Abstract
To explore whether the magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) loaded with cisplatin can reverse the diaminedichloro platinum (DDP) resistance to multidrug resistance of ovarian carcinoma cells and to investigate its mechanisms. The SKOV3/DDP cells were divided into DDP treatment (DDP group), MNPs-Fe3O4 treatment (MNPs-Fe3O4 group), DDP + MNPs-Fe3O4 treatment (DDP + MNPs-Fe3O4 group), and control group. After incubation with those conjugates for 48 h, the cytotoxic effects were measured by MTT assay. Apoptosis and the intracellular DDP concentration were investigated by flow cytometry and inductively coupled plasma atomic emission spectroscopy, respectively. The expression of apoptosis associated gene Bcl-2 mRNA was detected by reverse transcription polymerase chain reaction and the expressions of MDR1, lung resistance-related protein (LRP), and P-glycoprotein (P-gp) genes were studied by Western blot. Our results indicated that the 50% inhibition concentration (IC50) of the MNPs-Fe3O4 loaded with DDP was 17.4 μmol/l, while the IC50 was 39.31 μmol/l in DDP groups (p < 0.05); Apoptosis rates of SKOV3/DDP cells increased more than those of DDP groups. Accumulation of intracellular cisplatin in DDP + MNPs-Fe3O4 groups was higher than those in DDP groups (p < 0.05). Moreover, the expression of Bcl-2 mRNA and the protein expressions of MDR1, LRP, and P-gp were decreased when compared with those of DDP groups, respectively. Our results suggest that MNPs-Fe3O4 can reverse the DDP resistance to the ovarian carcinoma cell. The effects may be associated with over-expression of MDR1, LRP, P-gp, and Bcl-2, which can increase the intracellular platinum accumulation and induce the cell apoptosis.
Keywords: magnetic nanoparticles of Fe3O4, multidrug resistance reversal, SKOV3/DDP, MDR1, LRP, P-gp, Bcl-2
Introduction
Ovarian cancer is the leading cause of death from gynecologic malignancies and the fourth most common cause of death due to cancer among women. Treatment for ovarian cancer includes maximal cytoreductive surgery followed by combination chemotherapy. Unfortunately, the initial response rate is not durable; the majority will experience disease recurrence. The poor five-year survival rates seen in epithelial ovarian cancer are at least partly attributed to the development of platinum resistance.1 A major clinical obstacle in cancer therapy is the development of resistance to a multitude of chemotherapeutic agents, a phenomenon called multidrug resistance (MDR). Cisplatin (cis-diaminedichloro platinum [CDDP]) is one of the most potent antitumor agents to display high efficiency in the treatment of ovarian and testicular cancer. CDDP exerts its cytotoxity on ovarian cancer and induces apoptosis.2 Resistance to CDDP remains a major obstacle for the successful treatment of cancer. The mechanisms of CDDP resistance include reduced drug accumulation by changing the profile of uptake/efflux, inactivation of CDDP by increased levels of the intracellular thiols such as glutathione, metallothionein, or other sulfur-containing molecules, increased repair of CDDP adducts, increased tolerance to CDDP adducts, and failure of apoptotic response.
Reduced intracellular CDDP accumulation in resistant cells may ascribe to an inhibition of CDDP uptake, an increase in drug efflux, or both. Multidrug transporter P-glycoprotein (P-gp), which is encoded by the MDR1 gene, is a major organic action transporter in tissues responsible for the excretion of xenobiotics (both drugs and toxins) by the biliary tract and proximal tubule of the kidney.3 P-gp functions as transmembrane drug efflux pumps, decreasing intracellular drug accumulation. Lung resistance-related protein (LRP) is another MDR-related protein.
Human ovarian adenocarcinoma cells (SKOV3), detected by high expression of 110-kDa LRP protein and over-expression of P-gp,4 were incubated in a medium containing DDP to maintain the resistant characteristics. Clinical studies have demonstrated that the increased level of LRP/major vault protein (MVP) may be an important factor contributing to intrinsic CDDP resistance in SKOV3 cells.5
Apoptosis is a key determinant of chemosensitivity in ovarian cancer.6–9 It has been widely accepted that apoptosis is an active gene-directed cellular suicide mechanism and many human genes contribute to the regulation. Among them, Bcl-2 families draw particular attention because it is one of the key factors of the common final pathway involved in the regulation of cell apoptosis. Recent studies showed that over-expression of Bcl-2 caused increased MDR in cancerous cells. In the SKOV3/DDP cell line, over-expression of Bcl-2 was demonstrated but not in SKOV3 cell line alone.10
In spite of the mechanism for MDR being unveiled incompletely, avoiding the appearance of drug resistance and modulation of the MDR has been a great challenge to cancer therapy in the laboratory and clinic. Much effort has been extended to efficient cancer therapies. Unfortunately, many approved treatments for ovarian cancer have accumulative toxicities that leave patients more susceptible to adverse events during subsequent lines of therapy. It is generally accepted that any treatment which could increase the effective concentration of an intracellular chemotherapeutic agent should be pursued.11
Recently, the application of drug-coated polymer nanospheres and nanoparticles to inhibit related MDR has drawn much attention. As a promising drug delivery system, magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) have been studied for some years in our group.11–12 In our previous studies, we prepared MNPs-Fe3O4 loaded with Adriamycin (ADM) and/or tetrandrine (Tet) to reverse MDR of K562/A02 cells. The cytotoxicity test in vitro revealed that MNPs-Fe3O4 exhibited excellent biocompatibility.13–14 In previous work by our group,12 we found that MNPs-Fe3O4 loaded with ADM could enhance the effective accumulation of ADM in K562/A02 cells. It is demonstrated that ADM polymerized with MNPs-Fe3O4 have shown more chemosensitizing activities than those of ADM alone.
In this work, we present an evaluation of the potential of MNPs-Fe3O4 as a candidate agent for treatment of ovarian cancer and to investigate the role of MDR1, LRP, P-gp, and Bcl-2 in intracellular platinum accumulation and cell apoptosis.
Materials and methods
Cell line and cell culture
The MDR human ovarian cancer cell line, SKOV3/DDP, was obtained from the Chinese Academy of Medical Sciences and Peking Union Medical College. The SKOV3/DDP cell line was cultured in a flask in RPMI-1640 containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA), 2 μmol/l l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL, Grand Island, NY) at 37 °C, 5% CO2 with high humidity. For the maintenance of the MDR phenotype, 2 μg/ml cisplatin (Sigma Aldrich, St. Louis, MO) was added to the medium, which was passaged every 2∼3 days using 0.05% trypsin (Gibco BRL) and 0.01% EDTA (Sigma Aldrich).
Preparation of drug-loaded nanoparticles
The synthesis of MNPs-Fe3O4 was prepared by electrochemical deposition under oxidizing conditions (EDOC) based on our previous studies.11–12 Before application in the present experiment, the magnetic nanoparticles were well distributed in RPMI-1640 medium (Sigma Aldrich) with 10% heated inactivated FBS freshly added by using ultrasound treatment in order to obtain MNPs-Fe3O4 colloidal suspension. CDDP conjugated with MNPs-Fe3O4 (DDP + MNPs-Fe3O4) was prepared by mechanical absorption polymerization as previously reported.15 The temperature effect was also investigated and a temperature of 37 °C or 4 °C was chosen for our polymerization process. Briefly, different concentrations (V/V) of 25 μg/ml MNPs-Fe3O4 were respectively added under mechanical stirring to 200 μl of an aqueous medium with 20 μmol/l DDP that, in the final nanoparticle and cell suspension, was 50 μmol/l (pH = 7.4). At different temperatures (37 °C, 4 °C), the overall polymerization process lasted for 24 h. The RPMI-1640 medium was regarded as blank control and cells with no intervention were negative control.
MTT assay
For cell growth and viability assays, 5 × 104/ml cells were plated into Six-well flat-bottomed plates (Costar, Charlotte, NC), respectively. Different concentrations of DDP, MNPs-Fe3O4, and MNPs-Fe3O4 loaded with DDP or RPMI-1640 were added into these cells and cultured to measure their growth and viability. Based on these results, different concentrations of DDP and MNPs-Fe3O4 were combined to find the best combination concentration which can best kill tumor cells. After incubation for 48 h, 20 μl MTT solution (5 mg/ml)was added into each well at 37 °C in the dark for at least 4 h. Formazan crystals were solubilized in 200 μl dimethyl sulfoxide (DMSO) in every well and the reduction of MTT was quantified by absorbance at 540 nm using a plate reader (Model 550; Bio-Rad, Tokyo, Japan). The inhibition ratio of cells was determined as follows (1-A of tests cells/A of black control) × 100%. Each assay was repeated at least three times.
Annexin V–PI assays for apoptosis
As described before, cells were incubated and harvested. For Annexin V–propidium iodide (PI) assays, cells were stained and evaluated for apoptosis by flow cytometry according to the manufacturer’s protocol. Briefly, 1 × 106 cells were stained with 5 μl Annexin V–fluorescein isothiocyanate (FITC) and 10 μl PI (5 μg/ml) in 1 × binding buffer (1.0 mmol/L HEPES [4-(2-hydroxyethyl)-1-piperazineethanesul-fonic acid], pH = 7.4, 140 mmol/L NaOH, 2.5 mmol/L CaCl2) for 20 min at room temperature in the dark. The apoptotic cells were determined using flow cytometry (FACSCalibur™; Becton-Dickinson, Franklin Lakes, NJ).
Measurement of intracellular DDP accumulation
Briefly, cells (5 × 104/ml) were incubated with DDP, MNPs-Fe3O4, MNPs-Fe3O4 loaded with DDP or RPMI-1640 medium for 48 h at 37 °C in humidified 5% CO2 atmosphere. After incubation, an aliquot was taken, washed twice with 2 mol/l iced-cold isotonic buffer, the pellet was resuspended in 33% HNO3, and then the DDP content was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) in SPECTRO GENESIS SOP (SPECTRO Analytical Instruments, Marble Falls, TX) equipment.
Reverse transcription polymerase chain reaction
As described before, cells seeded at a density of 5 × 104 cells/well in 6-well plates were treated with one of the following treatments: (a) DDP + MNPs-Fe3O4, (b) DDP, (c) MNPs-Fe3O4, (d) RPMI-1640 for 48 h, and harvested. Cells were dissolved in TRIzol reagent (Gibco BRL). Total RNA was extracted according to the manufacturer’s instructions. The RNA A260/A280 ratios were between 1.6 and 1.8. The primers for human Bcl-2 (forward: 5’-GGGAGAACAGGGTAC GATAA-3’; reverse: 5’-CCACCGAACT CAAAGAAGG-3’), and β-actin (forward: 5’-TATGACTTAGTTGC GTTA-CACC-3’; reverse: 5’-CCTTCAC CGTTCCAGTTT-3’) were used. The amplified polymerase chain reaction (PCR) products were 452 bp and 155 bp, respectively. The copy number for each sample was calculated and all the data were normalized to β-actin. Briefly, cDNA was synthesized from 1 μg of total cellular RNA using TaKaRa RNA PCR kit (AMV) (Ver. 3.0; Dalian, China). The newly synthesized cDNA was amplified by PCR (TaKaRa). The PCR conditions were 95 °C for 3 min and 35 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min. Control amplifications were conducted either without reverse transcription (RT) or without RNA. Following PCR amplification, the reaction products were electrophoresed at 100 V on 1.5% agarose gels with 0.5 μg/ml ethidium bromide (Sigma Aldrich)16 and PCR fragments were visualized by UV illumination (GDS7500 Gel; UVP Inc., Upland, CA, USA). Densitometric analysis was performed using the electrophoresis image analysis system Smart View 2000 software (Furi, Shanghai, China).
Western blot
SKOV3/DDP cells were treated with DDP, MNPs-Fe3O4, DDP + MNPs-Fe3O4, and RPMI-1640 medium for 48 h, respectively. Whole cell extracts were harvested on ice, washed in phosphate-buffered saline (PBS), and lysed in 100 ml of lysis buffer (30 mM Tris, pH 7.5, 150 mM NaCl, 1 mM PMSF, 1 mM Na3VO4, 1% Nonidet P-40, and 10% glycerol) for 30 min at 48 °C, then centrifuged at 14,000 r/min for 10 min. The supernatant was collected and the amount of protein was measured using Bio-Rad protein assay (Bio-Rad, Hercules, CA). Equals amount (25 mg) of protein from each sample was separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels using modified radio immunoprecipitation assay (RIPA) buffer and transferred the proteins to a nitrocellulose membrane (Bio-Rad). Western blotting was performed with a 1:500–1:1,000 dilutions of monoclonal antibodies against either anti-human P-gp (NeoMarkers, Fremont, CA), MDR1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), LRP (Santa Cruz Biotechnology Inc.) or β-action anti-body (Santa Cruz Biotechnology Inc.) in 5% nonfat dry milk, followed by peroxidase-conjugated IgG-HRP (Santa Cruz Biotechnology Inc.) as a secondary antibody (peroxidase-conjugated swine anti-rabbit).The blots were developed by enhanced chemiluminescence (ECL system, Amersham, UK).
Statistical analysis
All data were presented as means ± standard deviation in triplicate. All analyses were performed with Statistical Package for Social Science (SPPS Release 11.5; SPSS Inc., IL, USA). Differences were evaluated using Student’s t-test or paired t-test and considered statistically significant for values of p < 0.05.
Results
Cell growth and inhibition
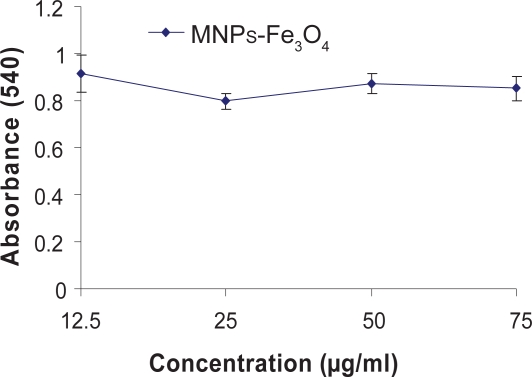
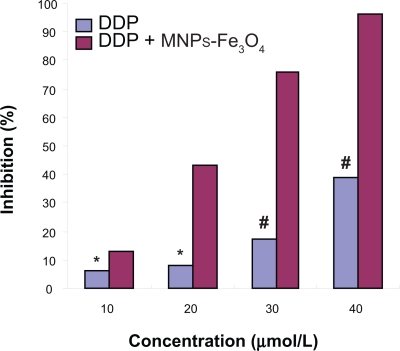
The MTT assay revealed that the growth and inhibition of DDP, MNPs-Fe3O4, or DDP + MNPs-Fe3O4 to SKOV3/DDP cells (as shown in Figures 1, 2). It was indicated that MNPs-Fe3O4 alone could hardly inhibit SKOV3/DDP cell proliferation when their doses were between 12.5 μg to 75 μg (p > 0.05) (Figure 1). SKOV3/DDP cells were significantly resistant to DDP when the concentration of DDP was not less than 10 μmol/l. The inhibition of SKOV3/DDP cells was in a dose-dependent manner and the 50% inhibition concentration (IC50) was 39.31 μmol/l. Furthermore, we found the optimum concentration of MNPs-Fe3O4 was 25 μg/ml from a different concentration of MNPs-Fe3O4 loaded with a different concentration of DDP. The inhibition of SKOV3/DDP cells in MNPs-Fe3O4 loaded with DDP group was significantly higher than that in DDP group and the IC50 of MNPs-Fe3O4 loaded with DDP was 17.4 μmol/l, which was 2.259-fold lower than that in the DDP groups (p < 0.05), suggesting that the reverse rate was 2.259 (Figure 2).
Figure 1.
Growth inhibition rates of SKOV3/DDP cells incubating with different concentrations of MNPs-Fe3O4 for 48 h by MTT assay.
Note: p > 0.05, when the concentration of MNPs-Fe3O4 is less than 75 μg (signal factor analysis of variance).
Figure 2.
Growth inhibition rates of SKOV3/DDP cells incubating with different concentrations of DDP and DDP + MNPs-Fe3O4 for 48 h by MTT assay, respectively.
Notes: p < 0.05, compared to DDP without MNPs-Fe3O4 treated-SKOV3/DDP cells (signal factor analysis of variance); *p > 0.05, 10 μmol/l DDP compared to 20 μmol/l DDP (signal factor analysis of variance); #p < 0.05, when the concentration of DDP is not less than 20 μmol/l (signal factor analysis of variance).
Annexin V–PI assays for apoptosis
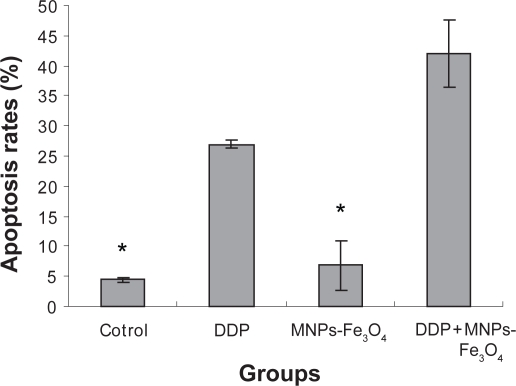
Annexin V–PI double-staining assays demonstrated that MNPs-Fe3O4 could hardly induce apoptosis of SKOV3/DDP cells compared to control group (p > 0.05). Only (4.4% ± 0.36%) apoptosis of SKOV3/DDP cells were observed under MNPs-Fe3O4. When DDP was loaded with MNPs-Fe3O4, apoptosis rates of SKOV3/DDP cells in the DDP+ MNPs-Fe3O4 group was significantly higher than that in DDP group alone (p < 0.05). The apoptosis rates were (42.07% ± 5.55%) and (26.93% ± 4.11%), respectively. There were all statistically significant when compared with (6.83% ± 0.64%) in the control group (p < 0.05) (Figure 3).
Figure 3.
Apoptosis of SKOV3/DDP cells incubating with 20 μmol/l DDP or DDP + 25μg/ml MNPs-Fe3O4 and Fe3O4-MNPs alone.
Notes: p < 0.05, compared to control with or without MNPs-Fe3O4-treated SKOV3/DDP cells (signal factor analysis of variance); *p > 0.05, MNPs-Fe3O4 alone compared to control (signal factor analysis of variance).
Intracellular DDP concentration
The intracellular DDP concentration in cells was explored by ICP-AES analysis when the SKOV3/DDP cells were incubated for 48 h.The intracellular DDP concentration of SKOV3/DDP cells in DDP with or without MNPs-Fe3O4 groups was founded to increase by (0.057 ± 0.003 μmol/l) and (0.074 ± 0.006 μmol/l), respectively. There were statistically significant differences between them when compared (p < 0.05) (Table 1).
Table 1.
Intracellular accumulation of DDP in SKOV3/DDP cells incubating with DDP or MNPs-Fe3O4 for 48 h
| Group | Intracellular DDP (μ mol/l) |
|---|---|
| DDP | 0.057 ± 0.003 |
| DDP + MNPs-Fe3O4 | 0.074 ± 0.006* |
Note: *p < 0.05, compared with DPP (signal factor analysis of variance).
RT-PCR for Bcl-2 mRNA
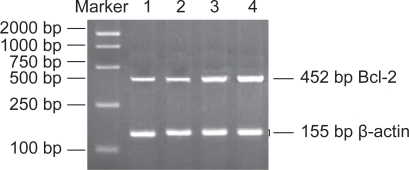
Based on computer-assisted image analysis, it appeared that single use of MNPs-Fe3O4 had no obvious effect on Bcl-2 mRNA in SKOV3/DDP cells (p > 0.05), but DDP + MNPs-Fe3O4 reinforced downregulation of Bcl-2 mRNA content significantly (p < 0.05), causing a 70-fold more decrease in Bcl-2 mRNA level and single use of DDP had less downregulation than the same concentration of DDP polymerized with MNPs-Fe3O4 (p < 0.05), suggesting that MNPs-Fe3O4 can enhance the action of DDP in downregulation of Bcl-2 mRNA in SKOV3/DDP cells (Figure 4).
Figure 4.
The expression of Bcl-2 mRNA in SKOV3/DDP cells by different interferences for 48 h. Lane 4 was the negative control group; lane 3 was the MNPs-Fe3O4 group; lane 2 was the DDP group; and lane 1 was the DDP + MNPs-Fe3O4 group.
Western blot
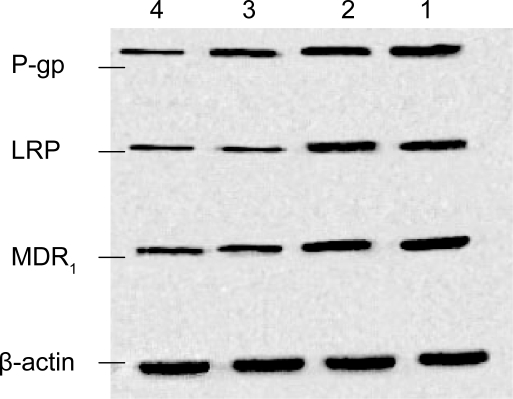
In order to examine the expression of the P-gp, LRP, and MDR1, we next performed Western blot analysis on whole cell protein extracts from cells treated for 48 h as described previously (Figure 5). Based on computer-assisted image analysis, it appeared that MNPs-Fe3O4 themselves could not lower the protein of P-gp, LRP, and MDR1 in SKOV3/DDP cells (p > 0.05), but they could downregulate the expression of P-gp, LRP, and MDR1 protein levels when loaded with DDP. We also found that the expressions of protein in DDP + MNPs-Fe3O4 group were lower than those in DDP-alone groups (p < 0.05), suggesting that MNPs-Fe3O4 can enhance the accumulation of DDP in SKOV3/DDP cells.
Figure 5.
The protein of P-gp, LRP, and MDR1 in SKOV3/DDP cells by different interferences for 48 h. Lane 1 was the negative control group; lane 2 was the MNPs-Fe3O4 group; lane 3 was the DDP group; and lane 4 was the DDP+ MNPs-Fe3O4 group. Blots were stripped and re-probed with β-actin to indicate relative amounts of protein loaded.
Discussion
It is well known that intrinsic and/or acquired chemoresistance is the major obstacle for successful treatment of patients with ovarian carcinoma.17 A low five-year overall survival rate of only 53% for woman suffering from ovarian cancer is related to the development of resistance of tumor cells to standard chemotherapeutic agents.18–19 Cisplatin was the first platinum-containing compound introduced into therapeutic trials for ovarian cancer. Resistance to cisplatin-based chemotherapy is a major cause of treatment failure in human ovarian cancer. Both intrinsic and acquired resistance to cisplatin occurs frequently.
Many studies have shown that the development of drug resistance of cancer cells is related to inhibition of cell apoptosis. Moreover, the apoptotic rate increased with the concentration of cisplatin, suggesting that cisplatin induced apoptosis in a dose-dependent fashion. So, the first step to successful reversal drug resistance is to increase cisplatin concentrations in the ovarian cancer cell. Nanotechnology and nanoscience have developed rapidly during the last few decades. The most important clinical application of nanotechnology is probably in pharmaceutical development.20 As demonstrated previously and as observed in this study, the intracellular cisplatin levels in DDP + MNPs-Fe3O4 groups were found to be higher than those of DDP-alone groups, suggesting that MNPs-Fe3O4 can increase cisplatin concentration in SKOV3/DDP cells and enhance the effective accumulation of anticancer agents in resistant cancer cells. We also found that the apoptosis rates of SKOV3/DDP cells were increased than those of DDP groups. All of these suggested that MNPs-Fe3O4 could reverse the DDP resistance to the ovarian carcinoma cell (SKOV3/DDP), and the effects are ascribed to increase the intracellular DDP accumulation, and to induce appoptosis, which were demonstrated by flow cytometry.
As one of the most commonly used magnetic nanoparticles, MNPs-Fe3O4 are obtained more easily than other MNPs and they may aggregate in water or tissue fluid spontaneously with good biocompatibility and low toxicity,21–23 which suggests MNPs-Fe3O4 were safe for the use of drug carriers. Moreover, MNPs-Fe3O4 are found readily to interact with proteins.24 The mechanism of MNPs-Fe3O4 in increasing the effective intracelluar concentration of DDP is still unclear. Nanoparticles loaded with an anticancer drug could readily approach the cell membrane, leading to drug concentrations at the cell surface higher than those obtained with the same amount of drug diluted in a culture medium, leading in turn to higher intracellular drug concentration.25–26 Not only have MNPs-Fe3O4 the ability to block P-gp function, but they also have potency in aggregation and drug-capsulation.27–28 The expression of P-gp and MDR-related proteins are associated with a poor prognosis in patients with ovarian cancer. P-gp and MDR protein (MRP) are known to be associated with MDR. P-gp, an integral membrane glycoprotein with a molecular mass of 170 kd, has been postulated to function as a pump to remove hydrophobic anticancer agents from drug-resistant cells. MRP, a 190-kd 1531-amino-acid membrane glycoprotein, is over-expressed in most non-P-gp-mediated multidrug resistant cell lines. MRP and P-gp are members of the adenosine triphosphate (ATP)-binding cassette superfamily of membrane transporter proteins. It is generally accepted that increased levels of P-gp expression have been observed in some tumors at the time of relapse after initial chemotherapy. The 110-kd LRP frequently is over-expressed in multidrug resistant cells. For occurrence of MDR, a key role was P-gp, which effluxes chemotherapeutic agents out through an ATP-dependent transport leading to intracellular deficient drug concentrations.29 Zhang and colleagues demonstrated that the SKOV3 cell line did not express P-gp, but over-expressed LRP/MVP. However, the SKOV3/DDP cell line has been proved to be resistant to CDDP and is characterized with high expression of P-gp, MRR1, and LRP.30,31 It was also demonstrated that down-regulation of MDR1 gene expression or inhibiting the function of MDR1 has a reversal effect on SKOV3/DDP cells, which was resistant to DDP.32,31 Our study demonstrated that MNPs-Fe3O4 themselves could not lower the protein of P-gp, LRP, and MDR1 in SKOV3/DDP cells (p > 0.05), but they could downregulate the expression of P-gp, LRP, and MDR1 protein levels when loaded with DDP. The expressions of proteins in the DDP + MNPs-Fe3O4 group were also found to be lower than those in DDP-alone groups, suggesting that the mechanism of increasing the DDP concentration in SKOV3/DDP cell line may be associated with the downregulation of expression of MDR1 gene, MRP1, and LRP.
To further evaluate the role of Bcl-2 family proteins in reversal effect of MNPs-Fe3O4 loaded with cisplatin on ovarian carcinoma cells, studies were performed using SKOV3 as model ovarian cancer cell lines. The Bcl-2 family proteins represent another class of key regulators of cell death and survival. Recent studies have shown that therapeutic agents may target proteins of the Bcl-2 family, affect mitochondrial outer membrane permeabilization, and initiate an apoptotic cell death.33 The dormant period of cell increased and enhanced expression of Fas, Fas-L, and Bcl-2 protein may be the partial mechanisms of the cisplatin-resistance of SKOV3/DDP cells. Li and colleagues found that expression of Bcl-2 protein in SKOV3/DDP cells was higher than that in SKOV3 cells.16 A more pronounced difference of Bcl-2 expression and over-expressing of Bcl-2 were not insensitive to chemotherapy and may have been observed in SKOV3/DDP cells had the time been lengthened as was observed in other studies.16 In contrast, in our study, there was only a slight effect on Bcl-2 mRNA in the enhanced group, but DDP + MNPs-Fe3O4 reinforced downregulation of Bcl-2 mRNA content significantly (p < 0.05), causing a 70-fold decrease in Bcl-2 mRNA level and single use of DDP had less downregulation than the same concentration of DDP polymerized with MNPs-Fe3O4 (p < 0.05), suggesting that MNPs-Fe3O4 can enhance the action of DDP in downregulation of Bcl-2 mRNA in SKOV3/DDP cells.
Conclusions
All these results suggest that MNPs-Fe3O4 can reverse DDP resistance to the ovarian carcinoma cell, and the mechanism of chemoresistance may be associated with the downregulation of MDR1, P-gp, and LRP gene expression, which revealed a potential application for reversal of MDR in human ovarian cancer cells. The results presented here warrant further investigation in animal tumor models and eventually in cancer patients.
Acknowledgments
This work was supported by National 863 Program Emphasis Project: Nanometer Biology Ware Study (No 007AA0222007), National Natural Science Foundation of PR China (No 30740062 and No 30872970) and High School Doctor Subject Special-purpose Scientific Research Foundation (No 20070286042).
References
- 1.Kurzeder C, Sauer G, Deissler H. Molecular targets of ovarian carcinomas with acquired resistance to platinum/taxane chemotherapy. Curr Cancer Drug Targets. 2006;6:207–227. doi: 10.2174/156800906776842975. [DOI] [PubMed] [Google Scholar]
- 2.Meyn RE, Stephens LC, Hunter MR, et al. Kinetics of cisplatin-induced apoptosis in murine mammary and ovarian adenocarcinomas. Int J Cancer. 1995;60:725–729. doi: 10.1002/ijc.2910600526. [DOI] [PubMed] [Google Scholar]
- 3.Sikic BI, Fisher GA, Lum BL, et al. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40:S13–S19. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinao O, Yoshihiro O, Yuji B, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: Their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer Sci. 2007;98:1020–1026. doi: 10.1111/j.1349-7006.2007.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath V, Blanarova O, Svihalkova-Sindlerova L, et al. Platinum (IV) complex with adamantylamine overcomes intrinsic resistance to cisplatin in ovarian cancer cells. Gynecol Oncol. 2006;102:32–40. doi: 10.1016/j.ygyno.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Kigawa J, Minagawa Y, et al. Chemosensistive and p53-dependent apoptosis in epithelial ovarian carcinoma. Cancer. 1999;86:1307–1313. [PubMed] [Google Scholar]
- 7.Sasaki H, Sheng Y, Kotsuji F, et al. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–5666. [PubMed] [Google Scholar]
- 8.Fraser M, Leung BM, Yan X, et al. P53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemo-resistance in human ovarian cancer cells. Cancer Res. 2003;63:7081–7088. [PubMed] [Google Scholar]
- 9.Dan HC, Sun M, Kaneko S, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 10.Villedieu M, Louis MH, Dutoit S, et al. Absence of Bcl-X-L down-regulation in response to cisplatin is associated with chemoresistance in ovarian carcinoma cells. Gynecol Oncol. 2007;105:31–34. doi: 10.1016/j.ygyno.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Dai YY, Chen BA, Wang XM, et al. Synergistic effect of magnetic nanopartical Fe3O4. Au and daunomycin on K562/A02. Southeast Univ (Med Sci Edu) 2007;26:157–60. [Google Scholar]
- 12.Chen BA, Sun Q, Wang XM, et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/AO2 leukemic cells. Int J Nanomedicine. 2008;3:277–286. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng SS, Mu L, Win KY, et al. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem. 2004;11:412–424. doi: 10.2174/0929867043455909. [DOI] [PubMed] [Google Scholar]
- 14.Cheng F, Su C, Yang Y, et al. Characterization of aqucous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26:729–738. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Wang J, Shen X, et al. Preparation of magnetic polybutylcyanoacrylate nanosheres encapsulated with aclacinomycin A and its effect on gastric tumor. World J Gastroenterol. 2004;10:2010–2013. doi: 10.3748/wjg.v10.i14.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li BH, Hu DX, Zhao LY. Investigate the molecular mechanism of the cisplatin-resistant with human epithelial ovarian cancer cell line SKOV3 and SKOV3 /DDP cultured in vitro. Zhong Hua Fu You Baojian. 2005;20:1195–1197. [Google Scholar]
- 17.Dalton WS. Mechanisms of drug resistance in hematologic malignancies. Semin Hematol. 1997;34:3–8. [PubMed] [Google Scholar]
- 18.Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. Int J Gynecol Obstet. 2003;83:135–166. doi: 10.1016/s0020-7292(03)90118-4. [DOI] [PubMed] [Google Scholar]
- 19.Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm. 2007;340:117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- 20.Thrall JH. Nanotechnology and medicine. Radiology. 2004;230:315–318. doi: 10.1148/radiol.2302031698. [DOI] [PubMed] [Google Scholar]
- 21.Dresco PA, Zaitsev VS, Gambino RJ, et al. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir. 1999;15:1945–1951. [Google Scholar]
- 22.Willner I, Willner B. Functional nanoparticle architectures for sensoric, optoelectronic and bioelectronic applications. Pure Appl Chem. 2002;74:1773–1783. [Google Scholar]
- 23.Thomas K, Sayre P. Research strategies for safety evaluation of nano-materials, Part I: Evaluating the human health implications of exposure to nanoscale materials. Toxicol Sci. 2005;87:316–321. doi: 10.1093/toxsci/kfi270. [DOI] [PubMed] [Google Scholar]
- 24.Chen DH, Liao MH. Preparation and characterization of YADH-bound magnetic nanoparticles. J Mol Catal B. 2002;16:283–291. [Google Scholar]
- 25.Hu Y, Jarillon S, Dubernet C, et al. On the mechnism of action of doxorubicin encapsulation in nanospheres for the reversal of mutidrugresistance. Cancer Chemother Pharmacol. 1996;37:556–560. doi: 10.1007/s002800050428. [DOI] [PubMed] [Google Scholar]
- 26.Soma CE, Dubernet C, Bentolila D, et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin Ain polyalkyleyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 27.Wong HL, Bendayan R, Rauth AM, et al. A mechaninstic study of enhanced doxorubicin uptake and potention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticles system. J Pharmacol Exp Ther. 2006;317:1372–1381. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 28.Wong HL, Rauth AM, Bendayan R, et al. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23:1574–1585. doi: 10.1007/s11095-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou CG, Shen P, Cheng YY, et al. Quantitative study of the drug efflux kinetics from sensitive and MDR human breast cancer cells. Biochim Biophys Acta. 2007;7:1011–1020. doi: 10.1016/j.bbagen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HR, Chen J, Li HJ. Establishment of drug-resistant ovarian cancer cell strain to cisplatin and expression of resistance related genes. China Tropical Medicine. 2006;6:2132–2134. [Google Scholar]
- 31.Wei SJ, Li HY, Shi J, et al. Recombin-antmutant human-TNF in reversing drug-resistance in ovarian cancer cell lineSKOV3 /DDP and the related mechanism. Chinese Journal of Cancer Biotherapy. 2008;15:150–154. [Google Scholar]
- 32.Ludwig JA, Szakács G, Martin SE, et al. Selective toxicity of NSE73306 inmdr1-positive cells a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627–6631. doi: 10.1038/sj.onc.1204087. [DOI] [PubMed] [Google Scholar]