Abstract
Breast cancer is the most common type of malignancy diagnosed in women. In the metastatic setting this disease is still uncurable. Taxanes represent an important class of antitumor agents which have proven to be fundamental in the treatment of advanced and early-stage breast cancer, but the clinical advances of taxanes have been limited by their highly hydrophobic molecular status. To overcome this poor water solubility, lipid-based solvents have been used as a vehicle, and new systemic formulations have been developed, mostly for paclitaxel, which are Cremophor-free and increase the circulation time of the drug. ABI-007 is a novel, albumin-bound, 130-nm particle formulation of paclitaxel, free from any kind of solvent. It has been demonstrated to be superior to an equitoxic dose of standard paclitaxel with a significantly lower incidence of toxicities in a large, international, randomized phase III trial. The availability of new drugs, such as Abraxane®, in association with other traditional and non-traditional drugs (new antineoplastic agents and targeted molecules), will give the oncologist many different effective treatment options for patients in this setting.
Keywords: paclitaxel, Abraxane, breast cancer, nanotechnology
Introduction and background
Breast cancer is the most common type of malignancy diagnosed in women1 with more than 180,000 estimated new cases in USA in 2008. Almost one third (32%) of all cancers diagnosed in women are breast cancer.1 In the metastatic setting this disease is as yet incurable, and the main objectives are the palliative prolongation of survival and improvement of quality of life. Other end points are response rate, time to progression, time to treatment failure and others; all of these are surrogate end points without any real advantage for the patients suffering from a metastatic and progressive disease.
Therefore breast cancer continues to be a major health problem although the mortality has decreased during recent years, probably because of clinically improved new treatments for early-stage disease and the availability of new drugs which have shown demonstrable benefit for women also with advanced disease.2,3
Taxanes, and in particular the currently available paclitaxel (Taxol®; Bristol-Myers Squibb Co, Princeton, NJ, USA)4 and docetaxel5 (Taxotere®; Aventis Pharmaceuticals Inc, Bridgewater, NJ, USA), represent an important class of antitumor agents which have proved to be fundamental in the treatment of advanced and early-stage breast cancer. Both these drugs are included in the treatment regimens for adjuvant chemotherapy and are indicated as preferred agents for recurrent and metastatic breast cancer by The National Comprehensive Cancer Network (NCCN) clinical practice guidelines for breast cancer.6
Taxanes are cell cycle-specific agents that bind with high-affinity to microtubules, stabilizing and enhancing tubulin polymerization and suppressing spindle microtubule dynamics.7–11 This effectively inhibits mitosis, motility, and intracellular transport within cancerous cells, leading to apoptotic cell death. Thus these drugs have shown antineoplastic activity against a wide variety of malignancies including also non-small-cell lung cancer and ovarian cancer.12–14
Paclitaxel is a naturally occurring complex product extracted from the bark of the western yew (Taxus brevifolia)15–17 and is widely used for the treatment of breast, lung, and advanced ovarian cancers10,18,19 while docetaxel was originally isolated in a precursor form from the needles of the European yew.20
The clinical advances of taxanes have been limited by their chemical formulation: they are highly hydrophobic molecules. To overcome this poor water solubility, lipid-based solvents are used as a vehicle. Solubility of paclitaxel is enhanced with a mixture of 50:50 Cremophor EL® (CrEL, a non-ionic surfactant polyoxyethylated castor oil; BASF, Florham Park, NJ, USA) and ethanol (Taxol® and generic equivalents),8 while docetaxel is formulated in polysorbate 80 (Tween® 80) and ethanol diluent (Taxotere®). For administration, both drugs must be further diluted 5- to 20-fold with normal saline or 5% dextrose solutions before intravenous infusion.
These solvent-based formulations, however, can be associated with serious and dose-limiting toxicities.
In particular polyoxyethylated castor oil is biologically and pharmacologically active and leaches plasticizers from standard intravenous (iv) tubing releasing di(2-ethylhexyl)phthalate (DEHP). Its infusion produces histamine release with consequent well-described hypersensitivity reactions, including anaphylaxis. In early phase I trials 20% to 40% of unpremedicated patients were affected10,11 by these reactions. Moreover it has been also associated with hyperlipidemia, abnormal lipoprotein patterns, aggregation of erythrocytes, and prolonged, sometimes irreversible sensory neuropathy which may be associated with demyelination and axonal degeneration.25,26,29 CrEL can also cause neutropenia.30
The CrEL–paclitaxel formulation thus requires special infusion sets (tubing and in-line filters) in order to minimize exposure to DEHP. On the other hand, longer infusion times (1 to 24 hours, median time 3 hours) with a large volume of iv fluid and premedication (including dexamethasone, diphenhydramine, and cimetidin) help to reduce the risk of hypersensitivity reactions. Despite these standard precautions hypersensitivity can, however, occur and rarely be fatal.21
Infusion schedules of paclitaxel seem to influence its clinical effectiveness, too. In fact, longer infusions of the drug produce greater clinical efficacy than more rapid injections.18
Hypersensitivity reactions can also occur with polysorbate 80, though to a lesser extent than with CrEL. Polysorbate 80 has also been associated with sometimes severe and irreversible sensory and motor neuropathies.31 Moreover polysorbate 80 can alter membrane fluidity,31 leading to cumulative fluid retention. This unique docetaxel toxicity may be reduced by prophylactic corticosteroids.32,33
Another important point is that CrEL and polysorbate 80 may limit tumor penetration with a negative impact on efficacy. In particular, the formation of large polar micelles of CrEL–paclitaxel in the plasma compartment entraps the drug and can lead to non-linear pharmacokinetics due to decreased drug clearance and decreased volume of distribution. This contributes to a lack of dose-dependent antitumor activity.22–24,28 Also co-administered drugs, such as anthracycline compounds, could be affected by this phenomenon.22
Finally, it has been recently demonstrated in vitro that polyoxyethylated castor oil inhibits endothelial transcytosis of paclitaxel that is mediated by an albumin receptor.27
Therefore during recent years a special effort has been made to avoid these problems. New systemic formulations are being developed, mostly for paclitaxel, which are highly soluble, Cremophor-free and increase the circulation time of the drug.
Nanotechnology
Nanotechnology is a new field of interdisciplinary research that has expanded rapidly and widely over the past 10 years to help overcome problems in medicine. The term “nano-technology” was initially introduced to refer to small-scale applicative materials (1 to 100 nm).34 Today a combination of four criteria have been suggested as essential definitions of a nanotechnology tool:35 the “nano” size of the device; its man-made character; the properties linked to its nanoscopic dimensions; and the ability of “ad hoc” mathematical models to predict its specific behavior.
There are many examples of the development of this discipline, with tools applicable to different diseases. Most well studied are liposomes,36 dendrimers,37,38 super paramagnetic nanoparticulates,39,40 polymer-based platforms,41,42 gold nanoshells,43,44 silicon- and silica-based nanoparticles,45–47 carbon-60 fullerenes,48 and nanocrystals.49
They can be divided into three generations of compound, according to whether or not they were developed to target a specific target which is expressed on the tumor cells or the endothelium.50
Among the “first generation” vectors (not specifically targeted), liposomal drug delivery is certainly the most successfully used in the clinic, as demonstrated by liposomal doxorubicin for breast, ovarian and Kaposi’s sarcoma.51
In particular for liposomal daunorubicin (DaunoXome®), liposomal doxorubicin (D-99, Myocet™), and pegylated liposomal doxorubicin (Doxil® and Caelyx®), the delivery system enables the enhanced permeation and retention (EPR) effect.52,53 In fact the small dimension (<300 nm) enables the drug to accumulate in the tumor mass by crossing passively the fenestrations in the diseased vasculature (passive targeting), avoiding or reducing the perfusion of normal tissue (mostly the heart with a consequent lower cardiotoxic effect).
The “second generation” of therapeutic nanovectors are constructed to succeed in “active targeting “of specific biological molecules of the tumor cell. The aim is to deliver higher drug concentrations to pathologic tissues, sparing the normal ones in order to enhance the effect on the tumor, thereby reducing systemic toxicity. Chemical binding of high affinity ligand (eg, folate or prostate-specific membrane antigen) on the surface of the nanoparticles,54,55 enhance the interaction of nanoparticles with tumor cells, greatly improving biodistribution of nanoparticles.
The so-called “third generation” of nanovectors has been developed56,57 and is based on a multi-stage strategy. The first-stage particle (biodegradable mesoporous silicon microparticles) can circulate within the blood flow. The particle specifically chooses the pathologic endothelium through a mathematically driven recognition of the physico-chemical and geometrical (size, shape) surface features. The second-stage nanoparticles that are loaded, alone or in a group, to the first-stage ones, are released through the mesoporous material in the tumor mass from the site of vascular adhesion (tumor endothelium). These latter nanoparticles are sufficiently small (<20 nm) to easily cross the inter-endothelial junctions and diffuse within the extravascular compartment, addressing all the possible therapies in a more specific manner.
Albumin-bound paclitaxel (ABI-007)
Albumin has a number of characteristics that make it an attractive drug vehicle in oncology. It is a natural carrier of endogenous hydrophobic molecules (such as vitamins, hormones, and other water-insoluble plasma substances), that are bound in a reversible non-covalent manner58– 60
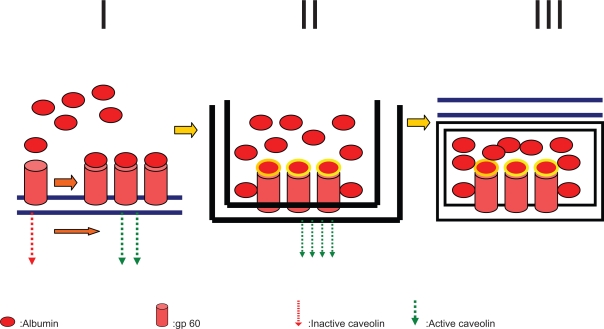
Moreover albumin seems to help endothelial transcytosis of protein-bound and unbound plasma constituents principally through binding to a cell-surface, 60-kDa glycoprotein (gp60) receptor (albondin). gp60 binds to caveolin-1 (an intracellular protein) with subsequent formation of transcytotic vesicles (caveolae) (Figure 1).61–64
Figure 1.
Mechanism by which gp60 protein-albumin complex induces caveolin-1-mediated membrane internalization of plasma components across the vascular endothelium. In detail, panel I shows the bond between albumin receptor (gp60) and albumin which recruits and activates caveolin-1. In panel II caveolin-1 leads to membrane invagination and internalization of free or protein-bound plasma molecules. Panel III: the so-formed caveolae mediate transocytosis and the extravascular deposition of their content.
Also, osteonectin (known as secreted protein acid rich in cysteine [SPARC]) has been shown to bind albumin because of a sequence homology with gp60. SPARC, as caveolin-1, is often present in some neoplasms (breast, lung, and prostate cancer), which could explain why albumin is known to accumulate in some tumors and thus facilitates intratumor accumulation of albumin-bound drugs.58
Albumin-bound (nab-)paclitaxel ABI-007 (Abraxane®; Abraxis BioScience and AstraZeneca) is another example of an EPR-based nanovector application for breast cancer. It represents one of the strategies adopted to overcome the solvent-related problems of paclitaxel and it has been recently approved by the US Food and Drug Administration for pretreated metastatic breast cancer patients.
ABI-007 is a novel, albumin-bound, 130-nm particle formulation of paclitaxel, free from any kind of solvent.66 It is used as a colloidal suspension derived from the lyophilized formulation of paclitaxel and human serum albumin diluted in saline solution (0.9% NaCl). In detail human serum albumin stabilizes the drug particle at an average size of 130 nm which prevents any risk of capillary obstruction and does not necessitate any particular infusion systems or steroid/antihistamine premedication before the infusion.67
Preclinical studies, conducted in athymic mice with human breast cancer, demonstrated that ABI-007 has a higher penetration into tumor cells with an increased anti-tumor activity, compared with an equal dose of standard paclitaxel.67, 68
A phase I clinical study by Ibrahim, conducted on 19 patients with solid tumors and breast cancer, showed a maximum tolerated dose of ABI-007 about 70% higher than that of CrEL paclitaxel formulation (300 mg/m2 for an every 3 weeks regimen). Dose-limiting toxicities were sensory neuropathy, stomatitis, and ocular toxicity (superficial keratopathy and blurred vision at a dose of 375 mg/m2). No patients experienced hypersensitivity reactions. ABI-007 was administered intravenously with no premedication, in shorter infusion periods (30 minutes vs 3 hours for polyoxyethylated castor oil-based paclitaxel) and with a standard infusion device. Moreover, pharmacokinetic parameters showed a linear trend.69
A phase II trial confirmed that ABI-007 has important antitumor activity in patients with metastatic breast cancer. The overall response rate (at a dose of 300 mg/m2 every 3 weeks) was 48% for all patients and 64% for patients in first-line therapy. Time to tumor progression was 26.6 weeks for all patients and 48.1 weeks for patients with confirmed tumor responses; median overall survival was 63.6 weeks. No severe ocular events were noted, and other common taxane-associated toxicities were less frequent and less severe (eg, myelosuppression, peripheral neuropathy, nausea, vomiting, fatigue, arthralgia, myalgia, alopecia).70
In a large international randomized phase III study, equitoxic doses of ABI-007 (260 mg/m2) and polyoxyethylated castor oil-based paclitaxel (175 mg/m2) were compared in 454 patients with metastitic breast cancer. ABI-007 was superior to standard paclitaxel for both overall response rate (33% vs 19%, respectively; p = 0.001) and time to tumor progression (p = 0.006) in all subgroups of patients, but mostly for those receiving the drug as first-line therapy (42% vs 27%, respectively; p = 0.029). Also in this trial the incidence of toxicities was significantly lower in the ABI-007 group than the polyoxyethylated castor oil-based paclitaxel group; in particular, grade 4 neutropenia was lower (10% vs 21%, respectively; p = 0.001) despite the approximately 50% higher dose. On the other hand, grade 3 sensory neuropathy was more frequent in the ABI-007 group (10% vs 2%, respectively; p = 0.001), but it was easily managed.71
The authors explained the increased antitumor activity of ABI-007 by the higher intratumor paclitaxel concentrations (as reported in preclinical studies) and higher dose administered.72
Neymann et al demonstrated also that weekly dosing of ABI-007 is safe and produces minimum toxic adverse effect with objective antitumor responses in patients previously exposed to paclitaxel.72
Future perspectives
Paclitaxel and docetaxel are hydrophobic antineoplastic agents with significant antitumor activity against a broad spectrum of human tumors; in recent years multiple studies have suggested the strategic role of taxanes in the treatment of breast cancer and other studies have evaluated these agents in order to better understand their preclinical and clinical pharmacology. Only 5 years ago, results of a clinical trial73 involving 3121 breast cancer patients showed that 4 cycles of paclitaxel after 4 cycles of doxorubicin and cyclophosphamide, compared with cyclophosphamide alone, were able to improve disease-free survival and overall survival of axillary node-positive patients. According to these data the therapy for axillary node-positive patients changed dramatically, with rapid adoption of paclitaxel plus cyclophosphamide as the new gold standard in clinical practice. In a relatively short time an impressive improvement occurred in the understanding of their mechanism of action, the mechanisms of tumor resistance and the toxicity profiles. Further laboratory and clinical research is necessary to advance the therapy of patients with breast cancer in order to improve the therapeutic index and the safety of this class of agents. In this context the knowledge and investigation of new taxanes constitutes today one of the most exciting strategies for improving the clinical control of breast cancer in association with other cytotoxic agents not usually used in this disease (cisplatin, carboplatin, and irinotecan, and molecularly targeted agents, such as inhibitors of epidermal growth factor receptor, angiogenesis, Src tyrosine kinase, and mTOR).
Abraxane® is a Cremophor-free, albumin-bound paclitaxel that is approved for the treatment of recurrent breast cancer after combination chemotherapy or relapse within 6 months of adjuvant chemotherapy. Abraxane® consists of the active ingredient paclitaxel, which is found in paclitaxel and its generic equivalents. However, in the formulation of Abraxane®, paclitaxel is delivered in a suspension of albumin particles, showing significant advantages to paclitaxel and its generic equivalents, in which polyethoxylated castor oil (Cremophor EL®) is used as the solvent.
Moreover Abraxane® has showed strong antitumor activity when associated with radiotherapy in a supra-additive manner.74 These effects were achieved without increased toxicity to normal tissues (the drug dose was 1.5 times higher than the maximum tolerated dose of traditional paclitaxel). According to these data, there is strong evidence that the association of Abraxane® with radiotherapy would improve the clinical results of taxane-based chemoradiotherapy. In our opinion in the near future this new taxane should be tested in large randomized clinical chemoradiotherapy trials.
Above all it is evident that treatment for breast cancer in future should be tailored to each patient, trying to select treatment strategies for cancer individually based on tumor-expressing factors and/or genomic and proteomic analysis. On the other hand, treatment strategies should be based not only on prognostic and predictive factors and prior adjuvant chemotherapy, but also on safety profile, impact on quality of life and patient preference. We believe that in this context, tolerability and compliance will probably become the most important factors in the future, according to the emerging value of quality of life in cancer care. The availability of new drugs, such as Abraxane®, in association with other traditional and non-traditional drugs (new antineoplastic agents and targeted molecules), will give the oncologist many different effective treatment options for patients in this setting.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Singletary SE. Overview of adjuvant systemic therapy in early stage breast cancer. Surg Clin North Am. 2007;87(2):499–509. doi: 10.1016/j.suc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Guarneri V, Conte PF. The curability of breast cancer and the treatment of advanced disease. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S149–S161. doi: 10.1007/s00259-004-1538-5. [DOI] [PubMed] [Google Scholar]
- 4.Princeton, NJ: Bristol-Myers Squibb Co; 2003. Taxol® (paclitaxel) injection [package insert] [Google Scholar]
- 5.Bridgewater, NJ: Aventis Pharmaceutical Products, Inc; 2003. Taxotere® (docetaxel) Injection Concentrate: Package Insert. [Google Scholar]
- 6.National Comprehensive Cancer Network, Clinical Practice Guidelines in Oncology: Breast Cancer v2 2008. Available at http://www.nccn.org/professionals/physician_gls/default.asp
- 7.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 9.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 10.Rowinsky EK, Donehower RC. Paclitaxel (Taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 11.Chu E, DeVita VT. Sudbury, MA: Jones and Bartlett Publishers; 2003. 2002. Physicians’ Cancer Chemotherapy Drug Manual; p. 284. [Google Scholar]
- 12.Choy H. Taxanes in combined-modality therapy for solid tumors. Oncology (Williston Park) 1999;13:23–38. [PubMed] [Google Scholar]
- 13.Hainsworth JD. Practical aspects of weekly docetaxel administration schedules. Oncologist. 2004;9:538–545. doi: 10.1634/theoncologist.9-5-538. [DOI] [PubMed] [Google Scholar]
- 14.Crown J, O’Leary M. The taxanes: An update. Lancet. 2000;355:1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- 15.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 16.Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr. 1993;15:141–147. [PubMed] [Google Scholar]
- 17.Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Therapeutic response to Taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol. 1986;17:137–142. doi: 10.1007/BF00306742. [DOI] [PubMed] [Google Scholar]
- 18.Spencer CM, Faulds D. Paclitaxel: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:794–847. doi: 10.2165/00003495-199448050-00009. [DOI] [PubMed] [Google Scholar]
- 19.Onetto N, Canett R, Winograd B, et al. Overview of paclitaxel safety. J Natl Cancer Inst Monogr. 1993;15:131–139. [PubMed] [Google Scholar]
- 20.Bissery MC. Preclinical pharmacology of docetaxel. Eur J Cancer. 1995;31A(Suppl 4):S1–S6. doi: 10.1016/0959-8049(95)00357-o. [DOI] [PubMed] [Google Scholar]
- 21.Kloover JS, den Bakker MA, Gelderblom H, et al. Fatal outcome of a hypersensitivity reaction to paclitaxel: A critical review of premedication regimens. Br J Cancer. 2004;90:304–305. doi: 10.1038/sj.bjc.6601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ten Tije AJ, Verweij J, Loos WJ, et al. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 23.Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a Cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136–4243. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 24.Winer E, Berry D, Duggan D, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B Trial 9342. J Clin Oncol. 2004;22:2061–2068. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 26.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from Taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 27.Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 28.Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor elmediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–1457. [PubMed] [Google Scholar]
- 29.Lorenz W, Reimann HJ, Schmal A, et al. Histamine release in dogs by Cremophor EL and its derivatives: Oxethylated oleic acid is the most effective constituent. Agents Actions. 1977;7:63–67. doi: 10.1007/BF01964882. [DOI] [PubMed] [Google Scholar]
- 30.Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- 31.Vaishampayan U, van Zuylen L, Verweij J, Sparreboom A. Role of formulation vehicles in taxane pharmacology. Invest New Drugs. 2001;19:125–141. doi: 10.1023/a:1010618632738. [DOI] [PubMed] [Google Scholar]
- 32.Piccart MJ, Klijn J, Paridaens R, et al. Steroids do reduce the severity and delay the onset of docetaxel (DXT) induced fluid retention: final results of a randomized trial of the EORTC investigational drug branch for breast cancer (IDBBC) Eur J Cancer. 1995;31A(suppl 5):S75. [Google Scholar]
- 33.Piccart MJ, Klijn J, Paridaens R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol. 1997;15:3149–3155. doi: 10.1200/JCO.1997.15.9.3149. [DOI] [PubMed] [Google Scholar]
- 34. http://nano.cancer.gov
- 35.Thei T, Peter D, Eric Drexler JK, et al. Nanotechnology Nat Nanotechnol 200618–10.18654128 [Google Scholar]
- 36.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist. 2003;8(Suppl 2):3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 37.Cloninger MJ. Biological applications of dendrimers. Curr Opin Chem Biol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- 38.Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R, et al. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007;67:8156–8163. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- 39.Oyewumi MO, Mumper RJ. Engineering tumor-targeted gadolinium hexanedione nanoparticles for potential application in neutron capture therapy. Bioconjug Chem. 2002;13:1328–1335. doi: 10.1021/bc025560x. [DOI] [PubMed] [Google Scholar]
- 40.Yan F, Xu H, Anker J, Kopelman R, Ross B, Rehemtulla A, et al. Synthesis and characterization of silica-embedded iron oxide nanoparticles for magnetic resonance imaging. J Nanosci Nanotechnol. 2004;4:72–76. doi: 10.1166/jnn.2004.074. [DOI] [PubMed] [Google Scholar]
- 41.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 42.Green JJ, Chiu E, Leshchiner ES, Shi J, Langer R, Anderson DG. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007;7:874–879. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 45.Yan F, Kopelman R. The embedding of meta-tetra(hydroxyphenyl)-chlorin into silica nanoparticle platforms for photodynamic therapy and their singlet oxygen production and pH-dependent optical properties. Photochem Photobiol. 2003;78:587–591. doi: 10.1562/0031-8655(2003)078<0587:teomis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Martin FJ, Melnik K, West T, Shapiro J, Cohen M, Boiarski AA, et al. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: preliminary findings. Drugs R D. 2005;6:71–81. doi: 10.2165/00126839-200506020-00002. [DOI] [PubMed] [Google Scholar]
- 47.Peng J, He X, Wang K, Tan W, Li H, Xing X, et al. An antisense oligonucleotide carrier based on amino silica nanoparticles for antisense inhibition of cancer cells. Nanomedicine. 2006;2:113–120. doi: 10.1016/j.nano.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yong KT, Qian J, Roy I, Lee HH, Bergey EJ, Tramposch KM, et al. Quantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cells. Nano Lett. 2007;7:761–765. doi: 10.1021/nl063031m. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 51.Di Paolo A. Liposomal anticancer therapy: pharmacokinetic and clinical aspects. J Chemother. 2004;16(Suppl 4):90–93. doi: 10.1179/joc.2004.16.Supplement-1.90. [DOI] [PubMed] [Google Scholar]
- 52.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 53.Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 54.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tasciotti E, Liu XW, Bhavane R, Plant K, Leonard AD, Price BK, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Decuzzi P, Cristofanilli M, et al. Nanotechnology for breast cancer therapy Biomed Microdevices 2008. DOI 10.1007/s10544-008-9209-0 [DOI] [PubMed] [Google Scholar]
- 58.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 59.Purcell M, Neault JF, Tajmir-Riahi HA. Interaction of Taxol with human serum albumin. Biochim Biophys Acta. 2000;1478:61–68. doi: 10.1016/s0167-4838(99)00251-4. [DOI] [PubMed] [Google Scholar]
- 60.Paal K, Muller J, Hegedus L. High affinity binding of paclitaxel to human serum albumin. Eur J Biochem. 2001;268:2187–2191. doi: 10.1046/j.1432-1327.2001.02107.x. [DOI] [PubMed] [Google Scholar]
- 61.John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 62.Minshall RD, Sessa WC, Stan RV, et al. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 63.Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512–L1522. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 64.Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 65.Vorum H. Reversible ligand binding to human serum albumin: Theoretical and clinical aspects. Dan Med Bull. 1999;46:379–399. [PubMed] [Google Scholar]
- 66.Schaumburg, IL: Abraxis Oncology, a Division of American Pharmaceutical Partners, Inc; 2005. Abraxane®: Prescribing information. [Google Scholar]
- 67.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 68.Desai N, Yao Z, Trieu V, et al. Evidence of a novel transporter mechanism for a Cremophorfree, protein-engineered paclitaxel (ABI-007) and enhanced in vivo antitumor activity in an MX-1 human breast tumor xenograft model. 25th Annual San Antonio Breast Conference Symposium; San Antonio, TX. December 11–14.2002. [Google Scholar]
- 69.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 70.Ibrahim NK, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 72.Nyman DW, Campbell KJ, Kristen Long EH, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 73.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 74.Wiedenmann N, Valdecanas D, Hunter N, et al. 130-nm albumin-bound paclitaxel enhances tumor radiocurability and therapeutic gain. Clin Cancer Res. 2007;13(6):1868–1874. doi: 10.1158/1078-0432.CCR-06-2534. [DOI] [PubMed] [Google Scholar]