Abstract
In this paper we establish the xenograft leukemia model with stable multidrug resistance in nude mice and to investigate the reversal effect of 5-bromotetrandrine (5-BrTet) and magnetic nanoparticle of Fe3O4 (MNP-Fe3O4) combined with daunorubicin (DNR) in vivo. Two subclones of K562 and K562/A02 cells were inoculated subcutaneously into the back of athymic nude mice (1 × 107 cells/each) respectively to establish leukemia xenograft models. Drug-resistant and sensitive tumor-bearing nude mice were assigned randomly into five groups which were treated with normal saline; DNR; NP-Fe3O4 combined with DNR; 5-BrTet combined with DNR; 5-BrTet and MNP-Fe3O4 combined with DNR, respectively. The incidence of formation, growth characteristics, weight, and volume of tumors were observed. The histopathologic examination of tumors and organs were detected. For resistant tumors, the protein levels of Bcl-2, and BAX were detected by Western blot. Bcl-2, BAX, and caspase-3 genes were also detected. For K562/A02 cells xenograft tumors, 5-BrTet and MNP-Fe3O4 combined with DNR significantly suppressed growth of tumor. A histopathologic examination of tumors clearly showed necrosis of the tumors. Application of 5-BrTet and MNP-Fe3O4 inhibited the expression of Bcl-2 protein and upregulated the expression of BAX and caspase-3 proteins in K562/A02 cells xenograft tumor. It is concluded that 5-BrTet and MNP-Fe3O4 combined with DNR had a significant tumor-suppressing effect on a MDR leukemia cells xenograft model.
Keywords: 5-bromotetrandrine, magnetic nanoparticle of Fe3O4, multidrug-resistance, xenograft model
Multidrug resistance (MDR) is a well-defined phenomenon of cross-resistance of mammalian cells to a number of anticancer agents following exposure to one such drug. It is a major obstacle to successful chemotherapy in leukemia.1 More than 90% of patients with malignant tumor die of MDR. A diverse range of agents involved in MDR include alkaloid compounds and bacterial and fungal antibiotics such as anthracyclines and etoposide. An accepted mechanism of MDR is a reduced cellular accumulation and an altered subcellular distribution of cytotoxic drugs. In many instances, this is mediated by increased expression at the cell surface of the MDR1 gene product, P-glycoprotein (P-gp), a 170-kD energy-dependent efflux pump. Different chemotherapeutic agents have been shown to induce the death of susceptible cells by apoptosis, and this can be inhibited by proteins such as Bcl-247 and Bcl-xL. The purpose of this paper is to find a low mammalian toxicity, high-efficiency, high-selectivity modulator.
Close attention has been paid to current nanoparticle techniques. The magnetic nanoparticle of Fe3O4 (MNP-Fe3O4)2,3 that we invented has good biocompatibility and no cytotoxicity. MNP-Fe3O4 can increase the intracellular effective concentration of chemotherapeutic drugs in vitro in order to reverse MDR.4,5 5-Bromotetrandrine (5-BrTet), when highlighted by isoquinoline alkaloid, is a hypotoxic calcium channel blocker.6 5-BrTet inhibits the function of P-gp in order to accumulate anticancer drugs in the body.7 Most research on the reversal of MDR was on the basis of experiments in vitro.
This paper was to establish the xenograft leukemia model with stable MDR in nude mice; to investigate the reversal effect of 5-BrTet and MNP-Fe3O4 combined with daunorubicin (DNR) in vivo and to search for possible reversal mechanisms in order to provide theoretic evidence for the clinical applications.
Material and method
Cell lines and cell culture
K562 cell was a cell line cloned from human chronic myelogenous leukemia by repeated passage in nude mice and in culture alternatively. The MDR leukemia cell K562/A02 was established by stepwise selection with an increasing concentration of adriamycin (ADM). K562 cells were maintained in RPMI 1640 medium with 10% newborn bovine serum at 37 °C in a humidified 5% CO2 atmosphere. K562/A02 was cultured in a medium containing 1 μg/ml ADM for maintaining MDR phenotype.
Experimental agents
Adriamycin (Hisun Phamaceutical Co., Zhejiang, China) and DNR (Main Luck Pharmaceuticals Inc., Shenzheng, China) 2 mg/ml stock solution were prepared with 0.01 mol/l phosphate buffer saline (PBS) (pH 7.4). Nutritional medium: RPMI1640 (Gibco Chemical Co., Carlsbad, CA, USA), calf serum (Gibco Chemical Co); MNP-Fe3O4 was provided by the Biological Science College at Southeast University, Nanjing; DNR conjugated with MNP-Fe3O4 colloidal suspension were prepared by mechanical absorption polymerization at 4 °C for 48 h as reported previously. 5-BrTet (Kanghong Chemical Co, Chengdou, China) was diluted with 0.01 mol/l PBS (pH 7.4).
Experimental animals
Female BALB/c nude mice which were age-matched (four weeks of age) and weight-matched (16–22 g) were purchased from Shanghai National Center for Laboratory Animals. They were maintained in specific pathogen-free (SPF) facilities and fed with irradiated food. Indoor temperature was maintained at 22 °C and humidity was controlled at 45 ± 5%.
Xenograft leukemia model in nude mice
The two subclones of K562/A02 and K562 cells were inoculated subcutaneously into the back of athymic nude mice (1 × 107 cells/0.2 ml/each) respectively to establish the human leukemia xenograft models: the drug-resistant xenograft nude-mice models and the sensitive tumor-bearing xenograft nude-mice models.
Using animal ultrasonic equipment to evaluate tumor formation
Six days after cell inoculation, pink neoplasm had emerged in the inoculated part. We used animal ultrasonic equipment to detect the neoplasm.
Tumor-bearing nude mice divided into groups. The effect of reversal of MDR in tumor-bearing nude mice determined
When the tumor size reached to 75–150 mm3, the drug-resistant and sensitive tumor-bearing nude mice were both assigned randomly to five groups. Each group contained eight nude mice. The drugs were administered by intraperitoneal injection except for MNP-Fe3O4 copolymerizating DNR delivered by vena caudalis injection. In group A, the control group was treated with normal saline (NS) 0.2 ml/each day; in group B, DNR 1 mg/(kg·day); in group C, 5-BrTet 2.5 mg/(kg·day) combined with DNR 1 mg/(kg·day), 5-BrTet was administered one hour before DNR; in group D, NP-Fe3O4 combined with DNR 0.63 mg/(kg·day); in group E, 5-BrTet 2.5 mg/(kg·day) and MNP-Fe3O4 combined with DNR 0.63 mg/(kg·day), 5-BrTet was administered one hour before MNP-Fe3O4. Each group was treated every day for 10 days. The incidence of formation, growth characteristics, weight, and volume of tumor were observed for each group, respectively. All the tumor-bearing nude mice were killed on the eleventh day to detect tumors and gains.
Tumor volume
Tumor dimensions were measured with digital calipers or animal ultrasonic inspection to obtain two diameters of the tumor formed. Tumor size was calculated by using the formula as follows: Tumor volume = l/2 × a × b2, where a is the longest diameter and b is the shortest. The inhibition rate (IR) (%) = (one-experimental group relative tumor volume [RTV]/control group RTV)·100%. RTV = Vx/V1, Vx and V1 represent tumor volume on the X day and the first day after treatment. Tumor weight was calculated by torsion balance and growth-inhibiting rat.
Histopathologic examination of tumors and organs
After the nude mice are killed, the tumors and organs must be fixed in formalin immediately, hematoxylin and eosin stained, and observed by 400x light microscope.
Apoptosis-related proteins detected by Western blot for resistant tumors
Cellular nucleic protein was obtained by grinding the tumors as discussed previously. Protein concentration (Bcl-2, BAX, Caspase-3) was settled to 2 μg/μl. Each lane contained 40 μg proteins. The proteins were resolved by 10% SDS/PAGE for one hour, and then transferred to nitrocellulose membrane. After being blocked in Trisbuffered saline/Tween-20 (TBST) with 5% skim milk overnight, the membrane was incubated with primary polyclonal antibody (1:500; Biolegend, San Diego, CA, USA) for one hour, and subsequently incubated with horseradish peroxidase-linked goat anti-rabbit secondary antibody (1:7500; Pierce, Rockford, IL, USA) for one hour. The protein bands were visualized by enhanced chemiluminescence regents (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Quantitative real-time PCR analysis
After killing the nude mice, total RNA of tumor was isolated using TRIzol® Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. One microgram of total RNA was used to generate cDNA using SuperScript™ II reverse transcriptase (Invitrogen Life Technologies). PCR primers were designed to amplify products within target and control sequences (Bcl-2, forward: 5′-GGGAGAACAGGGTACGATAA- 3′; reverse: 5′-CCACCGAACTCAAAGAAGG-3′. BAX, forward: 5′-TTTTGCTTCAGGGTTTCATC-3′; reverse: 5′-GACACTCGCTCAGCTTCTTG -3′. Caspase-3, forward: 5′-GCTATTGTGAGGCGGTTGT-3′; reverse: 5′-TGTTTCCCTGAGGTTTGC-3′. GAPDH, forward: 5′-CGGATTTGGTCGTATTG-3′; reverse: 5′-GAAGATGGTGATGGGATT- 3′). Quantitative real-time PCR (QPCR) was performed by real-time monitoring of the increase in fluorescence of SYBR Green I dye (Takara, Shiga, Japan) with Rotor-Gene 3000 (Corbett Research, Sydney, Australia).Each experiment was done in triplicate. The relative gene copy number was calculated by the concentration-CT standard curve method and normalized using the average expression of GAPDH.
Statistical analysis
The data was analyzed with SPSS v. 11.5 (SPSS Inc., Chicago, IL, USA) software. The significance test used factor variance analysis and the difference had statistical significance, p < 0.05
Results
The animal model of hematologic disease was established successfully
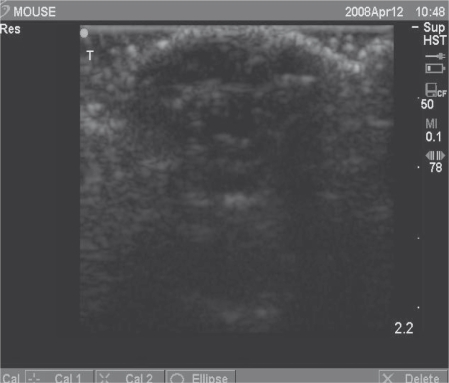
The median latency of K562 cells was six days when it formed neoplasm, which was shorter than that of K562/A02 cells at eight days. We used animal ultrasonic inspection to confirm the presence of tumor. Figure 1 displays the tumor formation incidences of K562 and K562/A02 cells at 100%.
Figure 1.
Animal ultrasonic inspection of pink neoplasm: tumor formation.
The relative tumor volume and inhibition rate of five experimental groups for K562 sensitive cells xenograft tumor
Six days after K562 cell inoculation, the tumors reached a volume of more than 100 mm3. The mice were divided into five groups and subject to 10 days of treatment. When the K562 cell tumor-bearing nude mice were killed, the relative tumor volumes of the five experimental groups were as follows: the control group A was 15.24 ± 0.10; groups B, C, D, and E were 5.79 ± 0.15, 5.77 ± 0.15, 5.78 ± 0.08, and 5.66 ± 0.06, respectively (p = 0.743). The inhibition rates of groups B, C, D, and E compared with the control group were 62.01 ± 0.31%, 62.12 ± 0.11%, 62.11 ± 0.22%, and 62.82 ± 0.18%, respectively (p = 0.678). The tumor weight was measured and the inhibition rate of tumor growth groups B, C, D, and E was calculated at 59.05 ± 0.14%, 59.64 ± 0.31%, 59.52 ± 0.37%, and 60.52 ± 0.44%, respectively (p = 0.748).
The relative tumor volume and inhibition rate of five experimental groups for K562/A02 resistant cells xenograft tumor
Eight days after K562/A02 cells inoculation, the tumors reached a volume of more than 100 mm3. The mice were divided into five groups and subject to 10 days of treatment. When K562/A02 cell tumor-bearing nude mice were killed, the relative tumor volumes of the five experimental groups were as follows: the control group A was 12.94 ± 0.14; groups B, C, D, E were 12.47 ± 0.30, 9.30 ± 0.21, 7.26 ± 0.34, and 4.89 ± 0.24, respectively (p = 0.024; Figure 2). The inhibition rates of groups B, C, D, and E compared with the control group were 3.68 ± 0.17%, 28.24 ± 0.32%, 40.80 ± 0.16%, 62.76 ± 0.42%, respectively. There were significant differences in groups B, C, D, and E (p = 0.015). The tumor weight was measured and the inhibition rates of tumor growth of groups B, C, D, and E were calculated at 1.34 ± 0.43%, 28.08 ± 0.41%, 46.40 ± 0.22%, 59.75 ± 0.16%, respectively (p = 0.021).
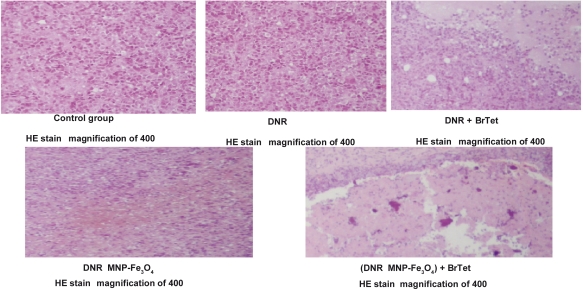
Figure 2.
Histopathologic examination of K562/A02 resistant tumors of different groups (hematoxylin and eosin stain, 400 × light microscope).
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; HE, hematoxylin and eosin; MNP-Fe3O4, magnetic nanoparticle of Fe3O4.
Histopathologic examination of K562/A02 resistant tumors and organs
In K562/A02-resistant tumors, the pathology of tumors from control group A and the DNR-alone group B showed more heterogeneity of neoplastic cells, karyomegaly, more mitotic figures, and vasiformation. Tumors from groups C and D showed a few necroses. The pathology of tumors from control group E showed obvious necrosis. There were no apparent histopathologic damage to organs such as the heart, liver, kidney, etc.
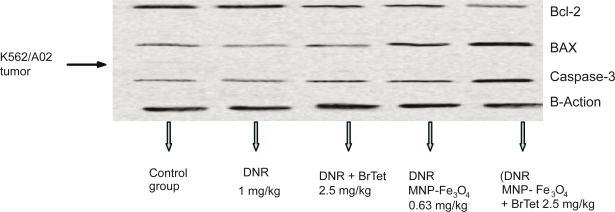
Western blot analysis of apoptosis-related protein expression in K562/A02 resistant tumors was performed. Application of 5-BrTet and MNP-Fe3O4 downregulated the expression of Bcl-2 protein compared with the control group and upregulated the expression of BAX and caspase-3 protein (Figure 3).
Figure 3.
Western blot of apoptosis-related protein expression in K562/A02-resistant tumors.
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; MNP-Fe3O4, magnetic nanoparticle of Fe3O4
Expressions of Bcl-2, BAX, and caspase-3 genes in K562/A02-resistant tumors and K562 tumors
The expression levels of Bcl-2, BAX, and caspase-3 genes were detected by PCR. Compared with the control group, both BAX and caspase-3 mRNA expression increased markedly in in K562 tumors in group E. The expression of Bcl-2 was significantly suppressed by application of 5-BrTet and MNP-Fe3O4 (Table 1).
Table 1.
Expressions of Bcl-2, BAX, and caspase-3 genes in different groups (x̄± SD)
| Gene expressions | k562/A02 control group | k562/A02 DNR 1 mg/kg | k562/A02 DNR MNP-Fe3O4 0.63 mg/kg | k562/A02 DNR 1 mg/kg + 2-BrTet 5 mg/kg | k562/A02 (DNR MNP-Fe3O4) 0.63 mg/kg + 5-BrTet 2.5 mg/kg | k562 control group |
|---|---|---|---|---|---|---|
| Bcl-2 | 9.364 ± 0.010 | 6.248 ± 0.120 | 4.012 ± 0.200 | 3.679 ± 0.138 | 2.004 ± 0.025 | 8.567 ± 0.225 |
| BAX | 2.013 ± 0.085 | 1.968 ± 0.038 | 6.025 ± 0.106 | 5.994 ± 0.173 | 11.256 ± 0.166 | 5.689 ± 0.281 |
| Caspase-3 | 2.063 ± 0.047 | 1.958 ± 0.057 | 3.668 ± 0.205 | 4.025 ± 0.193 | 12.334 ± 0.187 | 3.665 ± 0.043 |
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; MNP-Fe3O4, magnetic nanoparticle of Fe3O4.
Discussion
Chemotherapy is the main method of leukemia therapy. The clinical therapeutic effect is not as good as imagined, although more and more new chemotherapeutic drugs are emerging. One of the most important reasons for this clinical effect is MDR. The common cause of MDR is believed to be the enhanced expression of a transmembrane glycoprotein termed P-glycoprotein (P-gp) coded by the mdr1 gene in cancer cells.8,9 Some of the drug molecules that diffuse into cells are removed by P-gp. The industry development of MDR modulators is extremely fast, but their weak activity and toxic reaction limit clinical and therapeutic applications. Recently, exploratory research in nanoparticle medicine showed a reversal in MDR by the application of magnetic nanoparticles loaded with chemotherapeutics.10–12 MNP-Fe3O4 has been considered as a potential MDR cancer reversal agent, although its involved molecular mechanism has not been well elucidated. The diameter of MNP-Fe3O4 manufactured by the Biological Science College at Southeast University was 20–30 nm, which suits reversal of MDR.13 Some research reports that the fraction of nanoparticle-loaded doxorubicin (Dox), which enters cells by endocytosis, may be associated with solid lipids and is not easily cleared from the cell by P-gp drug efflux. Nanoparticle-loaded Dox may inhibit P-gp function. 5-BrTet could step down the toxicity of chemotherapeutics and may not impact the pharmacokinetics of chemotherapeutics in vivo.14
This experiment shows that the leukemia xenograft nude-mice model maintains MDR. 5-BrTet and MNP-Fe3O4 combined with DNR had a significant tumor-suppressing effect on the MDR leukemia cells xenograft model. This activity may enhance apoptosis. We propose further studies to determine whether 5-BrTet and MNP-Fe3O4 may suppress tumor cell proliferation and induce apoptosis by blocking multiple pathways. Our results indicated that application of 5-BrTet and MNP-Fe3O4 had a better effect in reversal MDR than DNR, 5-BrTet combined with DNR, or MNP-Fe3O4 combined with DNR. 5-BrTet and MNP-Fe3O4 may be the most promising MDR modulators for eventual assessment in the clinic. 5-BrTet and MNP-Fe3O4 combined with DNR had a significant tumor-suppressing effect on the MDR leukemia cells xenograft model.
Acknowledgments
This work was supported by National 863 Program Emphasis Project, Nanometer Biology Ware Study (No. 2007AA0222007), National Natural Science Foundation of PR China (No 30740062 and No 30872970), and High School Doctor Subject Special-purpose Scientific Research Foundation (No 20070286042). Contributions: Yanan Wu and Baoan Chen provided the concept, design, and analysis of the paper. Baoan Chen and Jian Cheng obtained a funding source and collected and assembled data. Xiaomao Li and Xuemei Wang provided the magnetic nanoparticles of Fe3O4. Feng Gao, Xinchen Sun and Guohong Li, provided study materials and technical support.
References
- 1.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 2.Cheng FY, Su CH, Yang YS, et al. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26(7):729–738. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Wang X, Wu C, et al. Synergistic enhancement effect of magnetic nanoparticles on anticancer drug accumulation in cancer cells. Nanotechnology. 2006;17:3622–3626. doi: 10.1088/0957-4484/17/14/043. [DOI] [PubMed] [Google Scholar]
- 4.Song M, Zhang R, Dai Y, et al. The in vitro inhibition of multidrug resistance by combined nanoparticulate titanium dioxide and UV irradition. Biomaterials. 2006;27(23):4230–4238. doi: 10.1016/j.biomaterials.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhang R, Wu C, et al. The application of Fe(3)O(4) nanoparticles in cancer research: A new strategy to inhibit drug resistance. J Biomed Mater Res A. 2007;80(4):852–860. doi: 10.1002/jbm.a.30901. [DOI] [PubMed] [Google Scholar]
- 6.Fu L, Liang Y, Deng L, et al. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53:349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Xu LZ, He KL, et al. Reversal effects of nomegestrol acetate on multidrug resistance in adriamycin-resistant MCF7 breast cancer cell line. Breast Cancer Res. 2001;3:253–263. doi: 10.1186/bcr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schdert T, Kurbocher RCM, Benzm, et al. Induction of MDR1 gene expression by antieoplatic agents in ovarin cancer cell lines. Anticancer Res. 2002;22:2199–2202. [PubMed] [Google Scholar]
- 9.Michale M, Gottesman G. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 10.Wong HL, Rauth AM, Bendayan R, et al. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23:1574–1585. doi: 10.1007/s11095-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 11.Alexiou C, Jurgons R, Schmid R, et al. [Magnetic drug targeting – a new approach in locoregional tumor therapy with chemotherapeutic agents. Experimental animal studies.] HNO. 2005;53(7):618–622. doi: 10.1007/s00106-004-1146-5. [DOI] [PubMed] [Google Scholar]
- 12.Omid CF, Cheng J, Benjamin AT, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas K, Sayre P. Research strategies for safety evaluation of nanomaterials, Part I: Evaluating the human health implications of exposure to nanoscale materials. Toxicol Sci. 2005;87:316–321. doi: 10.1093/toxsci/kfi270. [DOI] [PubMed] [Google Scholar]
- 14.Wang FP, Wang L, Yang JS, et al. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. J Biol Pharm Bull. 2005;28:1979–1982. doi: 10.1248/bpb.28.1979. [DOI] [PubMed] [Google Scholar]