Abstract
Aims: To investigate the relationship between the sweet liking/sweet disliking phenotype (a putative probe of brain opioid function), craving for alcohol and response to treatment with naltrexone in individuals with alcohol dependence. Methods: Forty individuals with alcohol dependence were enrolled in a 12-week open-label study of 50 mg of naltrexone with four sessions of motivational enhancement therapy. Prior to treatment, individuals completed a sweet preference test and the Penn Alcohol Craving Scale. Subjects were categorized as sweet liking (SL), n = 15, or sweet disliking (SDL), n = 25, via a standard sweet tasting paradigm. The sweet tasting results were blinded to the subjects and to treatment staff. SL status, pretreatment craving and their interaction were examined as predictors of frequency of abstinent days and heavy drinking days during treatment with naltrexone. Results: SL and SDL subjects achieved similar reductions in percent heavy drinking days with treatment. During treatment, SDL subjects had 48% abstinent days compared to 30% for SL subjects (P = 0.034). Pretreatment craving did not predict % heavy drinking days or % abstinent days. An interaction effect was found between the SL/SDL phenotype and pretreatment craving such that SL subjects with high craving demonstrated higher rates of percent abstinent days whereas SDL subjects with high craving demonstrated lower rates of percent abstinent days, P < 0.001. Conclusions: These findings indicate that the SL/SDL phenotype may predict variation in response to naltrexone and/or counseling treatment. Furthermore, the SL/SDL phenotype may interact with craving to provide a more robust prediction of outcome with naltrexone or counseling.
Introduction
Naltrexone was the first approved medication in the United States to address the central nervous system actions of alcohol. Its efficacy has now been investigated in around 3000 alcohol-dependent subjects in randomized, placebo-controlled, single-center and multicenter trials. Meta-analyses of these studies support the initial finding that naltrexone is modestly effective in reducing relapse to heavy drinking and reducing drinking frequency and less effective in enhancing abstinence (Kranzler and Van Kirk, 2001; Streeton and Whelan, 2001; Srisurapanont and Jarusuraisin, 2002; Bouza et al., 2004). Whereas the overall effect of naltrexone may be modest, for some individual patients the effect appears to be strong. Therefore, one of the challenges with using naltrexone in the clinical setting is assessing and then predicting which patients are likely to experience a good response to naltrexone.
To date, there has been limited information to help the clinician decide which patient should receive a trial of naltrexone. Several factors have been tentatively identified in the literature as positively associated with naltrexone response—high baseline craving for alcohol (Monterosso et al., 2001; Volpicelli et al., 1995; Jaffe et al., 1996, not confirmed by Keifer et al., 2005); increased density of familial alcohol problems (Monterosso et al., 2001; Rubio et al., 2005; Rohsenow et al., 2007; Tidey et al., 2008); early onset of alcohol problems (Rubio et al., 2005) and the A118G variant polymorphism in the μ-opioid receptor (Oslin et al., 2003; Anton et al., 2008; not confirmed by Gelernter et al., 2007). Many of these markers are associated with functional activity of the endogenous opioid system. For example, the 118G allele of the μ-opioid receptor gene (OPRM1) has been associated with stronger binding of β-endorphin to the μ-opioid receptor (Bond et al., 1998), a positive family history of alcoholism has been reported to be associated with low baseline β-endorphin levels in the brain (Gianoulakis et al., 1989) and severity of alcohol craving has been shown to be linked to availability (Heinz et al., 2005) and binding potential (Bencherif et al., 2004) of postsynaptic μ-opiate receptors. These findings indicate that predictors of naltrexone response may be identified through assessment of the functional activity of the endogenous opioid system.
A growing body of evidence indicates that the hedonic response to sweet taste reflects activity of the endogenous opioid system (Calcagnetti and Reid, 1983; Leventhal et al., 1995; Pecina and Berridge, 2005). The hedonic response to sweet taste is a stable and heritable trait (Mennella et al., 2005; Keskitalo et al., 2007), associated with genetic risk of excessive alcohol intake in animals (for review, see Kampov-Polevoy et al., 1999) and genetic risk of alcohol dependence (AD) in humans (Kampov-Polevoy et al., 2001, 2003a, 2003b; Pepino and Mennella, 2007; Wronski et al., 2007). In humans, hedonic response to sweet taste generally yields two broad types—SL and SDL (Thompson et al., 1976; Looy et al., 1992). SL individuals note increasing pleasantness of sucrose concentrations up to 2.0 M whereas SDL do not like sucrose concentrations above the 0.4 M range.
We know of no study that has compared outcomes from treatment according to sweet liking.
We hypothesize that hedonic response to sweet taste, as a phenotypic probe of the endogenous opioid system, may be useful to predict naltrexone response in patients with AD. To test this hypothesis we conducted an open-label trial of naltrexone in AD subjects using the SL/SDL phenotype as a blinded predictor of drinking during treatment. We also examined the effects of the SL/SDL phenotype on (1) the ability to achieve abstinence prior to treatment with naltrexone, i.e. during the period between eligibility assessment and randomization, and (2) the interaction between the SL/SDL phenotype and initial (pre-treatment) craving for alcohol in prediction of the effect of naltrexone.
Materials and Methods
Subjects
The study sample consisted of 40 AD subjects, who met inclusion/exclusion criteria and received at least one dose of naltrexone (for demographic characteristics, see Table 1).
Table 1.
Baseline Demographics of SL and SDL Subgroups
| SL/SDL subgroups | |||
|---|---|---|---|
| Total sample | |||
| (n = 40) | SDL (n = 25) | SL (n = 15) | |
| Age | 49 ± 9 | 48 ± 9 | 51 ± 7 |
| Males (%) | 73% | 64% | 86% |
| Married (%) | 68% | 64% | 73% |
| Baseline % heavy drinking days (%HDD) | 76% | 76% ± 23% | 75% ± 26% |
| Baseline % abstinent days (%ABST) | 11% | 12% ± 16% | 10% ± 17% |
| PACS | 14.6 ± 5.5 | 14.4 ± 4.4 | 14.8 ± 7.2 |
| Years alcohol dependent | 14 ± 11 | 12 ± 9 | 17 ± 14 |
| Abstinence as goal of treatment | 27% | 32% | 20% |
Screening and eligibility criteria
The study was conducted at the University of North Carolina, Chapel Hill, between November 2003 and January 2005. Subjects were recruited from newspaper and radio advertisements targeted to individuals who wished to change their drinking behavior. The protocol was approved by the Committee on the Protection of the Rights of Human Subjects, School of Medicine, University of North Carolina at Chapel Hill.
Men and nonpregnant women aged 21 years and older with AD were eligible. Potential subjects were initially screened on the telephone and those who appeared to meet broad study criteria and were interested in participation were scheduled for a screening visit (week −1). At the screening visit, subjects signed informed consent and received a breath alcohol test that had to be 0.0 g/dl in order to proceed with the screening visit. Subjects were then given a medical examination and medical history interview, were asked about prescription and over-the-counter medication use, and had blood drawn for aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), bilirubin, alkaline phosphatase and a serum pregnancy test. A urine toxicology screen was completed. Individuals also completed a 90-day timeline follow-back interview (TLFB; Sobell et al., 1988) and the Penn Alchol Craving Scale (PACS) (Flannery et al., 1999). Trained interviewers administered the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1999) to determine psychiatric diagnoses. Subjects were required to meet DSM-IV criteria for AD and to have at least two episodes of heavy drinking (defined as four standard alcohol drinks/day for women and five standard alcohol drinks/day for men) per week during the 30 days prior to screening. The subjects were excluded if they had a history in the past year of drug dependence other than nicotine or alcohol or if they had a positive urine toxicology test at screening. Subjects who had a positive urine cannabinoid test were allowed in the study if there was no evidence for cannabinoid dependence. Exclusion criteria included (1) clinically significant medical illness; (2) significant psychiatric disorder including depression with suicidal ideation, bipolar disorder or schizophrenia; (3) current use of psychotropic medication, including medication for alcohol dependence, with the exception of a stable dose for ≥ 8 weeks of a selective serotonin reuptake inhibitor (SSRI) and (4) elevated bilirubin, documented cirrhosis or an ALT or AST level >2.5× upper limit of normal.
Overall, 54 subjects were screened to yield 40 subjects who met inclusion/exclusion criteria and received at least one dose of naltrexone. Screen failures occurred for the following reasons: five patients had comorbid psychiatric illness, four had inadequate level of alcohol consumption, three withdrew consent, one did not reach DSM-IV criteria for AD and one had elevated liver enzymes. All subjects signed written informed consent.
Overall study design
The study was a 12-week, open-label trial of naltrexone. Following the screening visit, individuals were asked to achieve 3 days of sobriety before starting naltrexone. The length of time it took for individuals to achieve 3 days of sobriety varied; thus the time between screening and naltrexone initiation varied from one to several weeks. Though the trial was designed as a 12-week study, some individuals were followed for longer than 12 weeks before study termination because of missed visits and scheduling logistics.
Study procedures
At the initial treatment visit (week 0) and all subsequent study visits (weeks 1, 2, 3, 4, 6, 8, 10 and 12), a breath alcohol test was administered, and vital signs were recorded. The Clinical Institute for Alcohol Withdrawal scale, Revised (Sullivan et al., 1989), was administered to assess for symptoms of significant alcohol withdrawal. No one required referral for medication detoxification. Naltrexone dosing was titrated from 25 mg/day for the first 3 days to 50 mg/day for the remainder of treatment. Naltrexone blister packs were distributed four times during treatment: at weeks 0, 1, 4 and 8. Additionally, calendars were provided so that participants could record the number of pills taken, the number of drinks consumed and any side effects experienced. At each visit, subjects were asked about use of concomitant medications and completed the TLFB and PACS. At week 4, blood was drawn to assess AST, ALT, GGT and bilirubin values. At week 12, or early termination, a complete physical was conducted, and AST, ALT, GGT, bilirubin and alkaline phosphatase levels measured. Pregnancy testing was conducted each month for female participants of childbearing potential.
The first of four Motivational Enhancement Treatment (MET) sessions was conducted on day 0. Additional MET sessions were provided at weeks 1, 6 and 12. Counselors who were trained following MET manual guidelines published by NIAAA (NIH, 1994) provided MET therapy. Counseling included having each participant state his/her goal for treatment (i.e. abstinence, reduction in drinking) and encouragement to work toward his/her stated goal. Counselors did not push subjects for abstinence though this goal was discussed and encouraged as appropriate.
Sweet taste test methodology
At the initial study visit, participants’ sensitivity and hedonic response to sweet taste were tested using our standard methodology (Kampov-Polevoy et al., 2003b). Testing was conducted prior to taking naltrexone and after 3 days of abstinence from alcohol. Prior to testing, participants fasted for 2 h and abstained from smoking for 1 h. Each subject was instructed to sip, swish around his/her mouth and then spit out five different concentrations of a sweet solution (0.05, 0.10, 0.21, 0.42 and 0.83 M) each of which were presented five times in a pseudorandom order for a total of 25 tastings. After each tasting, participants rinsed their mouths with distilled water before proceeding to the next solution. The participants then rated the intensity and pleasureableness of each tasting using a 200 mm analog scale.
The Sweet Taste Test was conducted by a research assistant who was not involved in any other assessments. The subject and those staff involved in assessing and managing the subject were kept blind to the Sweet Taste Test results until the entire study was completed.
Determination of sweet liking status
Subjects were assigned to one of two categories—SL or SDL—based upon their hedonic response to the various sucrose concentrations. To be categorized as a sweet liker, a subject must have rated the highest concentration of sucrose (0.83 M) as the most pleasurable. Sweet-dislikers could prefer any of the other four concentrations (Kampov-Polevoy et al., 1997). Such categorization has been shown to be stable over time. For example, our previous study indicated a high correlation (r = 0.72; P < 0.0001) between the results of two sweet tests conducted in patients at admission and before discharge from a residential treatment program for substance use disorders (Kampov-Polevoy et al., 2003b).
Outcome measures
The primary drinking outcome measure was percent heavy drinking days. Secondary outcome measures included study retention, time to first heavy drinking day and to 2 consecutive heavy drinking days, percentage days abstinent, time to first day abstinent and time to 2 consecutive abstinent days and craving for alcohol prior to and during treatment. In addition, we noted how long it took for a patient to achieve 3 consecutive days of abstinence prior to the beginning of naltrexone treatment. Tolerability was assessed by records of side effects, dropouts due to side effects, laboratory values and serious adverse events.
Statistical analysis
All values are given as the mean ± standard deviation (SD) or, for model-based estimates, the values are given as the estimate followed by the standard error (SE) and this is noted. Model-based estimates allow for adjustments for predictors, covariates and complex designs such as repeated measures. Baseline drinking was defined as drinking during the period of time that included the 90-day period prior to the screening visit plus the period between the screening and initial study visits minus the mandatory 3 days of abstinence. The average duration of this period was 102 ± 5 days. Baseline differences in demographic variables and level of baseline drinking were investigated using independent sample t-tests for continuous variables and chi-square tests of independence for categorical variables. In the presence of small or empty cells in the tests of categorical variables, the chi-square test was replaced by Fisher's exact test.
For simple cross-sectional comparisons (e.g. comparison between groups at one point in time), independent sample t-tests were used for continuous outcome variables. In the presence of covariates, independent sample t-test was replaced by analysis of covariance (ANCOVA). The data provided weekly scores of abstinence and heavy drinking for each subject during the 12 weeks of medication.
For comparison between groups at one point in time, independent sample t-tests were used for continuous outcome variables. In the presence of covariates, independent sample t-tests were replaced by ANCOVA model. With the available data, we produced weekly scores of abstinence and heavy drinking for each subject during the 12 or more weeks of the medication period. With this sample, we implemented a general mixed-model analysis of variance (MMANOVA) to accommodate the within subject correlation present with the repeated measures (Schwarz, 1993). This framework models the means per group over the respective time period (i.e. weeks) and the covariance between the repeated measures over the assessments, and has been implemented in substance abuse research (Greenfield et al., 2007). For this sample, the MMANOVA approach estimates the on-average difference over the medication period between groups (sweet liking versus sweet disliking). To answer this difference between groups, a group main effect is modeled in the MMANOVA. The MMANOVA was implemented with SAS procedure PROC MIXED (Littell et al., 2006).
For the time to events outcomes, we implemented survival curves, which were estimated using the Cox proportional hazards model. As is typically done, patients lost to follow-up were treated as censored observations, as were patients who never achieved the event of interest. Survival models were implemented in SAS with the procedure PROC PHREG (Allison, 1997).
MMANOVA and Cox proportional hazards models allow for additional predictors or covariates to be included in all analyses. Specifically, one predictor of interest was the baseline craving. Our interest was in assessing the relationship between craving prior to treatment and its impact on abstinence and heavy alcohol use. For all analytical models, we included craving in the analysis to determine if it had an association with alcohol usage, or its association with alcohol usage was different across sweet liking classification. Determination of craving as a predictor of usage dependent on sweet liking status was inspected through including the interaction of craving with sweet liking status in the statistical model. A significant interaction would indicate the differential impact of craving on outcome as a function of sweet liking classification. If the interaction was non-significant, the interaction was removed from the statistical model. A significant main effect for craving would indicate craving as predictive of usage and that this prediction did not depend on the sweet liking status.
All analyses were conducted using SAS Version 9.1.3. Statistical significance was set at P < 0.05 (two-tailed) for all tests, unless specified otherwise.
Results
Demographics, sweet liking status, craving for alcohol and alcohol use history
Fifteen subjects were categorized as SLs and 25 as SDLs. Participants included 29 men and 11 women and 97.5% of the subjects were Caucasian. As shown in Table 1, there were no differences between SL and SDL subjects in demographic data (age, gender, marital status) and in drinking variables. Baseline PACS scores were similar between SL and SDL subjects. Fewer SL subjects identified abstinence as a goal of treatment but this was not statistically significant. The baseline levels of AST, ALT and bilirubin were within the protocol limits and remained within limits during treatment.
Retentions rates and tolerability
There were no differences in retention rates between groups: 82% of SL and 76% of SDL subjects completed the study. No differences in tolerability were noted between SL and SDL subjects.
Pre-naltrexone findings
All patients achieved the 3 abstinence days prior to the onset of medication; therefore, no censoring was present. SL patients took ∼40% longer (14.1 ± 6.0 days) to achieve the required 3 abstinent days before medication compared to SDL patients (10.1 ± 4.3 days; P = 0.02).
Naltrexone findings
Percent heavy drinking days
Both SL and SDLs demonstrated a sharp reduction in the percent heavy drinking days from pre-treatment to the active medication period from 75% ± 25.6% to 17% ± 28.8% for SL and from 76% ± 23.3% to 20% ± 24.6% for SDL. Overall, we found a significant reduction in heavy usage from the pre-treatment period to the medication period as assessed through a paired t-test [t(39) = 12.35, P < 0.0001]. The MMANOVA estimated proportion of heavy usage during the medication period was 0.190 (SE = 0.042) for SDL subjects and 0.128 (SE = 0.049) for SL subjects, with an estimated mean difference of 0.062 (SE = 0.056) that was not significant. To assess if this estimated difference between SL and SDL groups was consistent across the 12-week medication period, we included an interaction of the SL status indicator and week to the MMANOVA—no evidence of a group by time interaction was found.
Cox regression analysis indicated no significant difference between SLs (median = 10 ± 2.6 days with 33.3% survival) and SDLs (median time = 6 ± 1.1 days, with 34.6% survival) in time to first heavy drinking day or time to 2 consecutive drinking days’ SLs (median = 58 ± 6.9 days with 45.0% survival) and SDLs (median = 40 ± 6.5 days, with 39.9% survival).
Percent days abstinent
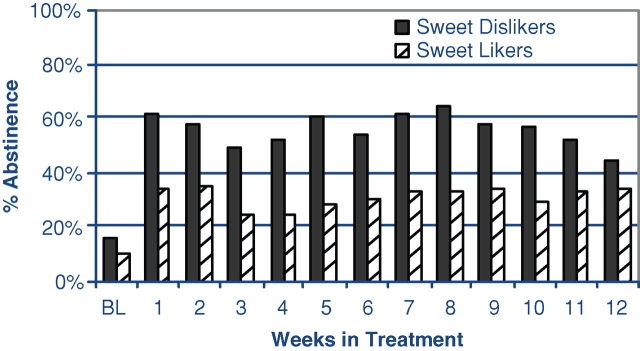
Both SL and SDLs demonstrated a sharp increase in the percent of abstinence from pre-treatment to the active medication period. Abstinence increased from 10% ± 17.3% to 29% ± 35.8% for SL and from 12% ± 15.9% to 48% ± 33.9% for SDL. Overall, we found a significant increase in abstinence from the pre-treatment period to the medication period as assessed through a paired t-test [t(39) = 6.47, P < 0.0001]. To assess if the amount of abstinence during the medication period varied across SL status we implemented the MMANOVA model, while controlling for the pre-treatment abstinence. The MMANOVA estimated a proportion of abstinence of 0.483 (SE = 0.055) for SDL subjects and 0.300 (SE = 0.068) for SL subjects, with a estimated mean difference of 0.183 (SE = 0.083), [F(1,37) = 4.84, P < 0.04]. Examination of change in abstinence over time by the SL/SDL group did not reveal any group differences, i.e. the differences in abstinent days persisted over the entire naltrexone treatment period, see Fig. 1.
Fig. 1.
Percent of abstinent days per week by sweet liking classification.
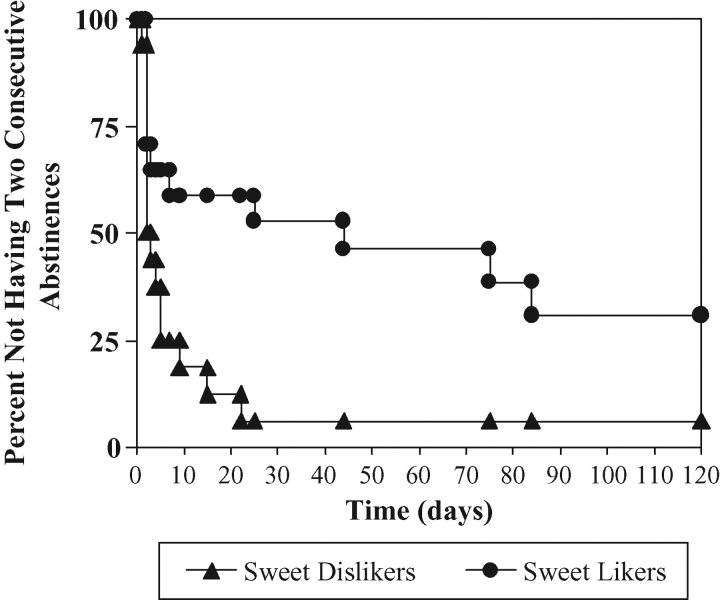
Cox regression analysis indicated no significant difference between SDLs (median = 2 ± 2.5 days, with 14.0% survival) and SLs (median = 1 ± 2.0 days, with 0% survival) in time to first drinking day. However, SL subjects were much less likely than SDL subjects to achieve 2 consecutive abstinent days, Cox regression difference between SDLs (median time = 4 ± 5.4 days, with 94.0% survival) and SLs (median = 44 ± 9.2 days with 63.4% survival) [chi(1) = 6.88, P < 0.01; Fig. 2].
Fig. 2.
Within-treatment time to 2 consecutive abstinent days by SL/SDL phenotype, Chi(1) = 6.88, P = 0.009.
Association between pill compliance and SL status
Independent sample t-test per assessment of compliance of medication indicated no significant difference between SDL and SL groups. At the first assessment a proportion of compliance of 0.92 ± 0.21 for SDL and of 0.93 ± 0.23 for SL was found. Similarly, at the last assessment of compliance, a proportion of compliance of 0.98 ± 0.05 for SDL and of 0.99 ± 0.01 for SL was found.
Association between alcohol craving as measured by PACS during the screening visit (PACS-T0) and outcome with naltrexone
We tested the hypothesis suggested by Monterosso et al. (2001) that severity of alcohol craving measured prior to treatment can predict outcome with naltrexone. A sequential regression analysis using the proportion of heavy usage and proportion of abstinence during the entire medication period showed that initial alcohol craving as measured by PACS-T0 does not predict treatment outcome either in terms of percent heavy drinking days or percent abstinent days.
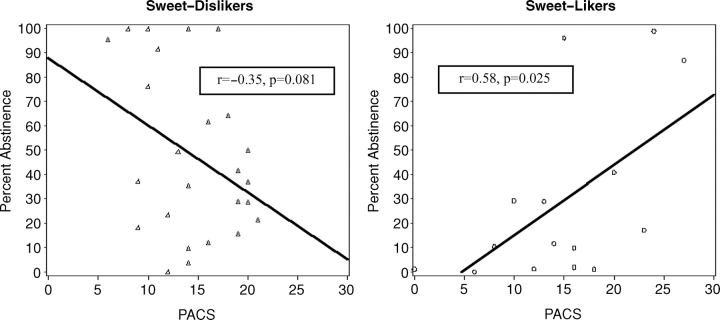
We then tested whether the association between initial alcohol craving and treatment outcome depended on the SL and SDL grouping by including the interaction between the craving status and SL/SDL grouping. We found a significant interaction between craving and SL/SDL grouping for percent abstinent days [F(1,36) = 8.84, P < 0.01] but a non-significant interaction for percent heavy drinking days. To further understand this relationship, we implemented the mixed-effects modeling framework on our 12 weeks of usage measures including the SL/SDL status and baseline craving in the model. The analysis showed a significant interaction between PACS-T0 and SL/SDL status in the prediction of percent abstinent days (but not percent heavy drinking days) [F(1,36) = 13.94, P < 0.001] (Fig. 3) indicating that differences between SL/SDL status on the likelihood of achieving abstinence interacted with initial alcohol craving score, see Fig. 3a and b. Extending this repeated measures analysis we found a non-significant three-way interaction of PACS-T0, SL/SDL status and week, indicating that the differential relationship of craving with abstinence per group (SL versus SDL) is consistent across the 12-week medication period. In the SL group, a positive correlation between PACS-T0 and percent abstinent days was present indicating that SL subjects with higher craving had more abstinent days when treated with naltrexone. In SDL subjects, the correlation was negative indicating that in SDL subjects higher craving was associated with less abstinence.
Fig. 3.
Association between initial alcohol craving (PACS-T0) and treatment outcome (percent abstinent days) in SL and SDL patients.
Discussion
Naltrexone, an antagonist at μ, δ and κ-opioid receptors, is thought to block alcohol-induced opioid activation of reward pathways thereby counteracting aspects of the positive response to alcohol. Subjects in clinical trials who receive naltrexone, overall, report diminished feelings of being ‘high’ after consuming alcohol (Volpicelli et al., 1995; O’Malley et al., 1996), note less craving for alcohol (Volpicelli et al., 1992; O’Malley et al., 1996), are less likely to relapse to heavy drinking (Kranzler and Van Kirk, 2001; Srisurapanont and Jarusuraisin, 2002) and achieve greater rates of abstinence (Kranzler and Van Kirk, 2001). However, for groups of patients, these actions are modest with mean effect sizes in the 0.1–0.2 range (Kranzler and Van Kirk, 2001) that has contributed to the low utilization of naltrexone in clinical practice (Mark et al., 2003).
Efforts to identify which patients respond to naltrexone have utilized two principal approaches—examination of clinical phenomenology and examination of biogenetic markers, (see the Introduction section). These predictors are not clearly established and, indeed, some have not been confirmed, e.g. Kiefer et al. (2005), failed to find an association between craving and naltrexone response. Nevertheless, these efforts suggest that predictors of naltrexone response can be identified and, if confirmed and refined, might have practical clinical value.
Variation in the brain's opioid system is an obvious target to identify naltrexone predictors. The hedonic response to sweet taste represents a heritable trait (Keskitalo et al., 2007) that likely provides information on brain opioid activity (see Pecina and Berridge, 2005). The results of the present study indicate that variation in the hedonic response to sweet taste is associated with the ability to achieve and maintain abstinence prior to treatment as well as during naltrexone treatment plus counseling.
Prior to initiating treatment, SL patients required a longer time to achieve 3 consecutive days of abstinence before starting naltrexone compared to SDL patients. This suggests that the propensity to drink alcohol may be stronger in SL subjects such that they have greater difficulty in achieving periods of abstinence. The biological underpinnings of this finding are not clear but could relate to altered opioid function in SL individuals (see below).
All patients demonstrated a sharp reduction in the percentage of heavy drinking days during the treatment period—from ∼75% to ∼20%, regardless of their SL/SDL status. The robust effect of this treatment is consistent with results reported in other published clinical trials, e.g. Garbutt et al., (2005).
However, SL and SDL individuals differed significantly in their ability to abstain from alcohol (see Fig. 1). The median time to achieve 2 consecutive abstinent days for SL patients was 10 times longer than for SDL patients (Fig. 2). In other words, for SL patients achieving and maintaining sobriety during treatment was considerably more difficult than for SDL patients—consistent with our finding that SL patients took longer to achieve sobriety prior to starting naltrexone.
The other major post-treatment difference between SL and SDL patients was in the relationship between baseline craving for alcohol and the likelihood of achieving abstinent days. Several groups have reported that the level of severity of the baseline craving for alcohol positively correlates with response to naltrexone (Volpicelli et al., 1995; Jaffe et al., 1996; Monterosso et al., 2001). In our trial we failed to find such an association in the whole sample though in the absence of a placebo-control we were unable to test for a relationship between craving and naltrexone effect size as Monterosso et al. (2001) did. However, when the SL/SDL phenotype was included in the analysis a highly significant interaction effect between craving, SL/SDL phenotype and percent abstinent days emerged. SL patients demonstrated a positive correlation between PACS-T0 and percent abstinent days, indicating that those with higher craving had more abstinent days when treated with naltrexone as suggested in the literature. On the other hand, SDL subjects showed a negative correlation indicating that, for SDL subjects, higher craving is associated with less abstinence.
The reason why the combination of the SL/SDL phenotype and craving improves predictive power is not clear. However, one hypothesis is that hedonic response to sweet taste and alcohol craving probe different components of the brain opioid system such that taken together they are more informative than taken separately. Whereas no direct studies in humans have yet been conducted to investigate the relationship of the SL/SDL phenotype to endogenous opioid function, there is extensive animal evidence demonstrating the critical role opioids play in mediating the hedonic response to sweets (see Pecina and Berridge, 2005). Furthermore, given the evidence that the SL phenotype is associated with familial risk for alcoholism, it could be hypothesized that SL is associated with an inherent dysfunction of β-endorphin release from presynaptic terminals as reported in individuals at high risk for alcoholism (Gianoulakis, 2004). Conversely, craving for alcohol has been linked to the availability (Heinz et al., 2005) and binding potential (Bencherif et al., 2004) of postsynaptic μ-opiate receptors. Therefore, a combination of the SL/SDL phenotype and craving may provide a more integrative measure of brain opioid function.
Another question is why the combination of hedonic response to sweet taste and initial alcohol craving had a predictive value regarding percent abstinent days but not percent heavy drinking days. One potential explanation of this phenomenon is that the overall treatment effect of counseling and naltrexone caused robust suppression of percent heavy drinking days in both SL and SDL groups but for different reasons—SL individuals responded to naltrexone while SDL individuals responded to counseling. Another potential explanation of this outcome is that loss of control over drinking within a drinking episode and inability to abstain from drinking may be determined by different opioidergic mechanisms that have different sensitivity to naltrexone treatment. For example, heavy drinking is often attributed to elevated sensitivity to the rewarding effect of alcohol stemming from increased β-endorphin release from presynaptic terminals in response to ethanol (Gianoulakis, 2004). Naltrexone treatment by reducing the pleasure or the ‘high’ associated with alcohol intake (King et al., 1997, 2002) may reduce alcohol consumption during a drinking episode. On the other hand, the effect of naltrexone treatment on the ability to abstain from alcohol may be related to its effect on alcohol craving that is associated with the availability (Heinz et al., 2005) and binding potential (Bencherif et al., 2004) of postsynaptic μ-opiate receptors. However, at this point these conclusions are still highly speculative.
Study limitations
The sample size of the study is modest at 15 SL and 25 SDL subjects. This could lead to a type 1 error though some of the statistical findings, e.g. the interaction effect between craving and SL/SDL status, revealed very robust results, which leads to greater confidence in the finding. With the limited sample size and the preliminary nature of this research, we did not adjust the alpha-level for any statistical contrast (Rothman, 1990). This lack of adjustment could lead to an inflated type I error as well. Another important limitation is the lack of a placebo group. Without a placebo group the effects of naltrexone versus counseling in SL versus SDL patients cannot be disentangled. This is particularly problematic for the interpretation of the heavy drinking results where both SL and SDL patients showed similar outcomes. As noted earlier, this result could be because both groups respond equally well to naltrexone or it could be, as we hypothesize, that SL patients show a superior response to naltrexone compared to placebo whereas SDL patients respond primarily to counseling.
In summary, we have found preliminary evidence that the SL phenotype predicts fewer days of abstinence in alcohol-dependent patients who are treated with naltrexone and counseling. Furthermore, we also found that the SL/SDL phenotype interacts with craving for alcohol such that SL patients who have high levels of craving are more likely to achieve abstinence whereas SDL patients with high levels of craving are less likely to achieve abstinence. These findings require confirmation and extension but support the hypothesis that the SL phenotype may be useful in advancing understanding of the biological heterogeneity of alcohol dependence and its relationship to naltrexone and treatment response.
Acknowledgments
This work was supported by GCRC grant RR00046 and CTSA grant UL1RR025747 from the National Institutes of Health.
References
- Allison PD. Survival analysis using the Sas system: A practical guide, SAS Publishing, Cary. 1997 North Carolina. [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Wand GS, McCaul ME, et al. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55:255–62. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu-opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, et al. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Reid LD. Morphine and acceptability of putative reinforcers. Pharmacol Biochem Behav. 1983;18:567–9. doi: 10.1016/0091-3057(83)90282-4. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, et al. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–12. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health. 1999;23:179–86. [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, et al. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 2003;64:120–6. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Roberts AJ, Cooney N, et al. The role of craving in alcohol use, dependence, and treatment. Alcohol Clin Exp Res. 2001;25:299–308. [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–95. [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, et al. Vivitrex Study Group Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, et al. VA Cooperative Study #425 Study Group Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31:555–63. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Béliveau D, Angelogianni P, et al. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci. 1989;45:1097–109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–7. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Trucco E, Lincoln M, et al. The Women's Recovery Group Study: a stage I trial of women-focused group therapy for substance use disorder versus mixed-gender group drug counseling. Drug Alcohol Depend. 2007;90:39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, et al. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005;62:57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- Jaffe AJ, Rounsaville B, Chang G, et al. Naltrexone, relapse prevention, and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol. 1996;64:1044–53. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky D. Evidence of preference for a higher concentration sucrose solution in alcoholic men. American Journal of Psychiatry. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–95. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003b;27:1743–9. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Ziedonis D, Steinberg ML, et al. Association between sweet preference and paternal history of alcoholism in psychiatric and substance abuse patients. Alcohol Clin Exp Res. 2003a;27:1929–36. doi: 10.1097/01.ALC.0000099265.60216.23. [DOI] [PubMed] [Google Scholar]
- Keskitalo K, Tuorila H, Spector TD, et al. Same genetic components underlie different measures of sweet taste preference. Am J Clin Nutr. 2007;86:1663–9. doi: 10.1093/ajcn/86.5.1663. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Helwig H, Tarnaske T, et al. Pharmacological relapse prevention of alcoholism: clinical predictors of outcome. Eur Addict Res. 2005;11:83–91. doi: 10.1159/000083037. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Sandstrom KA, Van Kirk J. Sweet taste preference as a risk factor for alcohol dependence. Am J Psychiatry. 2001;158:813–5. doi: 10.1176/appi.ajp.158.5.813. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–41. [PubMed] [Google Scholar]
- Leventhal L, Kirkham TC, Cole JL, et al. Selective actions of central mu and kappa opioid antagonists upon sucrose intake in sham-fed rats. Brain Res. 1995;685:205–10. doi: 10.1016/0006-8993(95)00385-4. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models Second Edition. 2006 Cary, NC: SAS Institute Inc. [Google Scholar]
- Looy H, Callaghan S, Weingarten HP. Hedonic response of sucrose likers and dislikers to other gustatory stimuli. Physiol Behav. 1992;52:219–25. doi: 10.1016/0031-9384(92)90261-y. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71:219–28. doi: 10.1016/s0376-8716(03)00134-0. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–22. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, et al. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–68. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Project Match Series, Volume 2 Motivational Enhancement Therapy Manual. 1994. NIH Pub. No. 94-3723.
- O’Malley SS, Jaffe AJ, Rode S, et al. Experience of a ‘slip’ among alcoholics treated with naltrexone or placebo. Am J Psychiatry. 1996;153:281–3. doi: 10.1176/ajp.153.2.281. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, et al. Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. J Clin Psychopharmacol. 2000;20:69–76. doi: 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–9. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Miranda R, Jr, McGeary JE, et al. Family history and antisocial traits moderate naltrexone's effects on heavy drinking in alcoholics. Exp Clin Psychopharmacol. 2007;15:272–81. doi: 10.1037/1064-1297.15.3.272. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jimenez R, et al. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40:227–33. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- Schwarz CJ. The mixed-model ANOVA: the truth, the computer packages, the books. Am Statistician. 1993;47:48–59. [Google Scholar]
- Scinska A, Bogucka-Bonikowska A, Koros E, et al. Taste responses in sons of male alcoholics. Alcohol Alcohol. 2001;36:79–84. doi: 10.1093/alcalc/36.1.79. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, et al. Mini International Neuropsychiatric Interview (M.I.N.I.) 1999 Tampa: University of South Florida. [Google Scholar]
- Sobell LC, Sobell MB, Leo GL, et al. Reliability of a timeline followback method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addictions. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2002;2:CD001867. doi: 10.1002/14651858.CD001867. [DOI] [PubMed] [Google Scholar]
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–52. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Moskowitz HR, Campbell RG. Effects of body weight and food intake on pleasantness ratings for a sweet stimulus. J Appl Physiol. 1976;41:77–83. doi: 10.1152/jappl.1976.41.1.77. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, et al. Moderators of naltrexone's effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Wildenberg E, Wiers RW, Dessers J, et al. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Clay KL, Watson NT, et al. Naltrexone in the treatment of alcoholism: predicting response to naltrexone. J Clin Psychiatry. 1995;56(Suppl 7):39–44. [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, et al. The μ-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–14. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wronski M, Skrok-Wolska D, Samochowiec J, et al. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol. 2007;42:75–9. doi: 10.1093/alcalc/agl097. [DOI] [PubMed] [Google Scholar]