Abstract
Background
Tamoxifen’s effect of reducing the risk of estrogen receptor (ER)–positive breast cancer is well established. Its effect on the time to first diagnosis of breast cancer has not been reported. We used information from the randomized, placebo-controlled Breast Cancer Prevention Trial (BCPT) to make that evaluation.
Methods
A total of 13 388 women enrolled in BCPT, of whom 174 were diagnosed with ER-positive tumors and 69 were diagnosed with ER-negative tumors. A flexible semiparametric cure rate model was used to assess the effects of tamoxifen vs placebo treatment on the time to disease diagnosis. Multivariable logistic regression, adjusted for age and tumor size at diagnosis, was used to assess the association between the mammography detection rate and treatment with tamoxifen. All statistical tests were two-sided.
Results
The median times to diagnosis of ER-positive tumors were similar in both treatment groups (43 months for the placebo arm and 51 months for the treatment arm). Times to diagnosis of ER-negative tumors, however, differed between treatment groups, with median times to diagnosis of 36 months in the placebo arm vs 24 months in the tamoxifen arm (difference = 12 months, 95% confidence interval [CI] = 3 to 17 months, P = .037). ER-negative breast cancers in the tamoxifen arm were more likely than those in the placebo arm to be detected by mammography than by clinical breast examination alone after adjustment for age and tumor size, but the increase was only marginally statistically significant (odds ratio for mammography detection = 4.68, 95% CI = 0.86 to 25.32, P = .073). No differences were found in the mammography detection rates for ER-positive tumors by treatment arm.
Conclusion
Although tamoxifen treatment does not reduce the incidence of ER-negative breast cancer, it may have advanced detection of such tumors by approximately 1 year, compared with that in the placebo arm. The time to diagnosis of ER-positive breast cancer was similar in both treatment arms.
Context and Caveats
Prior knowledge
The effect of tamoxifen chemoprevention treatment on the time to first diagnosis of breast cancer has not been reported.
Study design
Subset analysis of data from the phase 3 randomized placebo-controlled Breast Cancer Prevention Trial (BCPT), in which 13 338 women were enrolled and of whom 174 were diagnosed with estrogen receptor (ER)-positive tumors and 69 were diagnosed with ER-negative tumors.
Contribution
Times to diagnosis of ER-positive tumors were similar in both tamoxifen and placebo treatment groups. Times to diagnosis of ER-negative tumors differed between treatment groups, with a median time of 36 months in the placebo group and 24 months in the tamoxifen group. ER-negative tumors in the tamoxifen group were more likely than those in the placebo group to be detected by mammography than by clinical breast examination alone. No differences were found in the mammography detection rates for ER-positive tumors by treatment group.
Implication
Although chemoprevention with tamoxifen does not reduce the incidence of ER-negative breast cancer, it appears to have advanced the detection of ER-negative tumors by approximately 1 year. This result warrants further investigation.
Limitations
Breast density was not assessed in the BCPT. The number of ER-negative breast cancers diagnosed was relative small and so conclusions that are based on these data should be interpreted with caution.
From the Editors
The prevention of breast cancer in women who are at high risk for the disease has been substantially advanced in both primary prevention and early detection settings. Several randomized controlled primary prevention trials have shown that tamoxifen reduces the incidence of estrogen receptor (ER)–positive breast cancer by 30%–50% (1–7). In addition, breast cancer screening by mammography, with or without clinical breast examination, has been evaluated for its efficacy in several large randomized screening trials and has been recommended for the last two decades (8–11). However, to our knowledge, there is no literature evaluating the association between the primary prevention agent and the method of early detection and none on how tamoxifen may affect the time to breast cancer diagnosis. The Breast Cancer Prevention Trial (BCPT) (1,2) of the National Surgical Adjuvant Breast and Bowel Project (NSABP) is the largest randomized double-blinded placebo-controlled clinical trial to evaluate the efficacy of tamoxifen in the prevention of breast cancer. The same intensive surveillance schedule was implemented for both the placebo and tamoxifen treatment arms during the study follow-up. Therefore, the BCPT provides a substantial database to investigate not only the efficacy of tamoxifen on the incidence of breast cancer but also its impact on the time to detection of the disease. In this study, we used a retrospective analysis of BCPT data to evaluate the effect of tamoxifen on the time to diagnosis of breast cancer and the rate of cancer detection by mammography examination with or without clinical breast examination and by clinical breast examination alone among women with ER-positive or ER-negative breast cancer.
Patients and Methods
Study Population
Between 1992 and 1997, a total of 13 388 asymptomatic women aged 35 years or older who were at high risk for a first occurrence of invasive breast cancer were randomly assigned to receive either placebo or 20 mg of tamoxifen daily for 5 years. High risk was defined as having a projected 5-year breast cancer risk of at least 1.66% according to the modified Gail model (12). All participants were monitored for development of the disease by use of annual mammograms and follow-up clinical examinations at 3 months, 6 months, and every 6 months thereafter. Invasive breast cancer diagnosed during the study may have been detected by mammography only, clinical breast examination only, or both. When results of the trial were first reported (1), the average follow-up of the population was about 6 years. At that time, a total of 265 invasive breast cancers had been diagnosed (176 in the placebo arm and 89 in the tamoxifen arm). Among the 265 invasive breast cancers, 174 were ER positive, 69 were ER negative, and 22 had unknown ER status. Details of the design and implementation of the trial have been reported previously (1). This study was reviewed and approved by NSABP Operations Center and the Institutional Review Board of the M. D. Anderson Cancer Center.
Statistical Analysis
The primary endpoint was the time to diagnosis of breast cancer, as measured from the time of random assignment in the BCPT. Because only a small proportion of the women developed breast cancer during study follow-up, resulting in heavy censoring in the whole cohort, the difference in time to diagnosis between the treatment arms was difficult to discern. Also, if we had restricted our analysis to the patients diagnosed with breast cancer only during study follow-up, a simple comparison of the time to diagnosis between the two distributions for the treatment groups would have been biased by ignoring the potential right-censoring (ie, loss to follow-up) for participants who would have been diagnosed with breast cancer after the end of study follow-up. Therefore, we used a semiparametric cure rate model (13) to assess the treatment effects of tamoxifen in this mixed cohort of individuals who were both susceptible and unsusceptible to breast cancer. The mixture model allowed us to separately estimate the distribution for time to diagnosis among susceptible participants to the disease, as well as the disease incidence within each treatment arm. Among participants susceptible to the disease, we quantified the potential change in the time to disease diagnosis between the treatment and placebo arms by use of a flexible model, in which gc(t) was defined as exp{α + βt}fc(t), where gc and fc are the conditional probability density functions for the tamoxifen and placebo arms, respectively, t is time to the diagnosis of breast cancer as measured from the time of randomization, and α and β are parameters to quantify potential difference between the density functions gc(t) and fc(t). There is no parametric assumption for the two probability density functions. We used the conditional likelihood ratio test to assess the equality of the two density functions gc and fc. Details of the statistical inference and model checking have been described previously (13). We used a bootstrapping method to estimate 95% confidence intervals (CIs) for difference in the median times to diagnosis of breast cancer between the two treatment arms and for the estimated survival distributions of the two treatment arms (see Figure 1).
Figure 1.
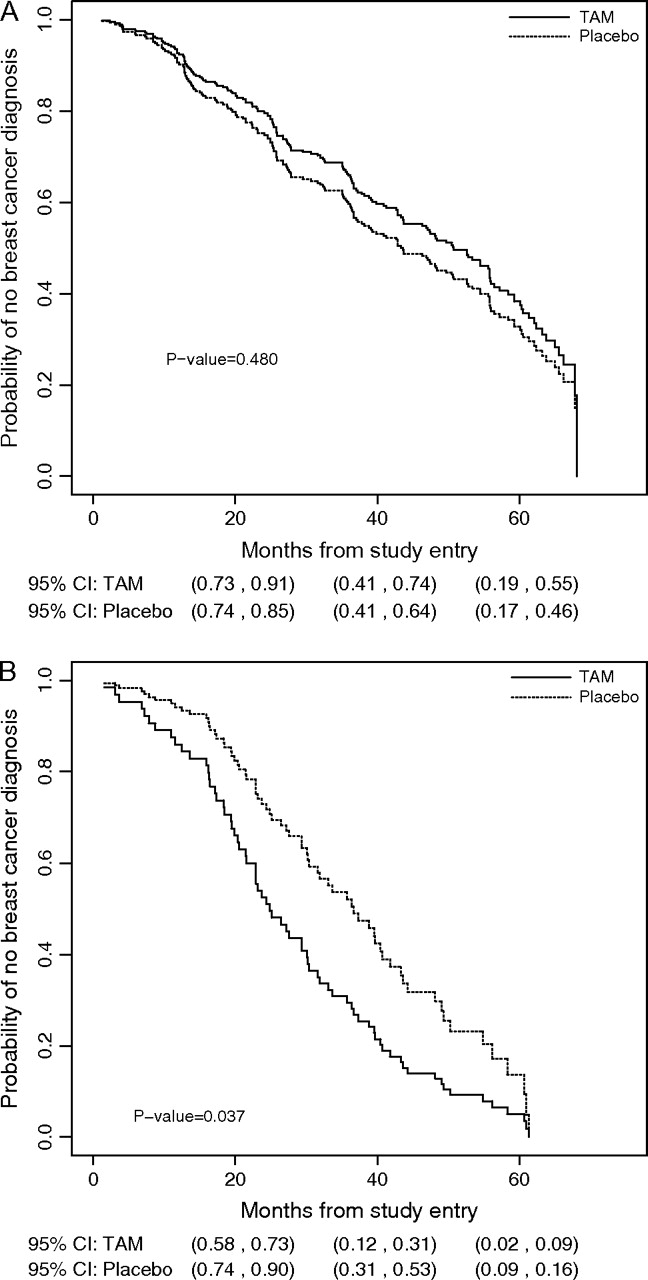
Estimated survival distributions for each Breast Cancer Prevention Trial treatment arm conditional on prevalent cases of breast cancer. A) Estrogen receptor (ER)–positive breast cancer. B) ER-negative breast cancer. The 95% confidence intervals for the probability of no breast cancer diagnosis are presented for 20, 40, and 60 months from the time of randomization. The conditional likelihood ratio test was used to assess the equality of the two density functions. All statistical tests were two-sided. Reprinted with permission from The Journal of American Statistical Association, Copyright 2007, the American Statistical Association. All rights reserved. TAM = tamoxifen.
We used descriptive analysis to compare tumor characteristics at the time of diagnosis by treatment arm and by tumor ER status. To compare tumor size distributions between the two arms by ER status, we used the Wilcoxon–Mann–Whitney exact test. Among women diagnosed with breast cancer, we used the Fisher exact test to compare the mammography detection rate with or without clinical breast examination between the two treatment arms, stratified by ER status. We used multivariable logistic regression to examine whether the probability of mammography detection with or without clinical breast examination was associated with treatment after adjustment for age (continuous variable) and tumor size (≤1.0 cm vs >1.0 cm) at diagnosis, stratified by ER status. We estimated the odds ratio (OR) and its 95% confidence interval and calculated the P value from Wald's test for each risk factor in the multivariable logistic models. We performed the statistical analyses with S-PLUS version 7.0 (Insightful Corporation, Seattle, WA) and SAS version 8.02 (SAS Institute, Inc., Cary, NC). All statistical testing was two-sided.
Results
Time to Diagnosis of Breast Cancer
A total of 243 women were diagnosed with invasive breast cancer of known ER status—174 with ER-positive tumors and 69 with ER-negative tumors. Among women with ER-positive tumors, the median time to disease diagnosis was similar in both treatment arms (43 months for the placebo arm and 51 months for the tamoxifen arm, difference = –8 months, 95% CI = –20 to 12 months, P = .48, conditional likelihood ratio test) (Figure 1, A). Among women with ER-negative tumors, however, the median times to diagnosis of disease differed between treatment groups. The median time to diagnosis of ER-negative breast cancer was 24 months for women in the tamoxifen arm and 36 months for women in the placebo arm (difference = 12 months, 95% CI = 3 to 17 months, P = .037, conditional likelihood ratio test) (Figure 1, B).
Mammography Detection Rate
The tumor characteristics at the time of diagnosis are shown by treatment and by ER status in Table 1. Among women with ER-positive tumors, the distribution of tumor sizes at diagnosis was similar in the two treatment arms (P = .823). Among women with ER-negative tumors, in contrast, a higher proportion of tumors in the tamoxifen arm than in the placebo arm were small (≤1.0 cm) at diagnosis: 39.5% of ER-negative tumors in the tamoxifen arm had diameters of 1 cm or less compared with 16.1% of ER-negative tumors in the placebo arm (difference = 23.4%, 95% CI = 0.94% to 41.2%, P = .069). No statistically significant difference between the placebo and tamoxifen arms was observed in the tumor–node–metastasis stage distribution or lymph node status.
Table 1.
Characteristics of invasive breast cancers at diagnosis by ER status among participants with known tumor ER status*
| ER-positive disease |
ER-negative disease |
|||||
| Characteristic | Placebo, No. (%) | Tamoxifen, No. (%) | P value† | Placebo, No. (%) | Tamoxifen, No. (%) | P value† |
| Lymph node status | ||||||
| Negative | 90 (68.2) | 32 (76.2) | 18 (58.1) | 22 (57.9) | ||
| Positive | 33 (25.0) | 10 (23.8) | 13 (41.9) | 15 (39.5) | ||
| Unknown‡ | 9 (6.8) | 0 (0) | .839 | 0 (0) | 1 (2.6) | .999 |
| Tumor size | ||||||
| ≤1.0 cm | 53 (40.2) | 18 (42.9) | 5 (16.1) | 15 (39.5) | ||
| 1.1–2.0 cm | 50 (37.9) | 12 (28.6) | 14 (45.2) | 13 (34.2) | ||
| 2.1–3.0 cm | 13 (9.8) | 6 (14.3) | 6 (19.4) | 7 (18.4) | ||
| ≥3.1 cm | 15 (11.4) | 6 (14.3) | 5 (16.1) | 3 (7.9) | ||
| Unknown‡ | 1 (0.8) | 0 (0) | .823 | 1 (3.2) | 0 (0) | .069 |
| TNM stage | ||||||
| I | 79 (59.8) | 27 (64.3) | 16 (51.6) | 17 (44.7) | ||
| II | 42 (31.8) | 13 (31.0) | 11 (35.5) | 18 (47.4) | ||
| III/IV | 7 (5.2) | 2 (4.7) | 3 (9.7) | 2 (5.3) | ||
| Unknown‡ | 4 (3.0) | 0 (0) | .953 | 1 (3.2) | 1 (2.6) | .607 |
| Detection method | ||||||
| Clinical breast exam only | 21 (15.9) | 6 (14.3) | 7 (22.6) | 2 (5.3) | ||
| Mammography only | 54 (40.9) | 19 (45.2) | 10 (32.3) | 12 (31.6) | ||
| Both | 57 (43.2) | 17 (40.5) | .999§ | 14 (45.2) | 24 (63.2) | .068§ |
| Total | 132 (100) | 42 (100) | 31 (100) | 38 (100) | ||
ER = estrogen receptor; TNM = tumor–node–metastasis.
The comparison was of the distributions by treatment group, excluding participants with unknown values. The Wilcoxon–Mann–Whitney exact test was used for comparing tumor sizes between the two arms, and the Fisher exact test was used for all other comparisons. All statistical tests were two-sided.
Excluded numbers of patients in the statistical tests are listed as unknown.
The comparison was of the combined categories of mammography only and both mammography and clinical breast examination to those of clinical breast examination only.
Among all participants diagnosed with breast cancer during the first 6 years of follow-up, more ER-negative breast cancers were detected by mammography with or without clinical breast examination in the tamoxifen arm than in the placebo arm (95% vs 77%, difference = 18%, 95% CI = 0.9% to 35.0%, P = .068, Fisher test), but the difference was only marginally statistically significant. However, ER-positive breast cancers were detected by mammography (with or without clinical breast examination) at approximately the same rate in both treatment arms (86% vs 84%, difference = 2%, 95% CI = –12.9% to 12.1%; P = .99, Fisher test). In multivariable logistic regression analyses, mammography detection of ER-positive breast cancer was not associated with treatment (OR = 1.16, 95% CI = 0.42 to 3.2, P = .768, Wald test). We also found that age at diagnosis appeared to play different roles by ER status (Table 2). Among women with ER-positive tumors, small tumors and tumors in older women were more likely to have been detected by mammography than by clinical breast examination alone, independent of treatment arm. ER-negative breast cancers in the tamoxifen arm were more likely than those in the placebo arm to be detected by mammography than by clinical breast examination alone after adjustment for age and tumor size, but the increase was only marginally statistically significant (OR = 4.68, 95% CI = 0.86 to 25.32, P = .073, Wald's test). Age and tumor size at diagnosis were not associated with the method used to detect ER-negative tumors.
Table 2.
Detection of invasive breast cancer by mammography vs clinical breast examination alone by estrogen receptor status*
| ER-positive disease (n = 174) |
ER-negative disease (n = 69) |
|||
| Risk factor | OR for mammography detection (95% CI) | P value† | OR for mammography detection (95% CI) | P value† |
| Treatment | ||||
| Placebo | 1.00 (referent) | 1.00 (referent) | ||
| Tamoxifen | 1.16 (0.42 to 3.20) | .768 | 4.68 (0.86 to 25.32) | .073 |
| Tumor size, cm | ||||
| ≥1 | 1.00 (referent) | 1.00 (referent) | ||
| <1 | 2.55 (0.96 to 6.80) | .061 | 1.89 (0.15 to 23.25) | .619 |
| Age at diagnosis per year‡ | 1.06 (1.01 to 1.11) | .024 | 1.03 (0.92 to 1.15) | .608 |
A multivariable model was used to investigate the detection of invasive breast cancer by mammography vs clinical breast examination alone. ER = estrogen receptor; OR = odds ratio; CI = confidence interval.
All P values were obtained from the Wald's test. All statistical tests were two-sided.
Range of age at diagnosis is 38–82 years. Age at diagnosis is a continuous variable. An odds ratio of 1.06 may be reported as follows: For every 1-year increase in age, women with ER-positive tumors are 1.06 times more likely to have their tumors detected by mammography than by clinical breast examination alone.
Discussion
We showed that tamoxifen treatment appeared to shorten the time to diagnosis of ER-negative breast cancer by approximately 1 year but was not associated with the time to diagnosis of ER-positive breast cancer. The earlier diagnosis of ER-negative breast cancer might have been a result of more diagnosable tumors or of faster- growing tumors associated with the use of tamoxifen. However, if tamoxifen had stimulated the growth of ER-negative tumors, we would have expected statistically significantly more ER-negative tumors to have been diagnosed during follow-up or at younger ages at diagnosis in the tamoxifen arm than in the placebo arm. We did not observe either of these events among women with ER-negative tumors. The estimated incidences of ER-negative breast cancer (7.4 per 1000 vs 7 per 1000 women) and the median ages at diagnosis (53.5 vs 52.7 years) were about the same in both the tamoxifen and placebo arms, respectively. The observation that more smaller-sized ER-negative tumors were diagnosed among women in the tamoxifen arm than in the placebo arm tends to support the interpretation that earlier diagnosis is a result of more “diagnosable” ER-negative tumors. The relative detection rate by mammography also confirmed that ER-negative tumors were more likely to be detected by mammography than by clinical breast examination in the tamoxifen arm than in the placebo arm, albeit not statistically significantly so. In contrast, no differences were found in the mammography detection rates of ER-positive tumors by treatment arm. Our analysis also showed that chemoprevention with tamoxifen, compared with placebo, reduced the number of invasive ER-positive breast cancers among participants in NSABP's BCPT and also concurred with previous findings that tamoxifen did not reduce the risk of ER-negative invasive breast cancer (1–7).
Because breast cancer is estrogen dependent, the biological function of endocrine therapies on the disease differs according to the ER status of the tumor. It is thus not surprising that the effects of tamoxifen on the reduction of disease incidence and mortality differed by the ER status of the tumor. Our study results also provide information on the effect of tamoxifen treatment on disease surveillance, which could be used to improve the early detection of breast cancer. Some studies (14,15) have hypothesized that ER-negative tumors are less likely to be detected by mammography than ER-positive tumors. In this study, we found a similar, although not statistically significant, pattern in the placebo arm: mammography detected 77.4% of ER-negative tumors and 84.1% of ER-positive tumors. Conversely, in the tamoxifen arm, mammography detected 94.7% of ER-negative tumors and 85.7% of the ER-positive tumors. Clearly, the mammography detection rate of ER-positive tumors was changed little by tamoxifen use, but the mammography detection rate of ER-negative tumors increased with tamoxifen use. Our multivariable logistic regression model also indicated that ER-negative breast cancers were more likely to be detected by mammography in the tamoxifen arm than in the placebo arm (OR = 4.68, 95% CI = 0.86 to 25.32, P = .073), although the difference did not reach statistical significance. These data appear to support the hypothesis that there are differences in mammographic detection of ER-negative breast cancer, but not for ER-positive breast cancer, by treatment group.
Our finding that tamoxifen treatment decreased the time to diagnosis of ER-negative breast cancers is supported by the findings that changes in breast density are associated with the use of tamoxifen (16–18) and that breast density appears to be a major factor influencing the sensitivity of mammography screening examinations (19,20). Tamoxifen treatment changes the density of normal breast tissue and thereby modifies the contrast between normal tissue and tumor tissue, which would increase the ability of mammography to detect tumors (5,16–18,21). Unfortunately, these studies (16–18,21) were not stratified by the ER status of the tumor and the BCPT trial did not collect data on breast density. It is therefore unclear why the potential change in breast density did not make a difference in the mammographic diagnosis of ER-positive breast cancer in the tamoxifen arm. One possibility is that tamoxifen treatment increases the contrast between normal tissue and ER-negative tumor tissue more than the contrast between normal tissue and ER-positive tumor tissue. An alternative explanation is that, as an antiestrogen agent, tamoxifen not only modifies breast density but also inhibits the growth of ER-positive breast cancers but not ER-negative breast cancers (22). The latter explanation somewhat supports the observations of Cuzick et al. (5) that the change in breast density associated with tamoxifen treatment predicted approximately one-third of the reduction in the incidence of breast cancer that was observed in the prevention trials and that the reduction of incidence occurred only in ER-positive tumors. If the earlier diagnosis of ER-negative breast cancer is a result of the reduction in breast density associated with the use of tamoxifen, then tamoxifen may not have a direct clinical benefit in the reduction of ER-negative breast cancer, even though breast density is an independent risk factor for breast cancer (23–25).
Our study has several limitations. Our analysis was limited by a lack of measurement of breast density either before or after treatment in the BCPT. In addition to its role as an important risk factor in predicting the occurrence of breast cancer, breast density also affects mammography sensitivity at diagnosis of breast cancer. Therefore, information of breast density should be collected at periodic screening examinations in future prevention trials. Another limitation to this study is that the number of ER-negative breast cancers diagnosed was moderately small (a total of 69 women with ER-negative tumors), and thus, any conclusions that are based on these data should be interpreted with caution.
The BCPT was the largest placebo-controlled chemoprevention trial to evaluate tamoxifen as a chemopreventive agent. We used BCPT data to investigate the effect of tamoxifen treatment in primary prevention and early detection settings by the ER status of the tumor. Findings from this study have the potential to increase our knowledge of tamoxifen's effects on the natural history of breast cancer, its primary prevention, and its early detection. The biologic mechanisms associated with the decreased time to diagnosis of ER-negative breast tumors in the tamoxifen arm, compared with that in the placebo arm, remain to be further investigated in treatment and prevention studies.
Funding
This project was supported in part by the National Cancer Institute grants (R01-CA079466 to Y.S., U10-CA-37377 and U10-CA69974 to J.P.C.).
Footnotes
The authors thank the National Surgical Adjuvant Breast and Bowel Project for providing data from the Tamoxifen Breast Cancer Prevention Trial. The authors had full responsibility for the design of the study, analysis and interpretation of data, the decision to submit a manuscript, and the writing of the manuscript.
References
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP. Re: tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2006;98(19):643–644. doi: 10.1093/jnci/djj167. [DOI] [PubMed] [Google Scholar]
- 3.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Maisonneuve P, Rotmensz N, et al. Italian Tamoxifen Study Group. Italian randomized trial among women with hysterectomy: tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst. 2003;95(2):160–165. doi: 10.1093/jnci/95.2.160. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9345):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Forbes JF, Sestak I, et al. International Breast Cancer Intervention Study I Investigators. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 7.Serrano D, Perego E, Costa A, Decensi A. Progress in chemoprevention of breast cancer. Crit Rev Oncol Hematol. 2004;49(2):109–117. doi: 10.1016/S1040-8428(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 8.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography. A meta-analysis. JAMA. 1995;273(2):149–154. [PubMed] [Google Scholar]
- 9.Humphrey LL, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Systematic Evidence Review No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2002. Sep, Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat3.chapter.27509. Accessed September 13, 2007. [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 11.Black WC, Haggstrom DA, Welch HG. All cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94(3):167–173. doi: 10.1093/jnci/94.3.167. [DOI] [PubMed] [Google Scholar]
- 12.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Qin J, Costantino JP. Inference of tamoxifen's effects on prevention of breast cancer from a randomized controlled trial. J Am Stat Assoc. 2007;102(480):1235–1244. doi: 10.1198/016214506000001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham JE, Butler WM. Racial disparities in female breast cancer in South Carolina: clinical evidence for a biological basis. Breast Cancer Res Treat. 2004;88(2):161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- 15.Porter PL, El-Bastwissi AY, Mandelson MT, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91(23):2020–2028. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8(10):863–866. [PubMed] [Google Scholar]
- 17.Brisson J, Brisson B, Coté G, Maunsell E, Bérubé S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9(9):911–915. [PubMed] [Google Scholar]
- 18.Chow CK, Venzon D, Jones EC, Premkumar A, O’Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9(9):917–921. [PubMed] [Google Scholar]
- 19.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996;276(1):33–38. [PubMed] [Google Scholar]
- 20.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 21.Chiarelli AM, Kirsh VA, Klar NS, et al. Influence of patterns of hormone replacement therapy use and mammographic density on breast cancer detection. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1856–1862. doi: 10.1158/1055-9965.EPI-06-0290. [DOI] [PubMed] [Google Scholar]
- 22.Radmacher MD, Simon R. Estimation of tamoxifen's efficacy for preventing the formation and growth of breast tumors. J Natl Cancer Inst. 2000;92(1):48–53. doi: 10.1093/jnci/92.1.48. [DOI] [PubMed] [Google Scholar]
- 23.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 24.Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98(17):1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 25.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. National Institutes of Health Breast Cancer Consortium. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]


