and marital status (married/cohabiting vs. other). Age was categorized into 4 5-year groups (34–40, 41–45, 46–50, and 51–55 years) because there was no evidence of a linear relation between age and BMI. Socioeconomic status, assessed by British civil service employment grade, was categorized into 3 groups in order of decreasing salary and work role: administrative (high), professional/executive (middle), and clerical/support (low)—a standard classification in the Whitehall II Study.
Statistical analyses
Differences in sample characteristics between men and women were assessed using a chi-square test. Mean BMI and its standard error in each phase were calculated separately among men and women and are shown graphically below. We first examined the association between the covariates (age, socioeconomic status, ethnicity, marital status, and BMI at age 25 years) and BMI trajectories. In these analyses of change in BMI over 4 waves of data collection spread over 19 years, we used mixed-models analysis of repeated measures in order to take into account the within-subject correlation between the measures of BMI. The dependent variables were the 4 repeated measures of BMI over the 19-year period, and the independent variables were time (exact time in years between phases, included as a continuous variable), the covariates, and interactions between time and these covariates.
We also conducted mixed-models analysis of repeated measures to explore the temporal association between hostility levels and subject-specific measures of BMI (variability in mean BMI) over 19 years of follow-up. In these analyses, we first used time, hostility level, and the interaction between time and hostility level as the independent variables (model 1). There were 3 coefficients of interest: 1) the coefficient for time assessed the change in BMI with time (a P value of 0.05 or less was taken to imply a significant change in BMI over follow-up); 2) the coefficient for hostility level estimated the association between the 4 hostility levels and BMI at baseline (phase 1); and 3) the coefficient for the interaction between time and hostility level assessed whether the mean annual rate of change in BMI over follow-up differed between hostility levels. A P value less than or equal to 0.05 for the interaction term indicated a significant difference in the mean annual rate of change in BMI over time between hostility levels. However, a P value greater than 0.05 suggested that the mean annual rate of change in BMI over time did not vary as a function of hostility level or that the association between hostility and BMI remained constant over time. These analyses were further adjusted (model 2) for covariates at baseline that had previously been shown to be associated with BMI trajectories over time. The procedure PROC MIXED in SAS was used to fit these models (version 9.1; SAS Institute Inc., Cary, North Carolina). As participants provided repeated BMI measurements, a covariance structure was specified for the error term in the mixed-effect model. An autoregressive order 1 model has been preferred because of correlation between BMI measurements. We used linear contrasts to test the effect of increasing hostility on BMI. The term for interaction between time, hostility, and sex was significant (P < 0.05); this interaction is illustrated below using line graphs of the mean BMI trajectory over time by quartile of hostility for men and women. All analyses were performed separately in men and women.
RESULTS
Only 75% of the 10,308 participants were asked to complete the hostility scale in phase 1, because this measure was introduced after the start of the baseline survey. A total of 6,484 participants responded to the hostility questions (84% of those asked). There were no differences between participants and those not included in terms of sex, socioeconomic status, ethnicity, marital status, or BMI at age 25 years. However, persons who were not included in the present study were more likely to be male (69.3% vs. 62.8%; P < 0.001). Table 1 presents descriptive data from baseline (phase 1) for persons included in the analyses.
Table 1.
Characteristics of Participants in Phase 1 (1985–1988) of the Whitehall II Study, by Sex, United Kingdom
| Men (n = 4,494) |
Women (n = 1,990) |
P Value | |||
| No. | % | No. | % | ||
| Age, years | |||||
| 35–40 | 1,245 | 27.7 | 467 | 23.5 | <0.001 |
| 41–45 | 1,260 | 28.0 | 494 | 24.8 | |
| 46–50 | 905 | 20.1 | 446 | 22.4 | |
| 51–55 | 1,084 | 24.1 | 583 | 29.3 | |
| Socioeconomic status | |||||
| High | 1,661 | 37.0 | 206 | 10.4 | <0.001 |
| Middle | 2,367 | 52.7 | 843 | 42.4 | |
| Low | 466 | 10.4 | 941 | 47.3 | |
| Ethnicity | <0.001 | ||||
| White | 4,141 | 92.2 | 1,710 | 85.9 | |
| Other | 349 | 7.8 | 280 | 14.1 | |
| Marital status | <0.001 | ||||
| Married/cohabiting | 3,582 | 80.0 | 1,169 | 59.1 | |
| Other | 895 | 20.0 | 808 | 40.9 | |
| Body mass indexa at age 25 years | <0.001 | ||||
| ≤19.9 | 550 | 12.6 | 489 | 25.3 | |
| 20–24.9 | 3,122 | 71.3 | 1,199 | 62.1 | |
| 25–29.9 | 635 | 14.5 | 197 | 10.2 | |
| ≥30 | 71 | 1.6 | 45 | 2.3 | |
| Quartile of hostility level | 0.480 | ||||
| Highest | 1,001 | 22.3 | 432 | 21.7 | |
| Middle highest | 1,210 | 26.9 | 531 | 26.7 | |
| Middle lowest | 1,143 | 25.4 | 508 | 25.5 | |
| Lowest | 1,140 | 25.4 | 519 | 26.1 | |
Weight (kg)/height (m)2.
BMI changes over time
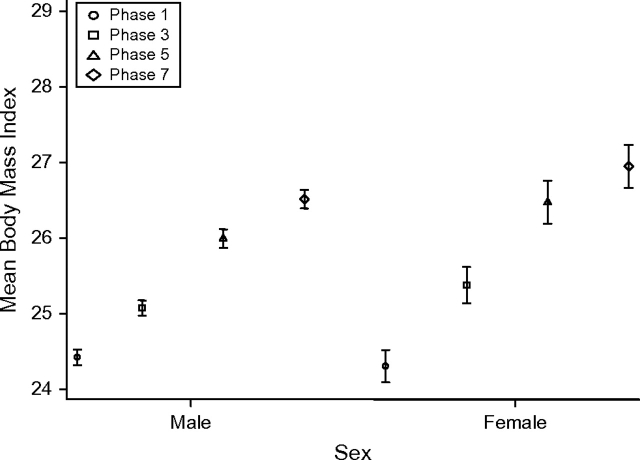
Figure 1 characterizes the dynamics of BMI change over time in men and women. In both sexes, mean BMI tended to increase over the 19 years of follow-up. For men, the mean BMI was 24.4 in phase 1, 25.1 in phase 3, 26 in phase 5, and 26.5 in phase 7. For women, the mean BMI was 24.3 in phase 1, 25.4 in phase 3, 26.5 in phase 5, and 27 in phase 7. Post-hoc paired t test analyses (results not shown) revealed that the mean BMI differences between phase 1 and phase 3, phase 3 and phase 5, phase 5 and phase 7, and phase 1 and phase 7 were all significant (P < 0.001) in both sexes.
Figure 1.
Mean body mass index (weight (kg)/height (m)2) among men (n = 3,323) and women (n = 1,356) in phases 1 (1985–1988), 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004) of the Whitehall II Study, United Kingdom. Bars, standard error.
Sociodemographic characteristics and BMI trajectories
Table 2 shows results from the mixed-models analysis used to assess associations between the covariates and BMI trajectories over time in men and women. The results indicate significant temporal effects (P < 0.001); the coefficient for time implies that the mean annual rate of increase in BMI was 0.138 in men and 0.260 in women over the 19 years of follow-up. BMI at baseline was lower in younger participants (P < 0.001), but the term for interaction between time and age showed a greater increase in mean BMI over time in the younger participants (P < 0.001). Higher socioeconomic status was associated with lower BMI at baseline in both sexes (P ≤ 0.025); the term for interaction with time suggested lower increases in mean BMI over time in the high socioeconomic status group compared with the low socioeconomic status group among women (P < 0.018). White participants had lower BMI at baseline in both sexes (P ≤ 0.04), but the term for interaction between time and ethnicity suggested a greater increase in mean BMI over time among white participants in both sexes (P ≤ 0.007). Neither marital status nor the interaction between time and marital status was associated with BMI. Finally, lower BMI at age 25 years was associated with lower BMI at baseline (P < 0.001), and the term for interaction between time and BMI at age 25 years suggested smaller increases in mean BMI during follow-up in participants with a lower BMI at age 25 years (P ≤ 0.005).
Table 2.
Associations (Mixed-Models Analyses) Between Sociodemographic Measures and Body Mass Index Trajectories From Phase 1 (1985–1988) to Phase 7 (2002–2004) of the Whitehall II Study, United Kingdom
| Men (n = 4,358) |
Women (n = 1,911) |
|||||
| β | SE | P Value | β | SE | P Value | |
| Time, per year | 0.138 | 0.025 | <0.001 | 0.260 | 0.038 | <0.001 |
| Age, years | ||||||
| 34–40 | −0.551 | 0.122 | <0.001 | −1.663 | 0.249 | <0.001 |
| 41–45 | −0.454 | 0.120 | <0.001 | −1.217 | 0.242 | <0.001 |
| 46–50 | −0.032 | 0.130 | 0.806 | −0.470 | 0.248 | 0.006 |
| 51−55a | 0 | 0 | ||||
| Time × age, years | ||||||
| 34–40 | 0.079 | 0.007 | <0.001 | 0.107 | 0.015 | <0.001 |
| 41–45 | 0.055 | 0.007 | <0.001 | 0.067 | 0.014 | <0.001 |
| 46–50 | 0.013 | 0.007 | 0.075 | 0.065 | 0.015 | <0.001 |
| 51–55a | 0 | 0 | ||||
| Socioeconomic status | ||||||
| High | −0.472 | 0.164 | 0.004 | −0.693 | 0.308 | 0.025 |
| Middle | −0.244 | 0.154 | 0.114 | −0.327 | 0.195 | 0.093 |
| Lowa | 0 | 0 | ||||
| Time × socioeconomic status | ||||||
| High | −0.010 | 0.010 | 0.365 | −0.040 | 0.017 | <0.018 |
| Middle | −0.008 | 0.010 | 0.670 | −0.031 | 0.012 | 0.010 |
| Lowa | 0 | 0 | ||||
| Ethnicity | ||||||
| White | −0.359 | 0.175 | 0.040 | −2.11 | 0.268 | <0.001 |
| Othera | 0 | 0 | ||||
| Time × ethnicity | ||||||
| White | 0.030 | 0.011 | 0.005 | 0.044 | 0.017 | 0.007 |
| Othera | 0 | 0 | ||||
| Marital status | ||||||
| Married/cohabiting | 0.078 | 0.111 | 0.484 | 0.056 | 0.179 | 0.757 |
| Othera | 0 | 0 | ||||
| Time × marital status | ||||||
| Married/cohabiting | 0.007 | 0.006 | 0.268 | −0.017 | 0.011 | 0.102 |
| Othera | 0 | 0 | ||||
| BMIb at age 25 years | ||||||
| ≤19.9 | −8.824 | 0.361 | <0.001 | −12.048 | 0.600 | <0.001 |
| 20–24.9 | −6.011 | 0.344 | <0.001 | −9.078 | 0.581 | <0.001 |
| 25–29.9 | −2.420 | 0.358 | <0.001 | −4.652 | 0.633 | <0.001 |
| ≥30a | 0 | 0 | ||||
| Time × BMI at age 25 years | ||||||
| ≤19.9 | −0.088 | 0.022 | <0.001 | −0.216 | 0.035 | <0.001 |
| 20–24.9 | −0.090 | 0.021 | <0.001 | −0.166 | 0.034 | <0.001 |
| 25–29.9 | −0.061 | 0.022 | 0.005 | −0.136 | 0.038 | 0.000 |
| ≥30a | 0 | 0 | ||||
Abbreviations: BMI, body mass index; SE, standard error.
Reference category.
Weight (kg)/height (m)2.
Hostility as a predictor of BMI trajectories
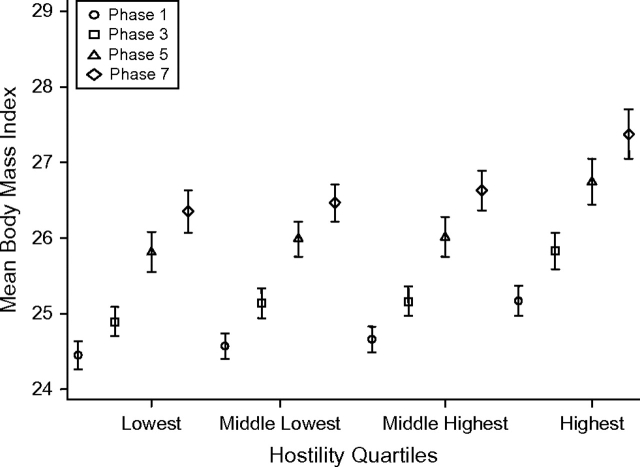
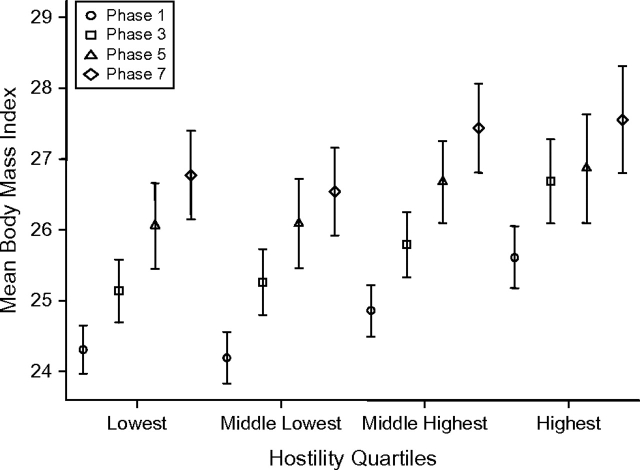
Table 3 shows results from the mixed-models analysis undertaken to assess associations between hostility levels and BMI trajectories over time in men and women. In model 1, men (β = 0.731, P < 0.001) and women (β = 1.326, P < 0.001) in the highest quartile of hostility had significantly higher BMIs at the start of follow-up. Furthermore, the term for interaction between time and hostility in men suggested a greater increase in BMI among those in the highest quartile of hostility, with an excess of 0.016 units in the mean annual increase of BMI (P = 0.023) over the duration of follow-up in comparison with the lowest quartile. This effect remained in the fully adjusted analysis (model 2; P = 0.043). Among women, there was no interaction between time and hostility, suggesting that the effect of hostility on the BMI trajectory over 19 years remained constant throughout the follow-up period. The dynamics of BMI change over time by hostility level are presented in Figure 2 (men) and Figure 3 (women).
Table 3.
Associations (Mixed-Models Analyses) Between Hostility Levels and Body Mass Index Trajectories From Phase 1 (1985–1988) to Phase 7 (2002–2004) of the Whitehall II Study, United Kingdom
| Men |
Women |
|||||
| β | SE | P Value | β | SE | P Value | |
| Model 1a | (n = 4,494) | (n = 1,990) | ||||
| Time, per year | 0.113 | 0.006 | <0.001 | 0.151 | 0.010 | <0.001 |
| Quartile of hostility level | ||||||
| Highest | 0.731 | 0.146 | <0.001 | 1.326 | 0.306 | <0.001 |
| Middle highest | 0.220 | 0.139 | 0.115 | 0.552 | 0.290 | 0.057 |
| Middle lowest | 0.132 | 0.141 | 0.352 | −0.100 | 0.293 | 0.732 |
| Lowestb | 0 | 0 | ||||
| P for linear contrast | <0.001 | <0.001 | ||||
| Time × quartile of hostility level | ||||||
| Highest | 0.016 | 0.007 | 0.023 | 0.006 | 0.014 | 0.709 |
| Middle highest | 0.012 | 0.006 | 0.052 | 0.016 | 0.014 | 0.238 |
| Middle lowest | 0.005 | 0.007 | 0.424 | 0.008 | 0.014 | 0.547 |
| Lowestb | 0 | 0 | ||||
| P for linear contrast | 0.011 | 0.562 | ||||
| Model 2c | (n = 4,374)d | (n = 1,924)d | ||||
| Time, per year | 0.174 | 0.391 | <0.001 | 0.133 | 0.028 | <0.001 |
| Quartile of hostility level | ||||||
| Highest | 0.555 | 0.124 | <0.001 | 0.333 | 0.255 | 0.191 |
| Middle highest | 0.212 | 0.116 | 0.068 | 0.240 | 0.232 | 0.302 |
| Middle lowest | 0.139 | 0.117 | 0.233 | −0.137 | 0.235 | 0.559 |
| Lowestb | 0 | 0 | ||||
| P for linear contrast | <0.001 | 0.055 | ||||
| Time × quartile of hostility level | ||||||
| Highest | 0.014 | 0.007 | 0.043 | 0.010 | 0.016 | 0.525 |
| Middle highest | 0.010 | 0.006 | 0.125 | 0.011 | 0.013 | 0.478 |
| Middle lowest | 0.006 | 0.006 | 0.309 | 0.009 | 0.013 | 0.491 |
| Lowestb | 0 | 0 | ||||
| P for linear contrast | 0.041 | 0.696 | ||||
Abbreviation: SE, standard error.
Results were adjusted for time, quartile of hostility level, and time × quartile of hostility level.
Reference category.
In addition to the factors listed above for model 1, results were adjusted for age, time × age, socioeconomic status, time × socioeconomic status (not in men), ethnicity, time × ethnicity, body mass index (weight (kg)/height (m)2) at age 25 years, and time × body mass index at age 25 years.
Figure 2.
Mean body mass index (weight (kg)/height (m)2) among men (n = 2,980–3,676) in phases 1 (1985–1988), 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004) of the Whitehall II Study, by quartile of hostility score, United Kingdom. Bars, standard error.
Figure 3.
Mean body mass index (weight (kg)/height (m)2) among women (n = 1,099–1,444) in phases 1 (1985–1988), 3 (1991–1993), 5 (1997–1999), and 7 (2002–2004) of the Whitehall II Study, by quartile of hostility score, United Kingdom. Bars, standard error.
DISCUSSION
In this study, we sought to examine longitudinal associations of hostility measured in adulthood with BMI assessed at 4 points over a 19-year follow-up period. In general terms, there was clear evidence of a trend of increasing mean BMI over time in both sexes. Higher levels of hostility were associated with higher mean BMI at the start of follow-up in both men and women. Furthermore, results from analysis of the interaction with time showed that in men, the highest hostility levels were associated with increasing BMI during the 19-year follow-up period when compared with the lowest hostility levels. In women, the association between hostility and BMI remained constant over time. This implies that the effects of hostility on BMI in men and women track over time, with an increasing effect on BMI over time among men with the highest levels of hostility.
The present findings are in line with some previous studies showing hostility to be associated with higher BMI (3, 13, 14, 16). However, to the best of our knowledge, this was the first large-scale study to examine the longitudinal relation of hostility to BMI assessed repeatedly over an extended follow-up period. Since BMI has been shown to change considerably over time (18), it is crucial to examine the dynamics of the association between hostility and BMI over time. The longitudinal modeling approach using time effects allowed us to control for weight gain over time, as well as to obtain precise estimates of effects. In contrast to some prior studies (3, 6), BMI in the present study was derived from height and weight assessed at a medical examination, thus minimizing measurement error or information bias and excluding the possibility of common-method bias. We were also able to control for self-reported BMI at age 25 years, which allowed us to examine the BMI trajectory over the adult life course.
As in other studies, BMI in the present study increased over time (18). The result showing the highest hostility level to be associated with increasing mean BMI during follow-up in men is consistent with our hypothesis that coronary heart disease behavioral risk factors associated with hostility do not remain constant throughout a person's life. The fact that high hostility was associated with higher BMI throughout the follow-up period and also, in men, with an excess annual increase in BMI during follow-up lends support to the hypothesis that hostility as an individual personality characteristic may influence the development and maintenance of behavior-related risk factors (6), evident in measures such as BMI.
There are several possible explanations for the link between hostility and BMI. First, the general cynicism and mistrust which characterize hostile persons may discourage them from following health promotion recommendations (35). Cynicism may decrease the perceived importance of health-enhancing behaviors such as diet and physical activity (14, 15)—factors which have been found to be associated with greater BMI and obesity (22, 26). Second, lower socioeconomic status is associated with higher hostility (12, 35) and greater BMI (36) and may be driving the association between hostility and BMI. However, in our analysis, this association remained robust to adjustment for socioeconomic status, either on its own or simultaneously with the other covariates. We assessed socioeconomic status using employment grade, the main measure of socioeconomic status in the Whitehall II Study. People in different grades differ with respect to salary, social status, and level of responsibility. Further research using repeated measures of socioeconomic status will be required to examine whether changes in socioeconomic circumstances mediate the association between hostility and BMI. An alternative explanation for this association is related to the psychosocial vulnerability model of hostility (14, 37). According to this model, hostile persons, given their oppositional attitudes and behaviors, are more likely to have increased interpersonal conflicts, lower levels of social support, more stressful life events, and a higher risk of depression (14, 37). The interrelations between these variables may influence BMI. For example, depression could result from a lack of social support or stressful life events and then affect diet or physical activity levels, which could in turn lead to higher BMI (38). Here again, further research using repeated measures of depression is needed to examine this possibility.
In women, we observed increases in mean BMI during the follow-up period, but the association between hostility and BMI remained constant throughout follow-up. In other words, the term for interaction between time and hostility did not suggest that the association between hostility and BMI observed at baseline increased or decreased over time, even though it tracked over time. These sex-specific results suggest that the influence of hostility on BMI may be patterned by sex, perhaps because of sex-specific biologic phenomena. Coronary heart disease affects men more than women (39), and it is not surprising that hostility, a risk factor for coronary heart disease (11, 14), is associated with an increasing effect on BMI over time in men alone. Menopause could be a confounder, as it has been found that at this time or several years before, women experience weight gain or have difficulty maintaining their usual weight (40).
In interpreting the present results, it is important to note 2 limitations. First, our cohort of civil servants did not include blue-collar workers and unemployed people and thus is not representative of the general population, which may limit the generalizability of our findings. Second, we were able to control for BMI at age 25 years, but this variable was derived from self-reported height and weight. However, in our results, BMI at age 25 years was found to be strongly associated with objectively measured BMI in phase 1, which supports the validity of this measure.
In summary, we found that mean BMI increased over a 19-year follow-up period among both men and women in the Whitehall II Study. We also found prospective evidence for an effect of hostility on BMI over the 19-year follow-up period. Finally, higher hostility was associated with significantly greater increases in BMI over time in men, suggesting that difference in BMI as a function of hostility is not stable over time. These results have implications for studies (e.g., studies on the association between hostility and coronary heart disease) in which the association between hostility and health outcomes is adjusted for health behaviors like BMI at baseline in order to assess the “independent” effect of hostility on health. Our findings suggest that controlling for baseline BMI might not be sufficient to address the mediation effect, particularly in men. Epidemiologic studies with repeated measures of covariates are now widespread. Going beyond baseline covariates might allow proper modeling of the mechanisms underlying the association between hostility and important health outcomes, such as coronary heart disease.
Acknowledgments
Author affiliations: Institut National de la Santé et de la Recherche Médicale, U687-IFR69, Villejuif, France (Hermann Nabi, Séverine Sabia, Aline Dugravot, Archana Singh-Manoux); Department of Epidemiology and Public Health, University College London, London, United Kingdom (Archana Singh-Manoux, Mika Kivimaki, Michael G. Marmot); and Centre de Gérontologie, Hôpital Sainte Périne, Paris, France (Archana Singh-Manoux).
The Whitehall II Study was supported by grants from the Medical Research Council; the British Heart Foundation; the United Kingdom Health and Safety Executive; the United Kingdom Department of Health; the National Heart, Lung, and Blood Institute, US National Institutes of Health (grant HL36310); the National Institute on Aging, US National Institutes of Health (grant AG13196); the Agency for Health Care Policy and Research, US Department of Health and Human Services (grant HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health. A. S.-M. was supported by a European Young Investigator Award from the European Science Foundation and a Chaire d'Excellence Award from the French Ministry of Research. M. M. was supported by a Medical Research Council Research Professorship. M. K. was supported by the Academy of Finland (grant 117604).
Conflict of interest: none declared.
Glossary
Abbreviation
- BMI
body mass index
References
- 1.Barefoot JC, Dahlstrom WG, Williams RB., Jr Hostility, coronary heart disease incidence, and total mortality: a 25-year follow-up study of 255 physicians. Psychosom Med. 1983;45(1):59–63. doi: 10.1097/00006842-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Shekelle RB, Gale M, Ostfeld AM, et al. Hostility, risk of coronary heart disease, and mortality. Psychosom Med. 1983;45(2):109–114. doi: 10.1097/00006842-198305000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Siegler IC, Peterson BL, Barefoot JC, et al. Hostility during late adolescence predicts coronary risk factors at mid-life. Am J Epidemiol. 1992;136(2):146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- 4.Bunde J, Suls J. A quantitative analysis of the relationship between the Cook-Medley Hostility Scale and traditional coronary artery disease risk factors. Health Psychol. 2006;25(4):493–500. doi: 10.1037/0278-6133.25.4.493. [DOI] [PubMed] [Google Scholar]
- 5.Matthews KA, Owens JF, Kuller LH, et al. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosom Med. 1998;60(5):633–638. doi: 10.1097/00006842-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Everson SA, Kauhanen J, Kaplan GA, et al. Hostility and increased risk of mortality and acute myocardial infarction: the mediating role of behavioral risk factors. Am J Epidemiol. 1997;146(2):142–152. doi: 10.1093/oxfordjournals.aje.a009245. [DOI] [PubMed] [Google Scholar]
- 7.Helmers KF, Krantz DS, Howell RH, et al. Hostility and myocardial ischemia in coronary artery disease patients: evaluation by gender and ischemic index. Psychosom Med. 1993;55(1):29–36. doi: 10.1097/00006842-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Barefoot JC, Larsen S, von der Lieth L, et al. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiol. 1995;142(5):477–484. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 9.Rosenman RH, Brand RJ, Sholtz RI, et al. Multivariate prediction of coronary heart disease during 8.5 year follow-up in the Western Collaborative Group Study. Am J Cardiol. 1976;37(6):903–910. doi: 10.1016/0002-9149(76)90117-x. [DOI] [PubMed] [Google Scholar]
- 10.Siegman AW, Dembroski TM, Ringel N. Components of hostility and the severity of coronary artery disease. Psychosom Med. 1987;49(2):127–135. doi: 10.1097/00006842-198703000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nabi H, Kivimäki M, Zins M, et al. Does personality predict mortality? Results from the GAZEL French prospective cohort study. Int J Epidemiol. 2008;37(2):386–396. doi: 10.1093/ije/dyn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabi H, Kivimäki M, Marmot MG, et al. Does personality explain social inequalities in mortality? The French GAZEL cohort study. Int J Epidemiol. 2008;37(3):591–602. doi: 10.1093/ije/dyn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niaura R, Banks SM, Ward KD, et al. Hostility and the metabolic syndrome in older males: The Normative Aging Study. Psychosom Med. 2000;62(1):7–16. doi: 10.1097/00006842-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Miller TQ, Smith TW, Turner CW, et al. A meta-analytic review of research on hostility and physical health. Psychol Bull. 1996;119(2):322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- 15.Leiker M, Hailey BJ. A link between hostility and disease: poor health habits? Behav Med. 1988;14(3):129–133. doi: 10.1080/08964289.1988.9935136. [DOI] [PubMed] [Google Scholar]
- 16.Scherwitz LW, Perkins LL, Chesney MA, et al. Hostility and health behaviors in young adults: The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 1992;136(2):136–145. doi: 10.1093/oxfordjournals.aje.a116480. [DOI] [PubMed] [Google Scholar]
- 17.Christensen U, Lund R, Damsgaard MT, et al. Cynical hostility, socioeconomic position, health behaviors, and symptom load: a cross-sectional analysis in a Danish population-based study. Psychosom Med. 2004;66(4):572–577. doi: 10.1097/01.psy.0000126206.35683.d1. [DOI] [PubMed] [Google Scholar]
- 18.Arnett DK, McGovern PG, Jacobs DR, Jr, et al. Fifteen-year trends in cardiovascular risk factors (1980–1982 through 1995–1997): The Minnesota Heart Survey. Am J Epidemiol. 2002;156(10):929–935. doi: 10.1093/aje/kwf133. [DOI] [PubMed] [Google Scholar]
- 19.Mokdad AH, Giles WH, Bowman BA, et al. Changes in health behaviors among older Americans, 1990 to 2000. Public Health Rep. 2004;119(3):356–361. doi: 10.1016/j.phr.2004.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serdula MK, Brewer RD, Gillespie C, et al. Trends in alcohol use and binge drinking, 1985–1999: results of a multi-state survey. Am J Prev Med. 2004;26(4):294–298. doi: 10.1016/j.amepre.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Sundquist K, Qvist J, Johansson SE, et al. Increasing trends of obesity in Sweden between 1996/97 and 2000/01. Int J Obes Relat Metab Disord. 2004;28(2):254–261. doi: 10.1038/sj.ijo.0802553. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292(10):1188–1194. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 23.Birmann BM, Giovannucci E, Rosner B, et al. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1474–1478. doi: 10.1158/1055-9965.EPI-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel S, Elwood P, Sweetnam P, et al. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348(9040):1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 25.Tohill BC, Seymour J, Serdula M, et al. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62(10):365–374. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Ledikwe JH, Blanck HM, Kettel Khan L, et al. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83(6):1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 27.Breslow RA, Smothers BA. Drinking patterns and body mass index in never smokers: National Health Interview Survey, 1997–2001. Am J Epidemiol. 2005;161(4):368–376. doi: 10.1093/aje/kwi061. [DOI] [PubMed] [Google Scholar]
- 28.Marmot M, Brunner E. Cohort profile: The Whitehall II Study. Int J Epidemiol. 2005;34(2):251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 29.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38(6):414–418. [Google Scholar]
- 30.Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol. 1992;11(3):139–150. doi: 10.1037//0278-6133.11.3.139. [DOI] [PubMed] [Google Scholar]
- 31.Everson-Rose SA, Lewis TT, Karavolos K, et al. Cynical hostility and carotid atherosclerosis in African American and white women: The Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152(5):982.. doi: 10.1016/j.ahj.2006.08.010. e7–982.e13. [DOI] [PubMed] [Google Scholar]
- 32.Yan LL, Liu K, Matthews KA, et al. Psychosocial factors and risk of hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA. 2003;290(16):2138–2148. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Dales R, Tang M, et al. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155(3):191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 35.Barefoot JC, Peterson BL, Dahlstrom WG, et al. Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychol. 1991;10(1):18–24. doi: 10.1037//0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Lynch J, Schulenberg J, et al. Emergence of socioeconomic inequalities in smoking and overweight and obesity in early adulthood: The National Longitudinal Study of Adolescent Health. Am J Public Health. 2008;98(3):468–477. doi: 10.2105/AJPH.2007.111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kivimäki M, Elovainio M, Kokko K, et al. Hostility, unemployment and health status: testing three theoretical models. Soc Sci Med. 2003;56(10):2139–2152. doi: 10.1016/s0277-9536(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 38.Pine DS, Goldstein RB, Wolk S, et al. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107(5):1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 39.Sytkowski PA, D'Agostino RB, Belanger A, et al. Sex and time trends in cardiovascular disease incidence and mortality: The Framingham Heart Study, 1950–1989. Am J Epidemiol. 1996;143(4):338–350. doi: 10.1093/oxfordjournals.aje.a008748. [DOI] [PubMed] [Google Scholar]
- 40.Panotopoulos G, Raison J, Ruiz JC, et al. Weight gain at the time of menopause. Hum Reprod. 1997;12(suppl 1):126–133. doi: 10.1093/humrep/12.suppl_1.126. [DOI] [PubMed] [Google Scholar]