Abstract
Polypeptide multilayer nanofilms were prepared using electrostatic layer-by-layer self-assembly nanotechnology. Small charged drug molecules (eg, cefazolin, gentamicin, and methylene blue) were loaded in polypeptide multilayer nanofilms. Their loading and release were found to be pH-dependent and could also be controlled by changing the number of film layers and drug incubation time, and applying heat-treatment after film formation. Antibioticloaded polypeptide multilayer nanofilms showed controllable antibacterial properties against Staphylococcus aureus. The developed biodegradable polypeptide multilayer nanofilms are capable of loading both positively- and negatively-charged drug molecules and promise to serve as drug delivery systems on biomedical devices for preventing biomedical device-associated infection, which is a significant clinical complication for both civilian and military patients.
Keywords: polypeptide, self-assembly, polyelectrolyte multilayer, nanofilm, charged molecule, tunable release
Introduction
Biomedical devices are indispensable in the care of patients. However, biomedical device-associated infection is a significant clinical complication. In orthopedics, infection prevention is one of the major goals of injury management. Infection rates are 7%–9% for elbow replacements and 1%–2% for hip replacements,1 and patients with open fractures have a high risk of infection due to bacterial contamination and soft tissue damage. The incidence of Gustilo grade III open fractures may exceed 30%2,3 and 2%–15% (or higher) of combat-related extremity injuries with developed osteomyelitis.4 To reduce the risk of biomedical device-associated infection, attention has turned recently to developing drug-containing films on biomedical devices,5–10 as such films can enhance the device’s specific functions including fighting infection and promoting wound-healing.5,7,10 For instance, dip coating,8 spin coating,9 spray coating,6 and covalent conjugation of antibiotics10 have been developed to prepare antimicrobial films on biomedical devices. Much effort has been devoted to controlling drug release via manipulating the dissolution or degradation of the films.
More recently, electrostatic layer-by-layer self-assembly nanotechnology has been developed11 and used to construct polyelectrolyte micro- or nanocapsules12,13 and multilayer films14,15 for drug delivery. Certain drug molecules, such as active proteins, enzymes, nucleic acids, and DNA, have been immobilized into polyelectrolyte multilayer films. The advantages of polyelectrolyte multilayer films as drug delivery systems include: (i) drug molecules can act as either functional drugs or components of the film, and they can also form a stable crosslinking structure with other film component(s) via multivalent interactions (eg, electrostatic or hydrogenbonding interactions), (ii) sustained drug release is possible through controlling the film properties,16 (iii) polyelectrolyte multilayer films have the potential to protect drug molecules from losing their biological functions,17–21 and (iv) the film preparation process is simple and can be automated.11,14–17,
The drug release behavior of polyelectrolyte multilayer films depends on the permeability, the disassembly or erosion of the multilayer structure, and other experimental variables. A variety of polyelectrolyte multilayer films has been studied to control drug release via ionic strength, temperature, pH, enzyme, and hydrolytical degradation,12,13 Hayne and colleagues have bonded a thiol-bearing molecule, 5,5’-dithiobis(2-nitrobenzoic acid) (DTNB), into multilayers and 2-nitro-5-thiobenzoate dianions were released from the films by the breakdown of disulfide bonds between the DTNB and one of the film components.22 Rubner and colleagues have loaded Ketoprofen or cytochalasin D into polyelectrolyte multilayers and have shown unique zero-order drug release over a period of a few days.23,24 Caruso and Quinn have developed thermo-responsive multilayers containing poly- (N-isopropylacrylamide-co-acrylic acid) and have achieved sustained drug release.16 In addition, hydrogels and micelles have been introduced into polyelectrolyte multilayers as “drug containers” to manipulate drug-loading capacity.25–27 However, only a few studies are reported on controlling the loading and release of small drug molecules.22,28–30 It is still challenging to achieve a controllable release of small charged drug molecules, probably due to the weak interactions between small drug molecules and the film components. All of these have limited the applications of polyelectrolyte multilayer films for controlled drug release, especially on biomedical devices. Introducing “binding-sites” with tunable properties within nanofilms could be very useful in achieving controllable drug loading and release in polyelectrolyte multilayer films on biomedical devices.
In this work, we prepared polypeptide multilayer nanofilms using weak polyelectrolytes of poly-l-lysine (PLL) and poly-l-glutamic acid (PLGA), and we studied the loading and release behavior of small charged drug molecules. One advantage of such biodegradable drug release systems is that drug “binding-sites” within the multilayer nanofilms can be created and tuned simply by immersing the multilayer nanofilms fabricated at one pH into an aqueous solution of a different pH. For instance, PLL/PLGA multilayer nanofilms were prepared at pH 4.0, and positivelycharged drugs including gentamicin and methylene blue (MB) were loaded into the multilayer nanofilms by immersing the nanofilms in a drug-containing solution of a higher pH (eg, pH 7.0). Negatively-charged drugs such as cefazolin were incorporated into nanofilms formed at pH 10.0 by incubating the nanofilms in a cefazolin-containing solution of a lower pH (eg, pH 7.0). We showed that the loading and release of small charged (positively and negatively) drug molecules could be tuned by changing the number of film layers, the pH of the application environment or the pH of drug solutions, and applying post-preparation heat-treatment of the nanofilms. The driving force of drug loading and release from the multilayer nanofilms is mainly electrostatic interaction, attraction or repulsion, between the small charged drug molecules and the charged side-chains (binding-sites) of PLL or PLGA. Moreover, we found that polypeptide multilayer nanofilms loaded with antibiotics presented antibacterial properties against Staphylococcus aureus (S. aureus). Therefore, the developed approach is promising for controlling the loading and release of small charged drug molecules and achieving drug release systems for preventing biomedical device associated infection.
Materials and methods
Materials
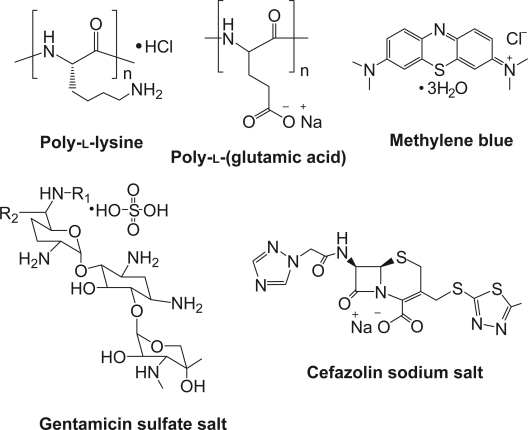
Poly-l-lysine (Mn = 150 kDa), PLGA (Mn = 50 kDa), MB, gentamicin, and cefazolin were used (Sigma Aldrich, St. Louis, MO). The structures of these polymers and drugs are shown in Figure 1, where cefazolin, a widely used antibiotic, is a negatively-charged small drug molecule, and gentamicin, another common antibiotic, and MB, a dye indicator, are positively-charged small molecules. Quartz slides were purchased from SPI Supplies, Inc. (West Chester, PA), cut into 25 mm × 10 mm × 1 mm, and cleaned by incubating in a piranha solution (4:1 H2SO4/H2O2) for 2 h at 80 °C followed by rinsing with deionized water. Stainless steel sheets were purchased (Small Parts, Inc., Miramar, FL) and cut into discs (10 mm × 0.25 mm), which were ultrasonicated in a 2% sodium dodecyl sulphate (SDS) solution for 30 min, washed in deionized water, and rinsed with an ethanol–NaOH solution and deionized water.
Figure 1.
Structures of studied polypeptides and drugs.
Buffer solutions in the pH range 4.0–10.0 were used throughout this study. Buffer solutions of pH 7.0–10.0 were prepared using 50 mM glycine–NaOH and buffer solutions of pH 4.0–7.0 were prepared using 10 mM Tris-HCl, 10 mM NaAc, and 130 mM NaCl. Gentamicin (10 mg/mL) and MB (3 mg/mL) solutions were prepared in Tris-HCl buffer solutions of pHs 4.0, 5.0, and 7.0. Cefazolin was dissolved in the glycine–NaOH buffer solutions of pHs 7.0, 8.0, and 9.0 at a concentration of 10 mg/mL. PLL (1 mg/mL) and PLGA (1 mg/mL) solutions were prepared by dissolving PLL and PLGA in the buffer solutions of pHs 4.0, 5.0, 7.0, 9.0, and 10.0.
Assembly of polypeptide multilayer nanofilms
Polypeptide multilayer nanofilms were prepared at pHs 4.0, 5.0, 7.0, 9.0, and 10.0 using a dipping-machine (Riegler and Kirstein GmbH, Berlin, Germany); all the aqueous media used were of the same pH for the same sample. In brief, pre-cleaned quartz slides or stainless steel discs were dipped in a PLL solution for 20 min followed by rinsing with corresponding buffer solution for 3 min and drying with air. The samples were then dipped in a PLGA solution for 20 min, rinsed with buffer solution for 3 min, and dried with air. These two dipping processes, ie, dipping in PLL and PLGA solutions, were referred to as one deposition cycle. By repeating the deposition cycle, polypeptide multilayer nanofilms, (PLL/PLGA)n, were prepared where n is the number of deposition cycles or bilayers.
The formation of polypeptide multilayer nanofilms on quartz slides was examined using UV-vis spectrometry. Images of PLL/PLGA multilayer nanofilms were obtained using atomic force microscopy (AFM, PicoSPM® II, Tempe, AZ) operating in a tapping mode with a silicon nitride cantilever tip. The growth of multilayer nanofilms on stainless steel discs with bilayers was measured using ellipsometry (M-2000, JA Woollam Co., Lincoln, NE).
Post-preparation heat-treatment of polypeptide multilayer nanofilms
Polypeptide multilayer nanofilms, (PLL/PLGA)10 and (PLL/PLGA)20, on quartz slides were treated at 120 °C in a vacuum oven (Isotemp Model 281, Fisher Scientific, Pittsburgh, PA) for 4 h. The vacuum applied was 380 Torr.
Stability of polypeptide multilayer nanofilms
The stability of polypeptide multilayer nanofilms in aqueous media was tested. In one set of studies, (PLL/PLGA)20 films on quartz slides were assembled at pHs 4.0, 7.0, and 10.0, and then incubated in a phosphate-buffered saline (PBS) of pH 7.0. In another set of experiments, (PLL/PLGA)20 films prepared at pH 10.0 on quartz slides were incubated in PBS solutions of pHs 4.0, 7.0, and 9.0. After incubating for 0, 0.5, 2.5, 8, 24, 48, 96, and 192 h, the samples were dried and their absorbances in the range of 190–290 nm were recorded using UV-vis spectrometry. All the data were averaged from three measurements. The absorbances of the same polypeptide multilayer nanofilm were compared between the time points studied.
Antibiotic- and MB-loading in polypeptide multilayer nanofilms
The loading of positively- and negatively-charged small drug molecules and drug models in polypeptide multilayer nanofilms was studied. The influences of number of film layers as well as the drug-loading time and drug solution pH were investigated. Polypeptide multilayer nanofilms prepared at pHs 4.0, 5.0, and 7.0 were used to load positively-charged drug molecules (ie, gentamicin and MB), and those at pHs 7.0, 9.0, and 10.0 were used to load negatively-charged drug molecules (ie, cefazolin). To determine the effect of drug solution pH on drug loading, (PLL/PLGA)20 films prepared at pH 4.0 were incubated in MB solutions of pHs 4.0, 5.0, and 7.0, and (PLL/PLGA)20 films prepared at pH 10.0 were immersed in cefazolin solutions of pHs 7.0, 8.0, 9.0, and 10.0.
In general, polypeptide multilayer nanofilms on quartz slides were incubated in the corresponding drug solutions at ambient temperature. At time periods of 2, 5, 10, 20, 40, and 60 min, the samples were rinsed with deionized water and dried with N2 gas followed by UV-vis absorbance measurements. The loading of cefazolin, gentamicin, and MB was determined by measuring the absorbance at 270 nm, 270 nm, and 665 nm, respectively, using UV-vis spectrometry.28,29
In order to obtain the total loading amounts of cefazolin, gentamicin, and MB in polypeptide multilayer nanofilms, the drug-loaded samples were ultrasonicated in 1 mL PBS for 30 min, and the ultrasonication process was repeated three or more times until no peak absorbance referring to the corresponding drugs on quartz slides could be observed using UV-vis spectrometry. The drug concentration in the PBS was analyzed by recording the peak absorbance of the drugs. Raw data were converted to concentration of drug (Cn, μg/mL) referring to the standard curves we obtained (data not shown). The drug released into the PBS solutions (Mn, μg/cm2) was calculated from an equation: Mn = Cn × V/An, where V is the total volume of the PBS and An is the surface area of the nanofilms on substrates. The total drug loaded in polypeptide multilayer nanofilms was determined as the cumulative amount of drugs released during the ultrasonication processes. It is worth noting that the peak absorbance of gentamicin at 270 nm was observed in gentamicin-loaded polypeptide multilayer nanofilms but the absorbance of the released gentamicin in the PBS solutions was hard to detect. As a result, the actual amount of gentamicin loaded was not reported in this study.
In vitro drug release from polypeptide multilayer nanofilms
Drug-loaded polypeptide multilayer nanofilms on quartz slides were incubated in 10 mL PBS of a certain pH (eg, pHs 4.0, 5.0, 7.0, 9.0, or 10.0). 0.6 mL of PBS solution was taken at a certain time period and 0.6 mL of fresh PBS was added to keep the volume of the release medium constant. The sample solutions of cefazolin and MB were analyzed using UV-vis spectrometry by measuring their absorbance at 270 nm and 665 nm, respectively. The total drug release (μg/cm2) was calculated as detailed before.
S. aureus Kirby–Bauer disk diffusion assay
A modified Kirby–Bauer technique was used to assess the antibacterial activity of polypeptide multilayer nanofilms loaded with antibiotics.31,32 A clinical isolate S. aureus was grown overnight in Mueller–Hinton broth, and the turbidity was adjusted to 0.5 McFarland. A cotton swab was dipped in the S. aureus suspension and rubbed across the surface of a Mueller–Hinton blood agar plate. Cefazolin- and gentamicinloaded polypeptide multilayer nanofilms on stainless steel discs were inserted parallel to the agar plate surface. The plates were inverted and incubated at 37 °C without shaking for 24 h before observation. The diameters of the zones of inhibition were measured six times from different directions, and the experiments were repeated at least three times. The average diameters of the zones were calculated.
Results
Growth curve and stability of PLL/PLGA multilayer nanofilms
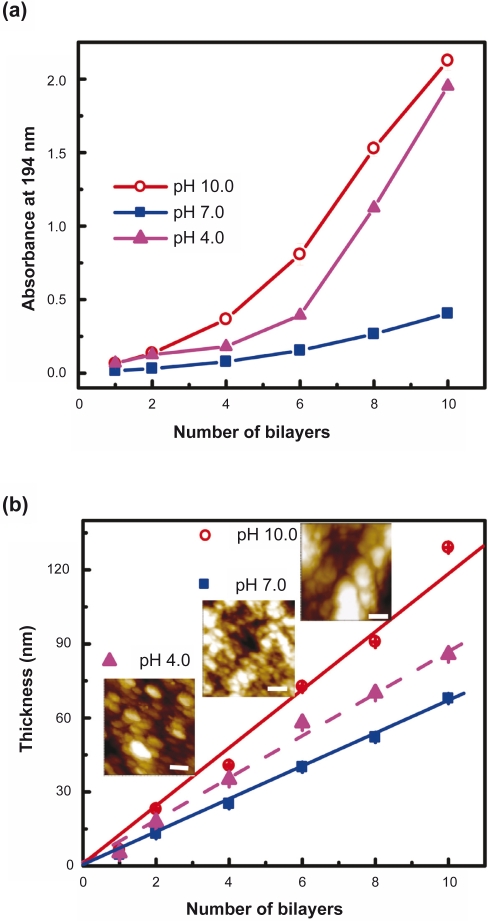
The formation of PLL/PLGA multilayer films was examined using UV-vis spectrometry, ellipsometry, and AFM. Figure 2a shows that the absorbance of PLL/PLGA multilayer films on quartz slides increased with increasing number of bilayers. In the pH values studied (ie, pH 4.0, 7.0, and 10.0), the smallest increase in absorbance was observed in the multilayer films constructed at pH 7.0. Figure 2b presents the thickness of polypeptide multilayer films. One can see that the thickness growth of PLL/PLGA multilayer films increased linearly with an increasing number of deposition bilayers. Similar to the UV-vis absorbance data, the least thickness was observed in the multilayer films constructed at pH 7.0 and the greatest thickness in the films prepared at pH 10.0. The thickness per layer of the PLL/PLGA multilayer films prepared at pHs 4.0, 7.0 and 10.0 were 4.3 ± 0.3 nm, 3.4 ± 0.1 nm, and 5.9 ± 0.2 nm, respectively.
Figure 2.
Growth profiles of pH-controllable polypeptide multilayer nanofilms a) on quartz slides and examined using UV-vis spectrometry, and b) on stainless steel discs and examined using ellipsometry. The inset in b) presents the atomic force microscope images of multilayer films formed at pHs 4.0, 7.0, and 10.0. The scale bars are 200 nm.
The surface morphology of polypeptide multilayer nanofilms formed at pHs 4.0, 7.0, and 10.0 was examined using AFM (Figure 2b insets). Particulate domains were observed. The size of the particulate domains of the nanofilms prepared at pH 7.0 was around tens of nanometers and was much smaller than those of the nanofilms assembled at pHs 4.0 and 10.0.
One concern in developing polyelectrolyte multilayer films is their stability. Our stability studies of the PLL/PLGA nanofilms in aqueous solutions showed no obvious changes in absorbance (data not shown) in the wavelength range of 190–290 nm after more than one week at all the pH values studied (ie, pH 4.0, 7.0, and 9.0). This means that PLL/PLGA multilayer nanofilms are stable and can tolerate pH shifts in our drug loading and release processes.
Tunability of drug loading in PLL/PLGA multilayer nanofilms
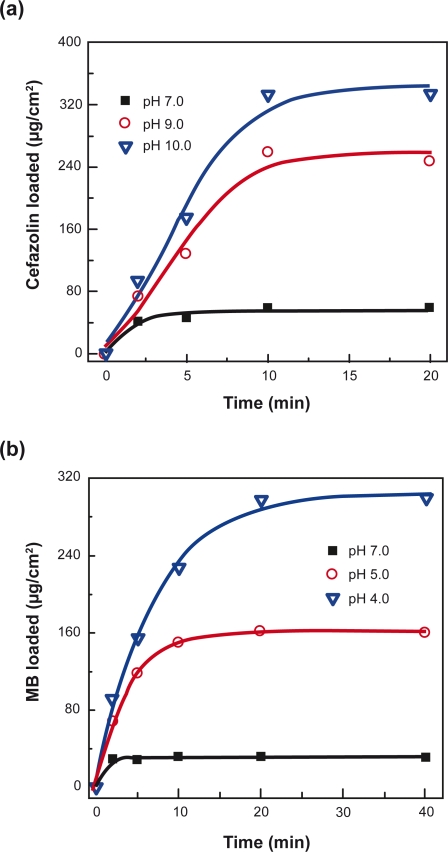
The influence of pH at which the multilayer nanofilms were prepared and time of incubation on drug loading was studied in (PLL/PLGA)20 nanofilms (Figure 3). It was found that the loading of drugs increased with the incubation time and the loading of cefazolin and MB could reach their maximum loading (ie, capacity) within 10 and 20 min, respectively. (PLL/PLGA)20 nanofilms formed at different pHs showed different drug-loading capacities. More drugs were captured in the nanofilms prepared at a pH away from pH 7.0 than at pH 7.0, and loading was faster in the nanofilms assembled at pHs 10.0 and 4.0 than at pH 7.0. Cefazolin-loading capacity in the nanofilms formed at pH 10.0 was ∼330 μg/cm2; it was the highest and it was almost six times that of nanofilms prepared at pH 7.0 (∼60 μg/cm2). Similarly, the MB-loading capacity in (PLL/PLGA)20 nanofilms assembled at pH 4.0 was about ten times that in the nanofilms prepared at pH 7.0.
Figure 3.
Effects of pH at which nanofilms were prepared on drug-loading profiles of a) cefazolin and b) MB in (PLL/PLGA)20 nanofilms. The pH of both cefazolin and MB solutions was 7.0.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
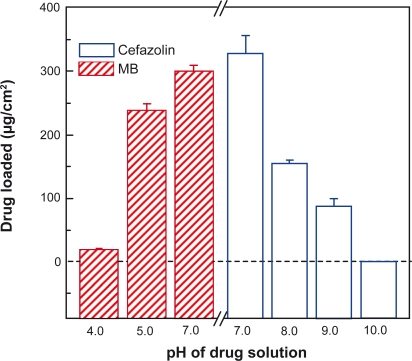
The influence of drug solution pH on drug loading was also investigated. Figure 4 shows that more cefazolin was loaded in (PLL/PLGA)20 nanofilms at a lower pH in the range of pH 7.0–10.0, and more MB was loaded at a higher pH in the range of pH 4.0–7.0. Cefazolin-loading capacity at pH 7.0 was about four times that at pH 9.0. Loading of cefazolin at pH 10.0 was not detected and the loading capacity of MB at pH 7.0 was about sixteen times that at pH 4.0.
Figure 4.
Effects of drug solution pH on drug-loading capacity of polypeptide multilayer nanofilms. Cefazolin was loaded at pHs 7.0, 8.0, 9.0, and 10.0 in (PLL/PLGA)20 nanofilms assembled at 10.0. MB was loaded at pHs 4.0, 5.0, and 7.0 in (PLL/PLGA)20 nanofilms prepared at pH 4.0.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
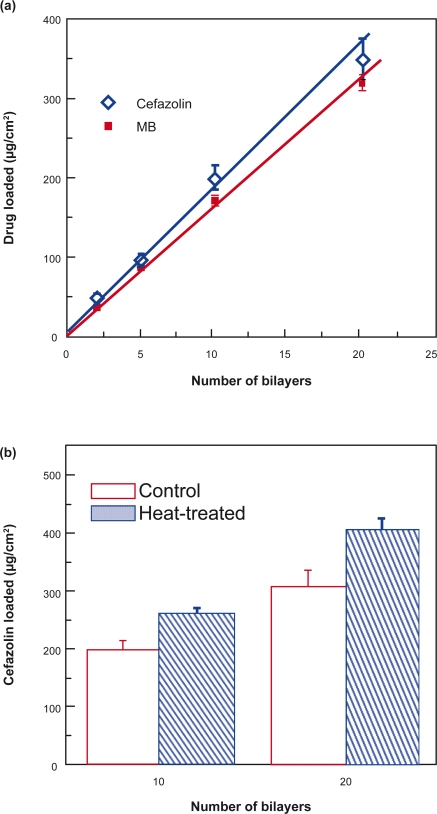
Figure 5a presents the drug-loading capacity versus bilayers of (PLL/PLGA)n nanofilms. The amounts of drugs, either cefazolin or MB, loaded increased approximately linearly with an increasing number of bilayers.
Figure 5.
Effects of a) film bilayers and b) heat-treatment on drug-loading capacity. The pH of both cefazolin-and MB-loading solutions was 7.0. Cefazolin was loaded in (PLL/PLGA)20 nanofilms assembled at pH 10.0. MB was loaded in (PLL/PLGA)20 nanofilms prepared at pH 4.0.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
The influence of post-preparation heat-treatment on drug loading is shown in Figure 5b. After heat-treatment at 120 °C for 4 h, both (PLL/PLGA)10 and (PLL/PLGA)20 nanofilms had ∼30% increase in drug loading.
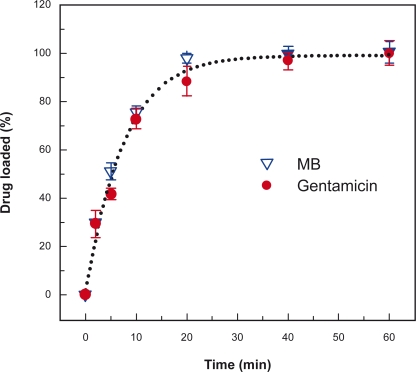
In addition, Figure 6 shows that gentamicin was loaded into PLL/PLGA multilayer nanofilms within 20 to 40 min, and gentamicin and MB, both positively-charged, had similar drug-loading kinetics in (PLL/PLGA)20 nanofilms.
Figure 6.
Loading profiles of gentamicin and MB in (PLL/PLGA)20 nanofilms assembled at pH 4.0. Both gentamicin and MB were loaded at pH 7.0. UV-vis absorbances of gentamicin at 270 nm and MB at 665 nm were recorded, and the highest absorbance values (ie, at 60 min) were set as 100% for gentamicin and MB.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
Tunability and kinetics of drug release from PLL/PLGA multilayer nanofilms
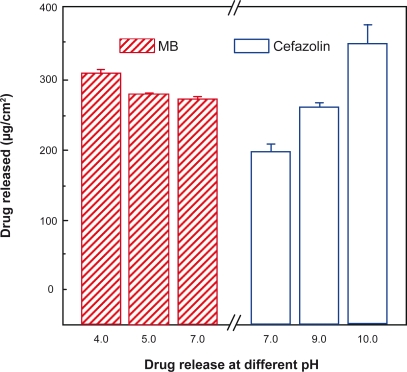
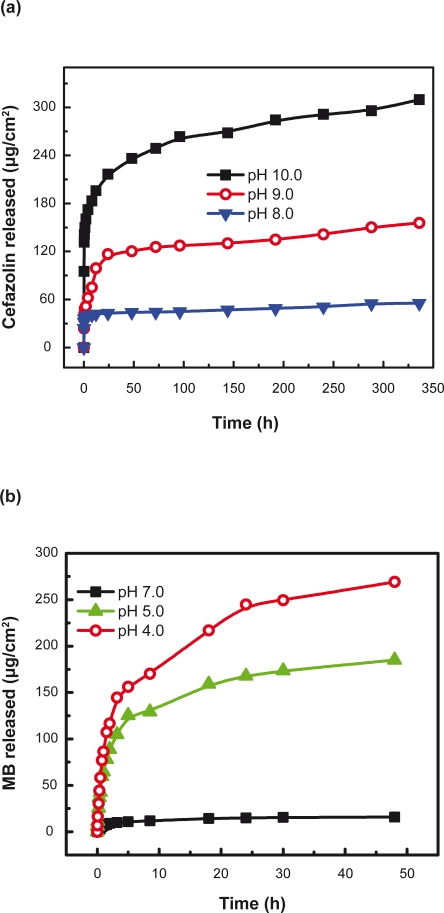
Environmental conditions (eg, pH) influenced the drug release from polypeptide multilayer nanofilms. We studied the release of cefazolin and MB from (PLL/PLGA)20 nanofilms in a wide range of pH values. Figure 7 shows that more cefazolin was released at a higher pH than at a lower pH in the pH range 7.0–10.0; the amount of cefazolin released at pH 10.0, ∼340 μg/cm2, was about twice that released at pH 7.0, ∼190 μg/cm2. Also, more MB was released at a lower pH than at a higher pH in the pH range 4.0–7.0.
Figure 7.
Effects of environmental pH on release of cefazolin and MB from polypeptide multilayer nanofilms. Cefazolin was released in PBS solutions of pHs 7.0, 9.0, and 10.0 for 48 h, and MB was released in PBS solutions of pHs 4.0, 5.0, and 7.0 for 48 h. Cefazolin was released from (PLL/PLGA)20 nanofilms that were assembled at pH 10.0 and loaded with cefazolin at pH 7.0 for 20 min. MB was released from (PLL/PLGA)20 nanofilms that were prepared at pH 4.0 and loaded with MB at pH 7.0 for 20 min.
Abbreviations: MB, methylene blue; PBS, phosphate-buffered saline; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
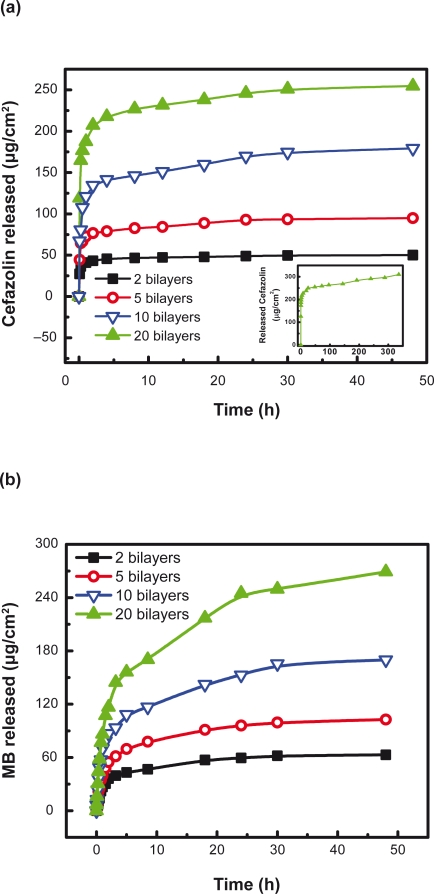
Moreover, the number of film bilayers, the pH at which the nanofilms were prepared, and post-preparation heat-treatment influenced drug release from polypeptide multilayer nanofilms. The number of film bilayers influenced the drug release behavior. Figure 8a shows that there was a burst release in the first few hours (mainly the first hour) in all the cefazolin-loaded samples. Up to 95% cefazolin was released from 2- and 5-bilayer PLL/PLGA nanofilms within 48 h, and 85% and 75% were released from the 10- and 20-bilyer PLL/PLGA nanofilms, respectively. More cefazolin was released from a higher number of bilayers of nanofilms. For instance, 50, 90, 180, and 250 μg/cm2 cefazolin were released at 48 h from 2, 5, 10, and 20-bilayer PLL/PLGA nanofilms, respectively. Similarly, there was a burst release in the first few hours in MB-loaded PLL/PLGA multilayer nanofilms, and more MB was released with an increasing number of bilayers (Figure 8b).
Figure 8.
Release profiles of (a) cefazolin and (b) MB from polypeptide multilayer nanofilms. Both cefazolin and MB were released at pH 7.0. Cefazolin was released from PLL/PLGA multilayer nanofilms that were assembled at pH 10.0 and loaded with cefazolin at pH 7.0 for 20 min. MB was released from PLL/PLGA multilayer nanofilms that were prepared at pH 4.0 and loaded with MB at pH 7.0 for 20 min. The inset presents the release, up to 336 h, of cefazolin from (PLL/PLGA)20 nanofilms.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
Figure 9 shows that the pH at which PLL/PLGA nanofilms were prepared also affected drug release. More cefazolin was released from (PLL/PLGA)20 nanofilms assembled at a higher pH in the pH range 7.0–10.0, and more MB was released from (PLL/PLGA)20 nanofilms deposited at a lower pH in the pH range 4.0–7.0.
Figure 9.
Effects of pH at which polypeptide multilayer nanofilms were assembled on release of (a) cefazolin and (b) MB. Both cefazolin and MB were released at pH 7.0. Cefazolin was released from (PLL/PLGA)20 nanofilms that were loaded with cefazolin at pH 7.0 for 20 min. MB was released from (PLL/PLGA)20 nanofilms that were loaded with MB at pH 7.0 for 20 min.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine
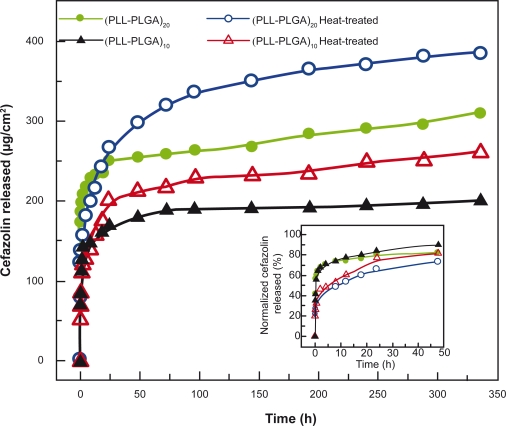
In addition, post-preparation heat-treatment influenced drug release (Figure 10). A greater amount of cefazolin was released from heat-treated samples than from untreated ones. Meanwhile, the heat-treatment slowed drug release from the PLL/PLGA nanofilms. Up to 80% of loaded cefazolin was released in the first 24 h from the 10- and 20-bilayer PLL/PLGA nanofilms without heat-treatment, while approximately 67% and 60% was released from the heat-treated 10- and 20-bilayer PLL/PLGA nanofilms, respectively (Figure 10 inset).
Figure 10.
Effects of heat-treatment on cefazolin release from polypeptide multilayer nanofilms. Cefazolin was released at pH 7.0. Cefazolin-containing (PLL/PLGA)10 and (PLL/PLGA)20 nanofilms were assembled at pH 10.0 and loaded with cefazolin at pH 7.0 for 20 min. The inset shows the release in percentage.
Abbreviations: PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
Kirby–Bauer assays
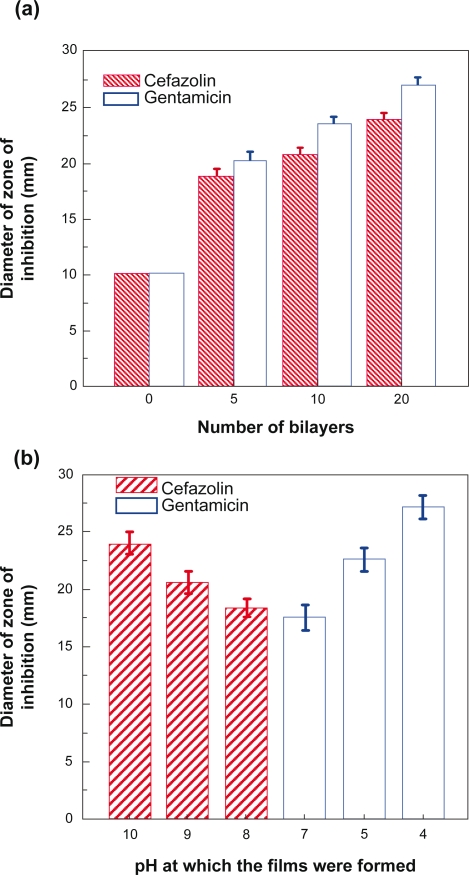
Studies were conducted to evaluate the antibacterial effects of cefazolin- and gentamicin-loaded polypeptide multilayer nanofilms. Figure 11a shows that the average diameter of zone of inhibition increased with increasing film bilayers. The discs with cefazolin had a zone of inhibition of 18.8 ± 0.8 mm, 20.7 ± 0.9 mm, and 23.9 ± 0.8 mm, respectively, for 5, 10, and 20-bilayer PLL/PLGA nanofilms. The average zone diameters, for 5, 10, and 20-bilayers, of gentamicin-loaded PLL/PLGA nanofilms were 20.2 ± 1.0 mm, 23.6 ± 0.7 mm, and 27.0 ± 0.9 mm, respectively. The cefazolin- and gentamicin-loaded 20-bilayer PLL/PLGA nanofilms assembled at different pHs also showed different antibacterial activity (Figure 11b). The average zone diameters for cefazolin-containing nanofilms assembled at pH 8.0, 9.0, and 10.0 were 18.3 ± 0.8 mm, 20.5 ± 1.0 mm, and 23.9 ± 0.8 mm, respectively, and the average zone diameters were 17.5 ± 1.1 mm, 22.5 ± 1.0 mm, and 27.0 ± 0.9 mm for gentamicin-containing (PLL/PLGA)20 nanofilms deposited at pH 7.0, 5.0, and 4.0, respectively.
Figure 11.
a) Diameter of zone of inhibition vs number of film bilayers. Cefazolin-containing PLL/PLGA nanofilms were assembled at pH 10.0 and loaded with cefazolin at pH 7.0 for 20 min. Gentamicin-containing PLL/PLGA nanofilms were assembled at pH 4.0 and loaded with gentamicin at pH 7.0 for 20 min. b) Diameter of zone of inhibition vs. pH at which drug-containing (PLL/PLGA)20 nanofilms were assembled. (PLL/PLGA)20 nanofilms were loaded with cefazolin or gentamicin at pH 7.0 for 20 min. The diameter of control samples was 10.0 mm.
Abbreviations: PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
Discussion
In this study, we developed biodegradable polypeptide multilayer nanofilms made of two weak polyelectrolytes as potential drug release systems on biomedical devices. Our developed polypeptide multilayer nanofilms on stainless steel discs and quartz slides possessed the capability to load both negatively- and positively-charged small drug molecules, and the drug loading and release were tunable and pH-dependent.
Mechanism of pH-dependent drug loading and release
Compared to strong polyelectrolytes, such as poly(dially ldimethylammonium chloride) (PDDA) and poly(styrene sulfonate) (PSS), weak polyelectrolytes such as PLL, PLGA, poly(ethyleneimine) (PEI), poly(acrylic acid) (PAA), and poly(allylamine hydrochloride) (PAH), may present various ionization statuses and surface charges as their environmental pH changes. At a pH below or above the isoelectric point (pI), a weak polyelectrolyte is partially charged and may adopt a coiled structure in polyelectrolyte multilayer nanofilms due to the decrease in charge repulsion among themselves.13,20 The coil-structured polymer contains uncharged segments that may be converted to be charged (“binding sites”) if the environmental pH is switched to a value at which the weak polyelectrolyte becomes more ionized. As a result, binding sites within multilayers made of weak polyelectrolytes can be created for capturing oppositely-charged drug molecules.
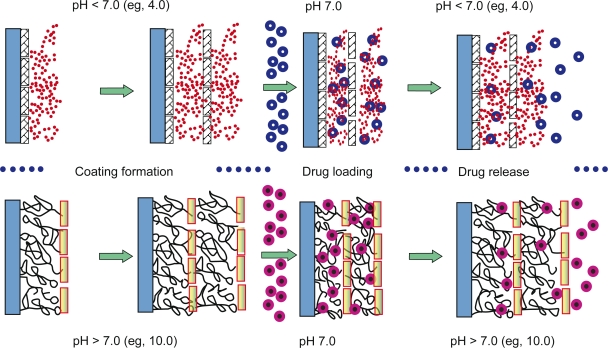
In our study, we prepared PLL/PLGA multilayer nanofilms using electrostatic layer-by-layer self-assembly based on alternative deposition of PLL and PLGA on a substrate. Mechanisms of drug loading and release from the developed PLL/PLGA multilayer nanofilms are proposed in Figure 12. PLL and PLGA are weak polyelectrolytes; PLGA possesses a net negative charge at a pH higher than pH 2.6, the pI of PLGA,33,34 and PLL presents a net positive charge at a pH lower than its pI, pH 12.5.32 Therefore, in the multilayer films formed in an acid solution, eg, pH 4.0, PLGA is partially charged and adopts a coiled structure (Figure 12 top part), and its net charge decreases as pH decreases in the pH range 2.6–7.0. This results in more deposition of PLGA and thicker films at pH 4.0 than at pH 7.0; PLL/PLGA multilayer nanofilms assembled at pH 4.0 presented a higher absorbance and higher thickness than those deposited at pH 7.0 (Figure 2). When incubating PLL/PLGA multilayer nanofilms prepared at pH 4.0 in a drug solution of pH 7.0, where both PLL and PLGA are highly ionized, the uncharged side chains of PLGA in the nanofilms become negatively charged thereby generating binding sites for positively-charged drug molecules. In the pH range 4.0–7.0, the higher the drug solution pH, the more binding sites are available and the more drugs can be captured. Therefore the capture of drugs is pH-dependent. Meanwhile, the release of drugs from PLL/PLGA multilayer nanofilms is also pH-dependent. When drug-loaded PLL/PLGA multilayer nanofilms serve in an environment of pH (eg, pH 4.0) lower than the pH (eg, pH 7.0) at which the drugs are loaded, the net charge of PLGA reverses and PLGA becomes less ionized. This leads to weakening of the interactions between the positively-charged drug molecules and the negatively-charged PLGA molecules. As a result, drugs are released from PLL/PLGA multilayer nanofilms.
Figure 12.
Mechanisms of pH-dependent drug capture and release from polypeptide multilayer nanofilms. PLL/PLGA multilayer nanofilms are assembled at a pH (eg, pH 4.0 or 10.0) away from the drug-loading pH, followed by incubating in a drug (eg, gentamicin, MB, or cefazolin) solution of pH 7.0 and releasing the captured drugs by altering the pH of releasing media.
Key:
 stretched PLL,
stretched PLL,
 coiled PLL,
coiled PLL,
 stretched PLGA,
stretched PLGA,
 coiled PLGA,
coiled PLGA,
 positively-charged drug,
positively-charged drug,
 negatively-charged drug.
negatively-charged drug.
Abbreviations: MB, methylene blue; PLGA, poly-l-glutamic acid; PLL, poly-l-lysine.
Similarly (Figure 12 bottom part), PLL/PLGA multilayer nanofilms assembled at a high pH (eg, pH 10.0) contain partiallyionized and coil-structured PLL. This allows the PLL/PLGA multilayer nanofilms the capability to load negatively-charged drugs at pH 7.0, and drug release can be induced in an environment of a higher pH, eg, pH 8.0, 9.0, or 10.0.
Tunable loading of charged drugs in polypeptide multilayer nanofilms
The pH at which polypeptide multilayer nanofilms are prepared may influence the amount of polymers deposited thereby influencing subsequent drug loading. Figure 3a shows that the drug-loading capacity of (PLL/PLGA)20 nanofilms prepared at pH 10.0 was approximately six times that of nanofilms assembled at pH 7.0. The higher loading of cefazolin in those nanofilms prepared at pH 10.0 was due to the higher amount of PLL assembled (corresponding to the higher absorbance and increased thickness at pH 10.0 in Figure 2) and the more ionization of PLL as the pH changes from film preparation at pH 10.0 to drug loading at pH 7.0, compared to those nanofilms prepared at pHs 9.0 and 7.0. As a result, binding sites on PLL molecules for negatively-charged drug molecules were created as the pH shifts from the film preparation pH to the drug-loading pH and more binding sites on PLL would be available at a lower pH in the pH range 7.0–10.0. This is consistent with the increased loading of cefazolin in PLL/PLGA multilayer nanofilms assembled at pH 10.0 than at pHs 9.0 and 7.0 (Figure 3a).
Similarly, (PLL/PLGA)20 nanofilms assembled at a lower pH in the pH range 4.0–7.0 would have more PLGA deposited, corresponding to higher absorbance and higher thickness at pH 4.0 than at pH 7.0 in Figure 2. This thereby leads to more binding sites on PLGA for positively-charged drug molecules when the drugs are loaded at pH 7.0. As shown in Figure 3b, the (PLL/PLGA)20 nanofilms formed at a lower pH in the pH range of 4.0–7.0 showed higher MB loading. Therefore, polypeptide multilayer nanofilms assembled at different pHs had various capacities for drug loading after film formation.
Meanwhile, the pH at which drugs are loaded could also be used to tune drug loading in polypeptide multilayer nanofilms. As shown in Figure 4, (PLL/PLGA)20 nanofilms prepared at pH 10.0 showed different capacities for loading negativelycharged cefazolin in the pH range 7.0–10.0, and (PLL/PLGA)20 nanofilms prepared at pH 4.0 allowed the tuning of positively-charged MB loading in the pH range 4.0–7.0. More side chains of PLL, assembled at pH 10.0, became ionized at a lower drug-loading pH in the pH range 7.0–10.0, therefore more binding sites were available for negatively-charged drug molecules and more cefazolin was captured at pH 7.0 than at pHs 8.0, 9.0, and 10.0 (Figure 4). At pH 10.0, no absorbance for cefazolin was detected in the nanofilms, because there was no pH shift between film preparation and drug loading and no binding sites were created; as a result, no cefazolin was captured in the nanofilms. This may mean that the driving force of drug loading in PLL/PLGA multilayers is mainly electrostatic attraction. Similarly, the drug solution pH also influenced the loading of positively-charged MB, where the PLL/PLGA nanofilms were assembled at pH 4.0. The change in ionization of PLGA led to the capture of more positivelycharged MB at pH 7.0 than at pHs 4.0 and 5.0 (Figure 4). The limited loading of MB at pH 4.0 might be related to the interaction of MB with the outermost layer, ie, PLGA, of the (PLL/PLGA)20 nanofilm.
Using the electrostatic layer-by-layer self-assembly technique, we can also control the amount of polymers deposited and tune the subsequent drug loading by manipulating the number of deposition bilayers. Figure 2 shows that the absorbance and thickness, and thereby the amount of polypeptides deposited, increased with increasing deposition bilayers. The increase in polypeptide deposition could lead to an increase in binding sites and drug loading when the pH shifts from the deposition pH to the drug-loading pH. As a result, the amounts of cefazolin and MB loaded in PLL/PLGA multilayer nanofilms increased approximately linearly with increasing deposition bilayers (Figure 5a).
In addition, heat-treatment after film formation may influence drug loading. We found that heat-treatment after multilayer film formation led to an increase (∼30%, Figure 5b) in cefazolin loading; however, the reason for the increase is unknown and will be studied in the future. In the literature, heat-treatment led to the formation of crosslinkings between polyelectrolyte multilayers due to the formation of amide bonds from carboxylic and amine groups within polyelectrolyte multilayers,35 and the swelling properties of heat-treated films changed significantly over a wide pH range.36
Tunable drug release from polypeptide multilayer nanofilms
In previous studies, drug release from polyelectrolyte multilayer films was mainly controlled by manipulating the permeability and degradation of the films. In our study, PLL/PLGA multilayer nanofilms were stable in aqueous solutions and could tolerate pH shifts in our drug loading and release processes. The release of drug molecules from polypeptide multilayer nanofilms is mainly due to the change of interaction between drug molecules and polypeptide nanofilms, and drug diffusion.
By changing the interaction between drug molecules and PLL/PLGA multilayer film components, one can tune drug release. As shown in Figure 12, when there is a pH shift from drug loading to drug release, the interaction between the drug molecules and the corresponding oppositely-charged polypeptides changes and drugs can be released. Cefazolin and MB were loaded at pH 7.0 and more cefazolin was released at pH 10.0 than at a lower pH in the pH range 7.0–10.0. More MB was released at pH 4.0 than at a higher pH in the pH range 4.0–7.0 (Figure 7), due to the change in the interaction between the drug molecules and the corresponding oppositely-charged polypeptides. For instance, as the pH changed from the drug loading (pH 7.0) to a higher drug release pH in the pH range 7.0–10.0, more binding sites of PLL were reversed and became uncharged; therefore, more cefazolin was released from the nanofilms.
Moreover, the history of polypeptide multilayer nanofilms had a significant impact on the amount of drugs released. Drug release could be tuned by controlling the number of film layers, the pH at which the nanofilms were prepared, and post-preparation heat-treatment. It was found that more drugs (cefazolin and MB) were released from PLL/PLGA multilayer nanofilms with more bilayers (Figure 8). More cefazolin was released from (PLL/PLGA)20 nanofilms assembled at pH 10.0 than those assembled at a lower pH in the pH range 7.0–10.0, and more MB was released from (PLL/PLGA)20 nanofilms assembled at pH 4.0 than those nanofilms prepared at a higher pH in the pH range 4.0–7.0 (Figure 9). The higher drug release was probably associated with the corresponding higher drug loading in the PLL/PLGA multilayer nanofilms under those conditions (Figure 3). The post-preparation heat-treatment also had some effect on drug release, as it seemed that heat-treatment slowed drug release compared to the control samples (Figure 10 inset). This was likely related to the possible crosslinking formation and the reduced swelling of polyelectrolyte multilayers in aqueous media after heat-treatment.36 The higher release amounts of cefazolin after the burst release period was because of higher amounts of cefazolin loaded in the nanofilms after heat-treatment (Figure 5b).
In vitro antibacterial activity against S. aureus
Quantitative assessment of the therapeutic activity of antibiotic- loaded polypeptide multilayer nanofilms was conducted. The diameter of a zone of inhibition provides a quantitative measure of the amount of in vitro active antibiotic (eg, cefazolin and gentamicin) released and diffused into the agar plates. Figure 11 shows that PLL/PLGA multilayer nanofilms containing cefazolin and gentamicin presented large zones of inhibition, and those samples without antibiotics had no antibacterial effects. The zone of inhibition became larger with increasing number of bilayers; this is because more drugs (eg, cefazolin) were loaded and subsequently released from the PLL/PLGA multilayer nanofilms with more bilayers (Figure 5a). The difference in the sizes of zone of inhibition in (PLL/PLGA)20 nanofilms assembled at different pHs was also related to the different amounts of antibiotics loaded and released from the PLL/PLGA multilayer nanofilms. The more antibiotics loaded, the bigger the zone of inhibition. Figure 3 shows that the (PLL/PLGA)20 nanofilms formed at different pHs possessed different drug-loading capacities, and more cefazolin was loaded at pH 10.0 than at pHs 9.0 and 7.0. As a result, a larger zone of inhibition was observed in cefazolin loaded (PLL/PLGA)20 nanofilms assembled at pH 10.0 than at pHs 9.0 and 7.0.
Our studies showed that stainless steel discs coated with antibiotic-loaded polypeptide multilayer nanofilms exhibited in vitro antibacterial activity against S. aureus. Stainless steel is one of the commonly used metal implants in orthopedics and S. aureus is the most common source of osteomyelitis and septic arthritis.37,38 Therefore, the developed antibioticloaded polypeptide multilayer nanofilms have the potential to prevent orthopaedic device-associated infection, and further studies will be carried out to investigate the efficacy of such antibiotic-loaded nanofilms in preventing infection in vivo in an open fracture rat model we recently developed.39
Conclusions
A multilayer self-assembly technology was applied to construct biodegradable polypeptide multilayer nanofilms made of PLL and PLGA, which are weak polyelectrolytes that enable the fine tuning of drug loading and release after film formation. Our studies showed that the loading kinetics of gentamicin and MB, both positively-charged, were very similar. The loading and release of both negatively- and positively-charged drug molecules (eg, cefazolin, gentamicin, and MB) could be tuned by several variables during and after the film preparation. Such variables include the number of deposition layers, pH of film preparation, and post-preparation heat-treatment. The loading of drugs (eg, cefazolin and MB) increased approximately linearly with an increasing number of layers, and heat-treatment before drug loading enhanced the drug-loading capacity. The pH of film preparation also significantly altered the film formation including surface morphology. In addition, the drug-loading pH and the incubation time in the drug solution could be used to tune the amount of drugs that could be loaded, and the pH of an application environment also had a significant impact on drug release. The developed antibiotic-loaded polypeptide multilayer nanofilms presented tunable antibacterial properties and potentially have significant applications in medicine, eg, antibacterial drug delivery systems for preventing biomedical device-associated infection.
Acknowledgments
The authors received financial support from National Science Foundation (NSF), West Virginia University Program to Stimulate Competitive Research (WVU PSCoR), West Virginia University Senate Grant, and National Aeronautics and Space Administration West Virginia Experimental Program to Stimulate Competitive Research (NASA WV EPSCoR). We also acknowledge the assistance of Rajiv Bhattarai (Davis and Elkins College, Elkins, WV) for film stability tests and staff support by Vincent Kish, Suzanne Smith, and Nina Clovis.
References
- 1.Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop Relat Res. 2005;437:105–110. doi: 10.1097/01.blo.0000175720.99118.fe. [DOI] [PubMed] [Google Scholar]
- 2.Zalavras CG, Patzakis MJ, Holtom PD, Sherman R. Management of open fractures. Infect Dis Clin North Am. 2005;19:915–929. doi: 10.1016/j.idc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. Prophylaxis and treatment of implant-related infections by antibioticcoated implants: a review. Injury. 2006;37:S105–S112. doi: 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Murray CK, Hsu JR, Solomkin JS, Keeling JJ, Andersen RC, Ficke JR, Calhoun JH. Prevention and management of infections associated with combat-related extremity injuries. J Trauma. 2008;64(3 Suppl):S239–S251. doi: 10.1097/TA.0b013e318163cd14. [DOI] [PubMed] [Google Scholar]
- 5.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 6.Sun YM, Chang CC, Huang WF, Liang HC. Fluidized-bed spray coated porous hydrogel beads for sustained release of diclofenac sodium. J Control Release. 1997;47:247–260. [Google Scholar]
- 7.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 8.Acharya G, Park K. Mechanisms of controlled drug release from drugeluting stents. Adv Drug Del Rev. 2006;58:387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Muller-Buschbaum P, Gebhardt R, Maurer E, Bauer E, Gehrke R, Doster W. Thin casein films as prepared by spin-coating: Influence of film thickness and of pH. Biomacromolecules. 2008;7:1773–1780. doi: 10.1021/bm060088u. [DOI] [PubMed] [Google Scholar]
- 10.Antoci V, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 2007;461:81–87. doi: 10.1097/BLO.0b013e3181123a50. [DOI] [PubMed] [Google Scholar]
- 11.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 12.Lynn DM. Layers of opportunity: nanostructured polymer assemblies for the delivery of macromolecular therapeutics. Soft Matt. 2006;2:269–273. doi: 10.1039/b517860f. [DOI] [PubMed] [Google Scholar]
- 13.De Geest BG, Sanders NN, Sukhorukov GB, Demeester J, De Smedt SC. Release mechanisms for polyelectrolyte capsules. Chem Soc Rev. 2007;36:636–649. doi: 10.1039/b600460c. [DOI] [PubMed] [Google Scholar]
- 14.Scranton AB, Rangarajan B, Klier J. Biomedical applications of polyelectrolytes. Adv Polym Sci. 1995;122:1–54. [Google Scholar]
- 15.Cai KY, Rechtenbach A, Hao JY. Polysaccharide-protein surface modification of titanium via a layer-by-layer technique: Characterization and cell behavior aspects. Biomaterials. 2005;26:5960–5971. doi: 10.1016/j.biomaterials.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Quinn JF, Caruso F. Thermoresponsive nanoassemblies: Layerby- layer assembly of hydrophilic-hydrophobic alternating copolymers. Macromolecules. 2005;38:3414–3419. [Google Scholar]
- 17.Chluba J, Voegel JC, Decher G, Erbacher P, Schaaf P, Ogier J. Peptide hormone covalently bound to polyelectrolytes and embedded into multilayer architectures conserving full biological activity. Biomacromolecules. 2001;2:800–805. doi: 10.1021/bm015529i. [DOI] [PubMed] [Google Scholar]
- 18.Serizawa T, Yamaguchi M, Akashi M. Alternating bioactivity of polymeric layer-by-layer assemblies: Anti- vs procoagulation of human blood. Biomacromolecules. 2002;3:724–731. doi: 10.1021/bm0200027. [DOI] [PubMed] [Google Scholar]
- 19.Jessel N, Atalar F, Lavalle P, et al. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv Mater. 2003;15:692–695. [Google Scholar]
- 20.Amiji M, editor. Polymeric gene delivery: Principles and applications. New York, NY: CRC Press; 2004. pp. 227–241. [Google Scholar]
- 21.Schultz P, Vautier D, Richert L, et al. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesis. Biomaterials. 2005;26:2621–2630. doi: 10.1016/j.biomaterials.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Zhong Y, Whittington CF, Haynie DT. Stimulated release of small molecules from polyelectrolyte multilayer nanocoatings. Chem Commun. 2007;14:1415–1417. doi: 10.1039/b615699a. [DOI] [PubMed] [Google Scholar]
- 23.Mendelsohn JD, Barrett CJ, Chan VV, Pal AJ, Mayes AM, Rubner MF. Fabrication of microporous thin films from polyelectrolyte multilayers. Langmuir. 2000;16:5017–5023. [Google Scholar]
- 24.Berg MC, Zhai L, Cohen RE, Rubner MF. Controlled drug release from porous polyelectrolyte multilayers. Biomacromolecules. 2006;7:357–364. doi: 10.1021/bm050174e. [DOI] [PubMed] [Google Scholar]
- 25.Qi B, Tong X, Zhao Y. Layer-by-layer assembly of two different polymer micelles with polycation and polyanion coronas. Macromolecules. 2006;39:5714–5719. [Google Scholar]
- 26.Schneider A, Vodouhe C, Richert L, et al. Multifunctional polyelectrolyte multilayer films: Combining mechanical resistance, biodegradability, and bioactivity. Biomacromolecules. 2007;8:139–145. doi: 10.1021/bm060765k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Wang X, Xu MF, Chen DD, Sun JQ. Layer-by-layer assembled microgel films with high loading capacity: Reversible loading and release of dyes and nanoparticles. Langmuir. 2008;24:1902–1909. doi: 10.1021/la7031048. [DOI] [PubMed] [Google Scholar]
- 28.Arici MK, Sumer Z, Guler C, Elibol O, Saygi G, Cetinkaya S. In vitro potency and stability of fortified ophthalmic antibiotics. Aust N Z J Ophthalmol. 1999;27:426–430. doi: 10.1046/j.1440-1606.1999.00239.x. [DOI] [PubMed] [Google Scholar]
- 29.Chung AJ, Rubner MF. Methods of loading and releasing low molecular weight cationic molecules in weak polyelectrolyte multilayer films. Langmuir. 2002;18:1176–1183. [Google Scholar]
- 30.Chuang HF, Smith RC, Hammond PT. Polyelectrolyte multilayers for tunable release of antibiotics. Biomacromolecules. 2008;9:1660–1668. doi: 10.1021/bm800185h. [DOI] [PubMed] [Google Scholar]
- 31.Sherertz RJ, Forman DM, Solomon DD. Efficacy of dicloxacillin-coated polyurethane catheters in preventing subcutaneous Staphylococcus aureus infection in mice. Antimicrob Agents Chemother. 1989;33:1174–1178. doi: 10.1128/aac.33.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang B, Li B. Polypeptide nanocoatings for preventing dental and orthopaedic device-associated infection. J Biomed Mater Res B. 2009;88:332–338. doi: 10.1002/jbm.b.31021. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann R, Kratzmuller T, Erickson D, Li DQ, Braun HG, Werner C. Ionic strength-dependent pK shift in the helix-coil transition of grafted poly(l-glutamic acid) layers analyzed by electrokinetic and ellipsometric measurements. Langmuir. 2004;20:2369–2374. doi: 10.1021/la035945j. [DOI] [PubMed] [Google Scholar]
- 34.Hahn SK, Hoffman AS. Preparation and characterization of biocompatible polyelectrolyte complex multilayer of hyaluronic acid and poly-l-lysine. Int J Biol Macromol. 2005;37:227–231. doi: 10.1016/j.ijbiomac.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Harris JJ, DeRose PM, Bruening ML. Synthesis of passivating, nylonlike coatings through cross-linking of ultrathin polyelectrolyte films. J Am Chem Soc. 1999;121:1978–1979. [Google Scholar]
- 36.Tong WJ, Gao CY. Stable microcapsules assembled stepwise from weak polyelectrolytes followed by thermal crosslinking. Polym Adv Technol. 2005;16:827–833. [Google Scholar]
- 37.Goldenberg DL. Septic arthritis. Lancet. 1998;351:197–202. doi: 10.1016/S0140-6736(97)09522-6. [DOI] [PubMed] [Google Scholar]
- 38.Marriott I, Gray DL, Tranguch SL, et al. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am J Pathol. 2004;164:1399–1406. doi: 10.1016/S0002-9440(10)63226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Jiang B, Boyce B, Lindsey B. Oral presentation, Local IL-12 incorporated in nanocoatings promising for preventing open fracture associated infection. San Francisco, CA. Orthopaedic Research Society (ORS) Annual Meeting; Mar, 2008. [Google Scholar]