Abstract
The purpose of this study is to assess the clinical value of spatiotemporal source analysis for analyzing ictal magnetoencephalography (MEG). Ictal MEG and simultaneous scalp EEG was recorded in five patients with medically intractable frontal lobe epilepsy. Dynamic statistical parametric maps (dSPMs) were calculated at the peak of early ictal spikes for the purpose of estimating the spatiotemporal cortical source distribution. DSPM solutions were mapped onto a cortical surface, which was derived from each patient's MRI. Equivalent current dipoles (ECDs) were calculated using a single-dipole model for comparison with dSPMs. In all patients, dSPMs tended to have a localized activation, consistent with the clinically-determined ictal onset zone, whereas most ECDs were considered to be inappropriate sources according to their goodness-of-fit values. Analyzing ictal MEG spikes by using dSPMs may provide useful information in presurgical evaluation of epilepsy.
1. Introduction
Investigating ictal onset zones is essentially important for characterizing epileptic seizures, which show various clinical symptoms. In presurgical evaluation of epilepsy, ictal onset zones are believed to be highly correlated with the epileptogenic zone (Lüders and Awad, 1991). The most precise localization of the ictal onset zone is traditionally obtained by invasive intracranial EEG recording (Rosenow and Lüders, 2001), however, non-invasive methods also may provide the localization in addition to or in lieu of intracranial EEG.
Magnetoencephalography (MEG) is a non-invasive method of recording magnetic fields emanating from the brain. It has been mainly used for source localization of interictal epileptic activities, whereas there are several studies of successful source localization of ictal MEG with a single-dipole model (Sutherling et al., 1987; Stefan et al., 1992; Eliashiv et al., 2002; Tilz et al., 2002; Assaf et al., 2003). However, ictal MEG occasionally failed to localize the ictal onset zone (Eliashiv et al., 2002; Tilz et al., 2002). Major possible explanations of this failure are considered (1) The early spikes of an ictal MEG may have low signal-to-noise ratio, lowering the localization precision, (2) Source localization using the single-dipole modeling may be misleading (Kobayashi, et al., 2005), by assuming that a single source can explain all the magnetic fields from the brain.
Dynamic statistical parametric maps (dSPMs) have recently been introduced for evoked MEG analysis (Dale et al., 2000). DSPM provides spatiotemporal source distribution with millisecond temporal resolution by imposing anatomical information about the cortical surface derived from magnetic resonance images (MRI) and noise normalization of the minimum norm estimate. Clinical studies of dSPM in patients with partial epilepsy suggest that it has advantages over a single-dipole model in analyzing interictal MEG spikes (Shiraishi et al., 2005a,b).
The purpose of the present study was to assess the possible localizing value of using dSPM-derived spatiotemporal maps in the evaluation of ictal MEG in patients with intractable frontal lobe epilepsy.
2. Patients and Methods
2.1. Patients
We retrospectively reviewed the MEG data from five patients with medically intractable frontal lobe epilepsy, who had clinical seizures during MEG recording. All patients underwent evaluation of their epilepsy, including medical history, long-term video-scalp EEG monitoring and structural MRI at Massachusetts General Hospital or Children's Hospital Boston. The ictal onset zone of each patient was determined clinically by considering the results of the evaluation prior to obtaining the MEG. Written informed consent was obtained before each data acquisition for the MEG research, which was approved by the institutional review board.
2.2. Data acquisition
MEG recordings were performed at Athinoula A. Martinos Center for Biomedical Imaging between 2005 and 2007. The details of simultaneous MEG and EEG recording have previously been described (Knake et al., 2006). MEG was recorded with a 306-channel (204 planar gradiometers and 102 magnetometers), whole-head MEG system (Elekta-Neuromag, Helsinki, Finland) in a magnetically shielded chamber. Scalp EEG was simultaneously recorded with a non-magnetic 70-channel electrode cap, based on the 10-10 system. The patients were recorded in supine position with a sampling rate of 600 Hz. Data were digitally filtered at a bandpass width 0.5-40 Hz in off-line data analysis. In all patients, high-resolution 3T MRI MPRAGE pulse sequences (TE 3.37 ms, TR 2000 ms, voxel size 1 × 1 × 1 mm3) were acquired with a high-resolution 3T MRI scanner (TIM TRIO, Siemens AG, Erlangen, Germany).
2.3. Data analysis
MEG data were visually examined, and segments containing epileptic discharges were selected from the early part of ictal MEG. For dSPM, an anatomically constrained linear estimation approach was applied, assuming the sources are distributed in the cerebral cortex (Dale and Sereno, 1993). The forward solution, which models the signal pattern generated by a unit dipole at each location on the cortical surface, was calculated by using a linear collocation single-layer boundary-element method (BEM) with the inner skull boundary approach, for each patient (Hamäläinen et al., 1989; Oostendorp et al., 1989). The surface was tessellated with 5120 triangles, providing adequate numerical accuracy (Crouzeix et al., 1999; Fuchs et al., 2001; Tarkiainen et al., 2003; de Jongh et al., 2005). The activation at each cortical location was estimated by using a noise-normalized minimum norm estimate (Dale et al., 2000; Liu et al., 2002), which results in an F-distributed estimate of the cortical current (Dale et al., 2000). Thus, dSPM identifies the locations of statistically increased current-dipolar strength relative to the noise level. The dSPM maps calculated for the peak of each spike were superimposed on inflated geometrical representations of cortical surfaces, which were reconstructed from the individual patients' MRI (Dale et al., 1999; Fischl et al., 1999; Fischl et al., 2001).
Equivalent current dipoles (ECDs) were also calculated at the peak of each spike using a single-dipole model without selecting a region of interest (i.e., all of the 306 sensors were used for the analysis). We estimated the numbers and locations of ECDs with a goodness of fit (GOF) better than 60%, 70% and 80%, separately.
In three patients we also analyzed interictal MEG spikes with both dSPM and single-dipole modeling. The ECDs with GOF > 70% were considered adequate as possible cortical sources.
3. Results
3.1. Overall results
Table 1 gives an overview of the clinical profiles of all patients. Four male and one female patients, 14-31 years old (mean age: 19.2 years) were studied. All clinical seizures occurred spontaneously during MEG recording, showing clinical symptoms consistent with the habitual seizures of each patient.
Table 1.
Clinical data of the patients.
| Patient | Age at seizure onset / MEG | Sex | Seizure type | Scalp EEG (interictal / ictal) | MRI | Clinically-determined ictal onset zone |
|---|---|---|---|---|---|---|
| 1 | 5 / 15 | M | CPS with unresponsiveness, sometimes accompanied by right hemi-convulsion | LF, RF / LF | Multiple subcortical lesions in LF lobe | LF lobe |
| 2 | 13 / 14 | M | EPC with left facial twitching | NA / RF | Atrophy of RF, RT lobes, insula | RF lobe |
| 3 | 8 / 15 | M | CPS with head and eye deviation (left or right) | LF, RF / LF, RF | Normal | LF and/or RF lobe(s) |
| 4 | 2 / 31 | M | Nocturnal CPS with abrupt arousal and non-patterned limb movements | NA / LF, RF | Normal | LF and/or RF lobe(s) |
| 5 | 12 / 21 | F | Nocturnal CPS with abrupt arousal and random body movement | LF, RF / RF | RF cortical dysplasia | RF lobe |
CPS: complex partial seizure, EPC: epilepsia partialis continua, LF: left frontal, RF: right frontal, NA: not available
The seizures of Patient 2 were characterized by frequent facial twitching of the left side. Ictal EEG started with rhythmic spikes or spike-and-slow-wave complexes (sp-w-c) in the right frontal area, spreading bilaterally in the latter part. MRI showed an atrophy of the right frontal and temporal lobes. Ictal onset zone was suggested to be in the right frontal lobe, based on semiology, ictal scalp EEG, and MRI. The patient underwent a hemispherectomy of the right side, and has been seizure-free for six months post surgery. Patient 3 showed two types of seizures. They were accompanied by head and eye deviation to the left or right, and ictal EEG showed rhythmic sp-w-c bursts dominantly in the right or left frontal area, respectively. Interictal EEG showed frequent sp-w-c in the left and the right frontal area. These seizures were considered to start from the left or right frontal lobe, corresponding to the two types of seizures. The seizure occurred during MEG recording was consistent with the former type, which was considered to arise from the right frontal lobe. The seizures of Patient 4 were accompanied by hypermotor movements of the body, typically seen during sleep. Ictal EEG showed mainly movement artifacts, preceded by small spikes in the bilateral frontal area. The semiology and ictal EEG were consistent with those of frontal lobe epilepsy. The seizure localization of Patient 1 and 5 are described below as illustrative cases.
Table 2 summarizes the results of MEG analyses. In each patient, 4-11 early ictal spikes were obtained. All dSPMs localized activity consistent with the clinically-determined ictal onset zone at a lobar level in four patients (Patient 1, 3, 4 and 5). In one patient (Patient 2), all dSPMs showed a localized activation at the same location as the ictal onset zone, with the exception of one dSPM showing larger activation areas.
Table 2.
Results of ictal MEG analyses by using dSPMs and single-dipole model.
| Patient | Interictal (ECD distribution) | Interictal (dSPM activation area) | No. of early ictal spikes | Ictal (No. of ECDs, location ) | Ictal (No. of dSPMs, activation area) | ||
|---|---|---|---|---|---|---|---|
| GOF>60 | GOF>70 | GOF>80 | |||||
| 1 | LF, RF | LF, BF | 7 | 2 LF, 2 RF | 1 LF | 0 | 7 LF |
| 2 | N/A | N/A | 7 | 4 RF, 2 LF | 3 RF, 2 LF | 1 RF | 6 RF, 1 BF |
| 3 | LF, RF | RF, BF | 6 | 1 RF, 2 RP, 1 LF | 1 RF, 2 RP | 0 | 6 RF |
| 4 | N/A | N/A | 4 | 2 LF, 1 LT | 2 LF, 1 LT | 1 LF | 4 LF |
| 5 | LF, RF | RF, BF | 11 | 4 RF, | 3 RF | 1 RF | 11 RF |
RF: right frontal lobe, LF: left frontal lobe, BF: bilateral frontal lobes, RP: right parietal lobe, LP: left parietal lobe, LT: left temporal lobe, N/A: not available
In three patients (Patient 1, 3 and 5), dSPMs obtained from interictal spikes showed widespread activation in unilateral or bilateral frontal lobe(s). These activation areas were larger than the activation in ictal dSPMs of each patient.
One ECD with GOF > 80 % were obtained in three patients (Patient 2, 4 and 5). Their locations were consistent with the clinically-determined ictal onset zone of each patient. In other two patients (Patient 1 and 3), there were no ECDs with GOF better than 80%. The numbers of ECDs were larger with the threshold of GOF > 70% and 60%. The locations of these ECDs were consistent with the ictal onset zone in one patient (Patient 5), and some ECDs were inconsistent in other four patients (Patient 1, 2, 3 and 4).
The distribution of ECDs obtained from interictal spikes was similar to the activation pattern in interictal dSPMs in each patient.
3.2. Illustrative cases
3.2.1. Patient 1
Patient 1 was a 15-year-old male with neurofibromatosis type I. Seizure onset was at age 5. He had daily seizures with unresponsiveness, sometimes accompanied with head drop and hemi-convulsion of the right side. Interictal EEG showed frequent spike-and-slow-wave complexes which were dominant in the left or right frontal areas. Ictal EEG showed rhythmic spike-and-slow-wave complex bursts dominant in the left frontal area. MRI showed multiple T2 hyperintense abnormalities especially prominent in the subcortical white matter of the left frontal lobe, which could affect the cortex adjacent to the lesion. Semiology was consistent with the left frontal lobe epilepsy. Ictal EEG showed the left frontal onset of seizures, and interictal EEG was also suggestive of frontal lobe epilepsy. MRI suggested the epileptogenic cortex in the left frontal lobe. Overall, the ictal onset zone was considered to be in the left frontal lobe.
The ictal MEG showed bilateral frontal spike bursts, which were initially predominantly on the left frontal sensors. Simultaneous EEG showed a similar pattern to the previous evaluation (Fig. 1A). Seven MEG spikes in the early part of the seizure were analyzed. The contour maps of magnetic fields showed a complicated pattern (Fig. 1B). All seven ictal dSPMs demonstrated activation in the left anterior frontal lobe (Table 2). A typical ictal dSPM is shown in Fig. 1C. The numbers of ECDs obtained with the threshold of GOF > 60%, 70% and 80% were four, one, and zero, respectively (Table 2). The ECD with GOF > 70% was located in the left frontal lobe (Fig. 1C), whereas ECDs with GOF > 60% were distributed in the left and right frontal lobes. The activation pattern of the interictal spikes dSPMs nearly always included a large portion of the left frontal cortex, and sometimes spread to the right side. ECDs obtained from the interictal spikes were mainly located in the left frontal lobe, but some were distributed in the right side (Table 2). A typical interictal dSPM and ECDs with GOF > 70% are shown in Fig. 1D.
Fig. 1.
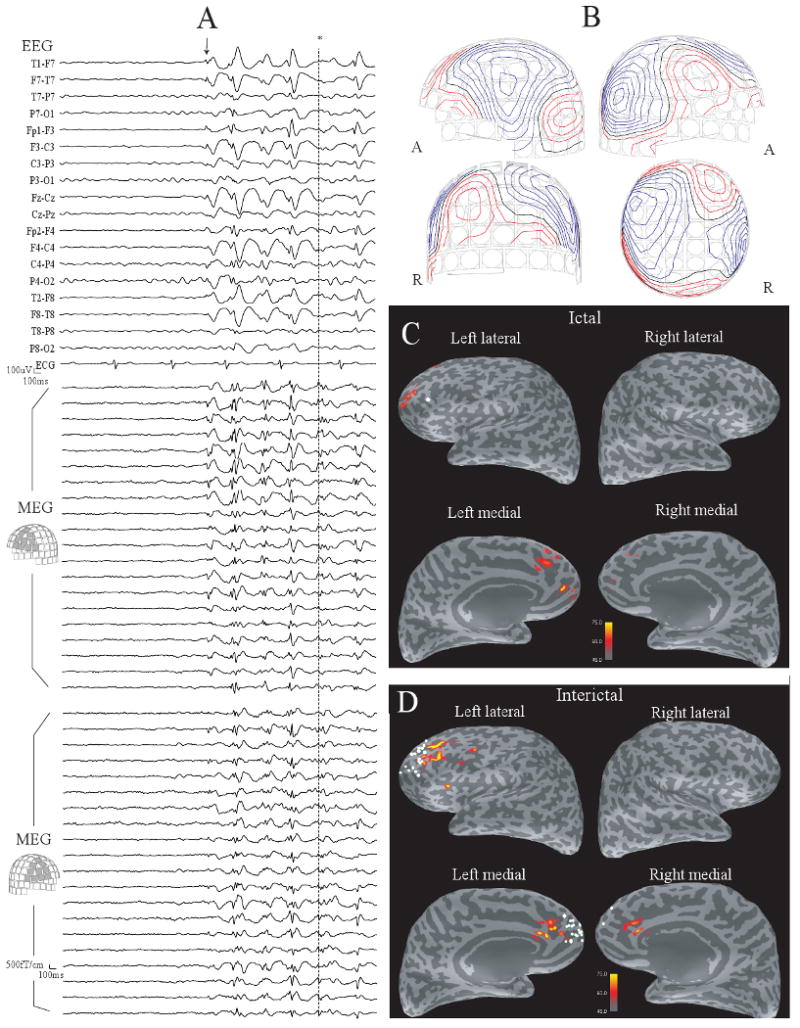
(A) The early part of ictal MEG and EEG in Patient 1. Rhythmic spike-and-slow-wave complexes are seen in the bilateral frontal areas after the point shown by an arrow. (B) Contour maps of magnetic fields at the peak of the MEG spike shown by * in (A). (C) The dSPM map at the same peak. The map are displayed on the inflated cortical surface, with darker gray indicating cortex buried in the sulci, and lighter gray indicating gyral cortex. Activation is seen in the left anterior frontal lobe. One ECD with GOF > 70% is obtained from seven ictal spikes (a white dot). (D) A dSPM map at the peak of a typical interictal spike. Activation is seen in a large part of the left frontal lobe and the right frontal lobe. ECDs with GOF > 70% obtained from interictal spikes (white dots) are distributed in bilateral frontal lobes. Note that the dSPM maps shown here are obtained from a single spike and ECDs are calculated from each individual spike.
3.2.2. Patient 5
Patient 5 was a 21-year-old female with frequent nocturnal seizures since age 12. Her habitual seizure consisted of hypermotor movements, sometimes accompanied with screaming. Interictal EEG showed frequent spikes in the bilateral frontal area. In previous evaluation, ictal EEG showed mainly movement artifacts, sometimes preceded by small spikes in the right frontal area. MRI showed a cortical dysplasia in the right anterior frontal lobe (Fig. 2E). The Semiology of this patient was consistent with frontal lobe epilepsy, as well as interictal EEG. Ictal EEG showed the right frontal onset of seizures. The MRI lesion suggested the epileptogenic cortex in the right frontal lobe. Based on these findings, ictal onset zone was considered to be in the right frontal lobe.
Fig. 2.
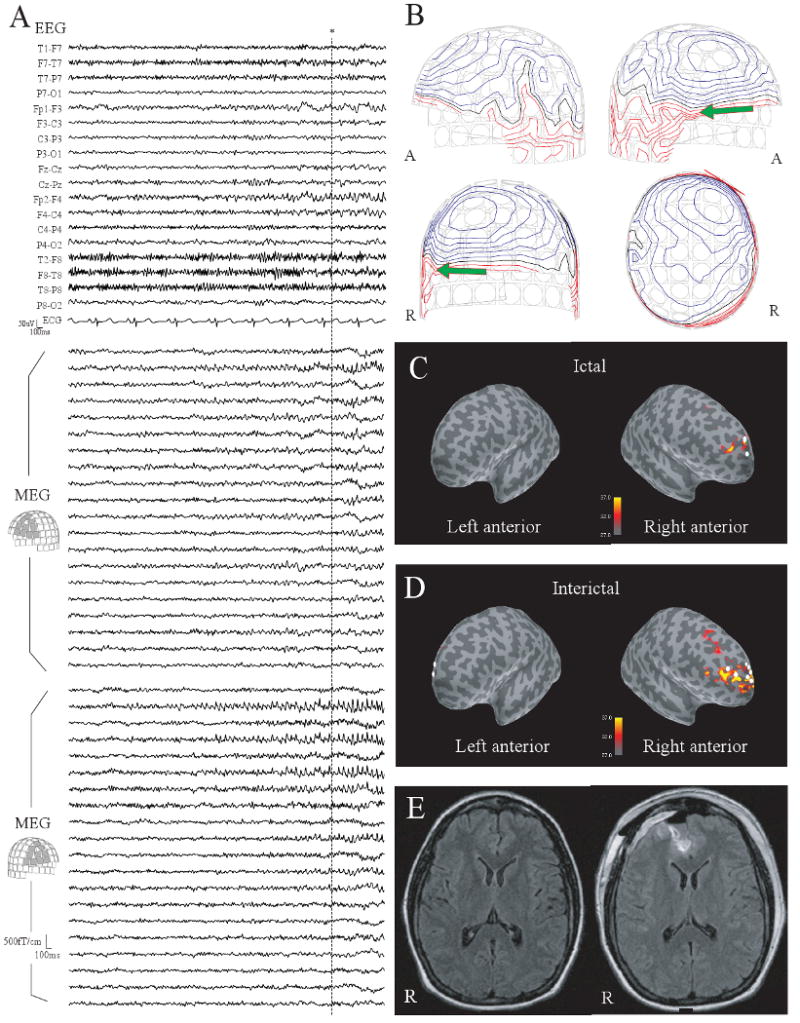
(A) The early part of ictal MEG and EEG in Patient 5. Small spike bursts are seen predominantly in the right frontal area. (B) Contour maps of magnetic fields at the peak of the MEG spike shown by * in (A). (C-upper) The dSPM map at the same peak. Activation in dSPM is seen in the right anterior frontal lobe. Three ECDs with GOF > 70% are obtained from 11 ictal spikes (white dots). (D) A dSPM map at the peak of a typical interictal spike. Activation is seen in a relatively a large part of the right frontal lobe. ECDs with GOF > 70% obtained from interictal spikes are distributed in the bilateral frontal lobes. Note that the dSPM maps shown here are obtained from a single spike and ECDs are calculated from each individual spike. (E-left) Presurgical FLAIR MRI. The abnormal signals in the right anterior frontal lobe suggest a cortical dysplasia. (E-right) Postsurgical FLAIR MRI. The lesion was removed by a surgical resection.
The early ictal EEG and MEG showed small spike bursts predominantly in the right frontal area (Fig. 2A). Eleven spikes were analyzed. The contour maps of magnetic fields showed a single-dipole pattern (Fig. 2B). All eleven dSPMs showed activation in the right anterior frontal lobe. Four, three and one ECD(s) were obtained with the threshold of GOF > 60%, 70% and 80%, respectively. These ECDs were located in the right frontal lobe. A typical ictal dSPM and ECDs with GOF > 70% are shown in Fig. 2C. The activation pattern of the interictal spikes dSPMs included a large portion of the right frontal lobe or bilateral frontal lobes. ECDs obtained from the interictal spikes were located mainly in the right frontal lobe, but some were seen in the left frontal lobe. A typical interictal dSPM and ECDs with GOF > 70% are shown in Fig. 2D.
This patient underwent a resection of the cortical dysplasia (Fig 2E) and has been seizure free for 13 months after surgery.
4. Discussion
In the present study, we estimated ictal onset zones in five patients with intractable frontal lobe epilepsy by using both dSPMs and a single-dipole model. In all spikes obtained from the early ictal discharges, the dSPM localized activity consistent with the clinically-determined ictal onset zone at a lobar level, except for one spike in Patient 2. The dSPM activation in this spike showed larger activation areas, including the clinically-determined ictal onset zone. Moreover, dSPMs had more restricted activation in these ictal spikes than interictal spikes. It is well known that the irritative zone obtained from the interictal spikes is often larger than the ictal onset zone (Lüders and Awad, 1991). DSPM findings of this study are in agreement with this phenomenon.
Since the single-dipole analysis always has the difference between measured and forward-calculated magnetic fields, many ictal MEG studies have applied a threshold of GOF values for selecting ECDs as appropriate sources (Oishi et al., 2002; Tanaka et al., 2004; Yoshinaga et al., 2004; Tanaka et al., 2005). The threshold has been set at 80-90% in these studies. This is a form of appropriate bias for increasing the likelihood of single-dipole analysis, when many ECDs are available after eliminating ECDs with a low GOF value. Several MEG studies have selected the channels of MEG to be used in the single-dipole analysis for obtaining better GOF values (Bast et al., 2004). This is another bias which may be useful only when the selection is adequate for representing the epileptic activity. In this study, we used all the MEG channels for single-dipole analysis. The number of ECDs decreased as the threshold of goodness-of-fit (GOF) values increased. No ECDs or only one ECD with the threshold of GOF > 80% in each case. The locations of some ECDs obtained with the thresholds of GOF > 60% and 70% were inconsistent with the clinically-determined ictal onset zones. These results may suggest the usefulness of ECDs may be limited in the evaluation of these ictal MEG spikes. Theoretically, dSPMs can represent all the magnetic fields by applying a source distribution model, assuming that the sources are distributed in the large patch of cortex (Dale and Sereno, 1993; Dale et al., 2000). Therefore, thresholds such as GOF values are considered unnecessary for dSPM analysis. On the other hand, determination of the appropriate amount of activated cortex is an important issue in distributed source models including dSPM. In dSPM, this issue is highly dependent on F values for statistical mapping. Smaller F values would show a larger area of activation, and larger F values would show no activation. In any case, the inverse solution used for making dSPMs explains all magnetic fields. We applied F values for representing the strongest activation, based on the assumption that epileptic activities are larger than other background activities. In addition, the activation areas were consistent at a lobar level under several different F values in all patients. Further study will be needed for investigating the accuracy of dSPM at a sublobar level by comparing dSPMs and the spike involvement on intracranial EEG.
Source localization of ictal discharges becomes difficult after seizure activity propagates (Tanaka et al., 2004), thus early ictal spikes may be more accurate for estimating the seizure onset zone (Tilz et al., 2002; Assaf et al., 2003). However, the low signal-to-noise ratio in the early spikes may complicate obtaining appropriate sources using single-dipole modeling. This may explain the low GOF values and widespread distribution of ECDs in the present study. In contrast, dSPMs showed a localized activation which was reproducible between the different ictal spikes, suggesting that they may be useful for analyzing these spikes with the low signal-to-noise ratio.
The small number of patients and the lack of a confident ictal onset zone determined by intracranial EEG may cause a limitation to the results of this study. However, we consider this study is still valuable for improving MEG analysis since this is the first report in which dSPMs are applied for analyzing ictal data. Two patients underwent surgery with a favorable outcome, removing the area of cortex which was consistent with the results of dSPMs. Further investigation in a larger patient group with intracranial EEG will provide an improvement on these issues.
Overall, the results of our patients suggested that dSPM provided superior source localization of early ictal spikes compared to single-dipole source analysis. By using dSPM, ictal MEG may be more informative than the single-dipole analysis alone in presurgical evaluation of epilepsy.
Acknowledgments
This work was supported by National Institutes of Health (P41RR14075, RO1NS037462-07), Mental Illness and Neuroscience Discovery Institute, NARSAD Young Investigators Award and Japan Epilepsy Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assaf BA, Karkar KM, Laxer KD, Garcia PA, Austin EJ, Barbaro NM, Aminoff MJ. Ictal magnetoencephalography in temporal and extratemporal lobe epilepsy. Epilepsia. 2003;44:1320–1327. doi: 10.1046/j.1528-1157.2003.14303.x. [DOI] [PubMed] [Google Scholar]
- Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, Fauser S, Zentner J, Rating D, Scherg M. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004;45:621–631. doi: 10.1111/j.0013-9580.2004.56503.x. [DOI] [PubMed] [Google Scholar]
- Crouzeix A, Yvert B, Bertrand O, Pernier J. An evaluation of dipole reconstruction accuracy with spherical head model and realistic head models in MEG. Clin Neurophysiol. 1999;110:2176–2188. doi: 10.1016/s1388-2457(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- de Jongh A, de Munck JC, Gonçalves SI, Ossenblok P. Differences in MEG/EEG epileptic spike yields explained by regional differences in signal-to-noise ratios. J Clin Neurophysiol. 2005;22:153–158. doi: 10.1097/01.wnp.0000158947.68733.51. [DOI] [PubMed] [Google Scholar]
- Eliashiv DS, Elsas SM, Squires K, Fried I, Engel J., Jr Ictal magnetic source imaging as a localizing tool in partial epilepsy. Neurology. 2002;59:1600–1610. doi: 10.1212/01.wnl.0000032493.83875.0b. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kastner J. Boundary element method volume conductor models for EEG source reconstruction. Clin Neurophysiol. 2001;112:1400–1407. doi: 10.1016/s1388-2457(01)00589-2. [DOI] [PubMed] [Google Scholar]
- Hamäläinen M, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Knake S, Halgren E, Shiraishi H, Hara K, Hamer HM, Grant PE, Carr VA, Foxe D, Camposano S, Busa E, Witzel T, Hamäläinen MS, Ahlfors SP, Bromfield EB, Black PM, Bourgeois BF, Cole AJ, Cosgrove GR, Dworetzky BA, Madsen JR, Larsson PG, Schomer DL, Thiele EA, Dale AM, Rosen BR, Stufflebeam SM. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res. 2006;69:80–86. doi: 10.1016/j.eplepsyres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Ohtsuka Y, Gotman J. Dipole modeling of epileptic spikes can be accurate or misleading. Epilepsia. 2005;46:397–408. doi: 10.1111/j.0013-9580.2005.31404.x. [DOI] [PubMed] [Google Scholar]
- Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp. 2002;16:47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders HO, Awad I. Conceptual considerations. In: Lüders H, editor. Epilepsy surgery. Raven Press; New York: 1991. pp. 1063–1070. [Google Scholar]
- Oishi M, Kameyama S, Morota N, Tomikawa M, Wachi M, Kakita A, Takahashi H, Tanaka R. Fusiform gyrus epilepsy: the use of ictal magnetoencephalography. Case report. J Neurosurg. 2002;97:200–204. doi: 10.3171/jns.2002.97.1.0200. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Van Oosterom A. Source parameter estimation in inhomogeneous volume conductors of arbitrary shape. IEEE Trans Biomed Eng. 1989;36:382–391. doi: 10.1109/10.19859. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Ahlfors SP, Stufflebeam SM, Takano K, Okajima M, Knake S, Hatanaka K, Kohsaka S, Saitoh S, Dale AM, Halgren E. Application of magnetoencephalography in epilepsy patients with widespread spike or slow-wave activity. Epilepsia. 2005;46:1264–1272. doi: 10.1111/j.1528-1167.2005.65504.x. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Stufflebeam SM, Knake S, Ahlfors SP, Sudo A, Asahina N, Egawa K, Hatanaka K, Kohsaka S, Saitoh S, Grant E, Dale AM, Halgren E. Dynamic statistical parametric mapping for analyzing the magnetoencephalographic epileptiform activity in patients with epilepsy. J Child Neurol. 2005;20:363–369. doi: 10.1177/08830738050200041601. [DOI] [PubMed] [Google Scholar]
- Stefan H, Schneider S, Feistel H, Pawlik G, Schuler P, Abraham-Fuchs K, Schlegel T, Neubauer U, Huk WJ. Ictal and interictal activity in partial epilepsy recorded with multichannel magnetoelectroencephalography: correlation of electroencephalography/electrocorticography, magnetic resonance imaging, single photon emission computed tomography, and positron emission tomography findings. Epilepsia. 1992;33:874–887. doi: 10.1111/j.1528-1157.1992.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Sutherling WW, Crandall PH, Engel JJ, Darcey TM, Cahan LD, Barth DS. The magnetic field of complex partial seizures agrees with intracranial localizations. Ann Neurol. 1987;21:548–558. doi: 10.1002/ana.410210605. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kamada K, Takeuchi F. Ictal magnetoencephalographic study in a patient with ring 20 syndrome. J Neurol Neurosurg Psychiatry. 2004;75:488–490. doi: 10.1136/jnnp.2003.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kamada K, Takeuchi F, Takeda Y. Magnetoencephalographic analysis of secondary bilateral synchrony. J Neuroimaging. 2005;15:89–91. doi: 10.1177/1051228404271007. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Liljestrom M, Seppa M, Salmelin R. The 3D topography of MEG source localization accuracy: effects of conductor model and noise. Clin Neurophysiol. 2003;114:1977–1992. doi: 10.1016/s1388-2457(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Tilz C, Hummel C, Kettenmann H, Stefan H. Ictal onset localization of epileptic seizures by magnetoencephalography. Acta Neurol Scand. 2002;106:190–195. doi: 10.1034/j.1600-0404.2002.02047.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga H, Ohtsuka Y, Watanabe Y, Inutsuka M, Kitamura Y, Kinugasa K, Oka E. Ictal MEG in two children with partial seizures. Brain Dev. 2004;26:403–408. doi: 10.1016/j.braindev.2003.11.003. [DOI] [PubMed] [Google Scholar]


