Abstract
The activation threshold for antigen-specific T cell responses is dependent on the avidity of the trimolecular interaction between TCR, antigen, and MHC. We compared CD4+ T cell avidities for the diabetes-associated autoantigen glutamic acid decarboxylase 555-567 (GAD 555) among serial samples from autoantibody-positive subjects at high risk of progression to type 1 diabetes (T1D). T cells from three at-risk subjects demonstrated significant avidity increases (p<0.05 by F test) over time. This avidity shift correlated with the outgrowth of T cells expressing TCR BV 9, 15, 17 or 20 that demonstrated higher GAD 555 tetramer-binding levels compared to cells expressing other TCR BV genes. Similar analysis of autoantibody-negative, first-degree relatives and T1D patients did not demonstrate similar changes in avidity. These data implicate the outgrowth of T cells expressing higher affinity TCR in a process of antigen-specific T cell avidity maturation during the pre-clinical stage of T1D.
Keywords: Type 1 diabetes, CD4+ T cells, Autoimmunity, glutamic acid decarboxylase
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by the destruction of insulin-producing beta cells of the pancreatic islets, resulting in hyperglycemia. At the time of diagnosis with T1D, approximately 20% of beta cells’ insulin secretory capacity may remain [1]. The pre-clinical phase of T1D is marked by the presence of autoantibodies and frequently, T cells with specificity for beta cell antigens. In pre-diabetic patients, CD4+ T cell responses directed against pro-insulin and glutamic acid decarboxylase 555-567 (GAD 555) have been reported [2, 3], and we observed that a cohort of autoantibody positive, at-risk subjects exhibited a significantly increased frequency of CD8+ T cells responding to an epitope of prepro-islet amyloid polypeptide [4]. However, T cells with the same specificities have also been isolated from healthy individuals, demonstrating that the mere presence of autoreactive T cells is not sufficient for diabetes induction [5, 6]. Instead, it is more likely that the ability of a T cell population to induce and potentiate an autoaggressive response is dictated, in large part, by the threshold and magnitude of antigen responsiveness.
Experimentally, thresholds for antigen response can be expressed in terms of T cell avidity for antigen, as the concentration of peptide that elicits 50% of a maximal functional response (EC50). T cell avidity is dependent upon T cell receptor (TCR) affinity for peptide-MHC, in combination with other parameters which sustain signaling, including activity of co-stimulatory and adhesion molecules, and the density of TCR expression [7]. The avidities of T cells with specificity for self-peptides are typically lower than those with specificity for foreign antigens as a result of central and peripheral tolerance mechanisms [8]. However, not all self-reactive T cells are deleted or rendered anergic, and T cells with a range of avidities persist. For example, in NOD mice, the avidity distributions of distinct autoreactive T cell populations have been demonstrated to correlate with the chronology of epitope spreading [9].
The range of T cell avidities displayed during an immune response can change following multiple antigen encounters in a process termed avidity maturation. Several mechanisms have been demonstrated to mediate avidity maturation including an increase in intracellular expression levels of the protein tyrosine kinase Lck [10] and activation-induced changes in T cell membrane cholesterol content [11]. Another mechanism of avidity maturation is the selective expansion of T cells expressing TCR with optimal affinity for antigen [12, 13]. In NOD mice, up to 30% of CD8+ cells in the pancreas react with a peptide of islet-specific glucose-6-phosphatase catalytic subunit-related protein comprised of residues 206-214 (IGRP 206), and the prevalence of these cells in the peripheral blood is a positive predictor of autoimmune diabetes development [14]. A longitudinal study of IGRP 206-responsive CD8+ cells in NOD mice demonstrated an increase in avidity prior to diabetes onset due to the sequential expansion of populations expressing successively higher affinity TCR [15]. These data imply that avidity maturation correlates with the progression of a benign self-reactive response to overt autoimmunity and that disruption of T cell avidity maturation may serve as an effective goal of preventive therapies.
The role of T cell avidity maturation in the development of human T1D has not been characterized. Analyses of GAD-reactive CD4+ cells from T1D patients using HLA/peptide tetramers revealed both high and low avidity populations with the former being more susceptible to the induction of activation-induced cell death (AICD) [16, 17]. It is possible that high avidity T cells play a more prominent role in islet destruction in later disease stages when antigen levels are limiting while low avidity cells predominate early in the pre-clinical phase of T1D. This would have important implications in the scheduling and dosing of antigen-specific preventive therapeutics in clinical trials.
To ascertain if GAD 555-reactive T cell populations undergo avidity maturation during diabetes development, we compared the CD4+ T cell avidities of serial samples from autoantibody-positive, at-risk subjects to those of T1D patients and autoantibody-negative relatives of patients. We observed that only T cells from at-risk subjects demonstrated significant avidity increases over time. The mechanism of avidity maturation in one of the at-risk patients, who subsequently developed diabetes, was investigated and determined to be the result of the outgrowth of T cells expressing higher affinity TCR.
Research design and methods
Subjects
All subjects were consented for study participation according to Benaroya Research Institute Institutional Review Board-approved protocols. Peripheral blood was collected by venous puncture into heparinized tubes and stored overnight. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-hypaque density gradient centrifugation, frozen in a 90% bovine calf serum: 10% DMSO solution, and kept in liquefied nitrogen until use. At-risk subjects carried the HLA susceptibility alleles for T1D and tested positive for at least one diabetes-associated autoantibody (GAD, IA-2 or insulin) on more than one occasion. Subject characteristics are detailed in Table 1.
Table 1.
Characteristics of subjects enrolled in study
| Subject | Group | Autoantibody1 | Gender | Age2 | DRB1 haplotype |
|---|---|---|---|---|---|
| 5574 | At-risk | GAD, IA-2 | M | 13 | *0401/*0301 |
| 6211 | At-risk | IA-2 | F | 15 | *0401/*0701 |
| 6827 | At-risk | IAA | M | 16 | *0401/*1502 |
| 6212 | At-risk | GAD, IA-2 | F | 17 | *0404/*0405 |
| 6899 | At-risk | GAD | F | 17 | *0401/*0403 |
| 0909 | At-risk | GAD, IA-2 | M | 17 | *0401/*1301 |
| 2824 | At-risk | GAD, IA-2 | M | 18 | *0401/*0301 |
| 5571 | At-risk | GAD, IA-2 | F | 19 | *0401/*0301 |
| 5725 | At-risk | GAD | F | 22 | *0401/*0401 |
| 4845 | At-risk | GAD | F | 39 | *0401/*0301 |
| 0901 | At-risk | GAD, IA-2 | F | 40 | *0401/*0301 |
| 8617 | At-risk | GAD, IA-2 | M | 45 | *0401/*0301 |
| 7625 | Diabetes (3 mo. post-diag.) | GAD, IA-2 | F | 14 | *0401/*0301 |
| 6754 | Diabetes (11 mo. post-diag.) | GAD, IA-2, IAA | M | 15 | *0401/*1302 |
| 0912 | Diabetes (16 mo. post-diag.) | GAD, IA-2 | M | 15 | *0401/*0901 |
| 0904 | Diabetes (38 mo. post-diag.) | GAD, IA-2 | M | 16 | *0401/*0101 |
| 5573 | Diabetes (1 mo. post-diag.) | GAD, IA-2, IAA | F | 18 | *0401/*0801 |
| 7553 | Diabetes (1 mo. post-diag.) | IA-2 | M | 19 | *0401/*0101 |
| 7581 | Diabetes (1 mo. post-diag.) | GAD, IA-2 | M | 24 | *0401/*1101 |
| 0908 | Diabetes (14 mo. post-diag.) | GAD, IA-2 | M | 24 | *0401/*0401 |
| 0907 | Diabetes (45 mo. post-diag.) | GAD, IA-2 | M | 28 | *0401/*1302 |
| 7472 | First-degree relative | Negative | F | 26 | *0401/*0301 |
| 6660 | First-degree relative | Negative | F | n/d | *0401/*0301 |
| 7052 | First-degree relative | Negative | F | n/d | *0401/*1302 |
| 0913 | First-degree relative | Negative | M | 57 | *0401/*1501 |
presence of GAD 65, IA-2, or insulin autoantibodies at time of initial sample collection;
age in years at time of initial sample collection; n/d: not determined
Cell culture
CD4+ cells were isolated from thawed PBMC using the Dynal® CD4 Positive Isolation Kit (Invitrogen, Carlsbad, CA). The non-CD4 cell fraction was irradiated (5000 Rads), plated at 1.5×106/well in a 48-well plate and incubated in culture medium (RPMI-1640 + 10% pooled human serum) at 37°C in 6% CO2. After one hour, non-adherent cells were aspirated from wells, and fresh culture medium was added along with 10 μg/ml of peptide and purified CD4+ T cells (5×105). Peptides, GAD 555-567 (NFFRMVISNPAAT) and HA 306-318 (PKYVKQNTLKLAT), were synthesized by AnaSpec (San Jose, CA) using standard fMOC chemistry, and identities were verified using mass spectrometry and HPLC analysis. After 4–5 days of culture, 48 ng/ml recombinant human IL-2 (Chiron Novartis, Emeryville, CA) was added to each well. Cells were harvested on day 10 for ELISPOT and tetramer staining.
ELISPOT assays
The frequency of cytokine-secreting CD4+ cells was quantified using the human IFN- ELISPOT kit according to manufacturer’s protocols (BD Pharmingen, San Diego, CA). Autologous PBMC cleared of CD3-expressing cells using MACS anti-CD3 beads (Miltenyi Biotech, Cologne, Germany) were used as antigen-presenting cells (APC) in ELISPOT assays. Non-CD3 cells were incubated with diluted peptides for one hour at 37°C in 6% CO2 prior to adding 5×104 cells per well to antibody-coated, blocked ELISPOT plates. Peptide-cultured CD4+ cells were then harvested and plated in triplicate at 1×104 cells per well. Plates were incubated for 24 hours at 37°C in 6% CO2 prior to washing and developing with AEC substrate according to manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Development reactions were monitored via dissection microscope and stopped by addition of distilled water after approximately nine minutes.
Well images were captured using a CTL-ImmunoSpot® S5 Micro Analyzer (Cellular Technology Limited, Shaker Heights, OH), and quantities of spot-forming cells (SFC) in each well were calculated using ImmunoSpot 5.0 Professional analysis software. Six wells were selected from each longitudinal culture, and the global detection sensitivity level for quantifying SFC was calculated using the SmartWell™ algorithm. At least 300 SFC from wells containing high and low peptide concentrations were chosen, and the Autogating function was utilized to set gate sizes such that ≥ 95% of all sampled spots are recognized. Gate sizes typically ranged from 0.001–0.1 mm2. Frequencies of SFC were plotted against antigen concentrations, and a curve was fit to the data using a 3-parameter logistic model, assuming a Hill coefficient of one (GraphPad Prism 7, GraphPad Software, La Jolla, CA). EC50 values were calculated for each curve and compared utilizing an F test that enables calculation of p-values.
Flow cytometry
Tetramers were synthesized by the BRI Tetramer Core Laboratory as previously described [18]. Following a 10 day expansion period with peptide, approximately 50,000 CD4+ cells were washed and incubated with 10 μg/ml of PE-labeled tetramer for 2–3 hours at 37°C. Cells were then stained for 15 minutes on ice with fluorochrome-labeled antibodies prior to washing with 1% BSA in PBS. As a negative control, cells were incubated with HLA-matched tetramers presenting a non-specific peptide. The following antibodies were used: PECy7 or APC anti-CD4 (Beckman Coulter, Fullerton, CA), APC anti-CD25 (BioLegend, San Diego, CA), APC anti-CD5, FITC anti-CD81 (BD Pharmingen, San Diego, CA), FITC anti-TCRαβ, FITC anti-IL-15Rα, APC anti-CD3ε (eBioscience, San Diego, CA), PE anti-TCRVβ9, FITC anti-TCRVβ17, FITC anti-TCRVβ20 (Beckman Coulter, Fullerton, CA). All flow cytometry experiments were performed using a BD FACSCalibur (Becton Dickinson, San Diego, CA) and data were analyzed using FlowJo software version 7.5.2 (TreeStar Inc., Ashland, OR). Gates were set with the frequency of irrelevant tetramer-stained cells equal to 0.1%, and these gates were used to calculate the percentage of cells stained with GAD peptide-loaded tetramers.
TCR Vβ repertoire analysis
Expression of TCR BV genes was assessed using the Super TCRExpress™ Clonality Detecting Kit according to manufacturer’s protocol (BioMed Immunotech, Alachua, FL). In brief, CD4+ T cells were cultured with GAD and autologous APC for 10 days prior to tetramer-staining as detailed above. After sorting approximately 1.5–6×103 of both tetramer-stained and unstained CD4+ cells, RNA was extracted, cDNA was synthesized, and TCR genes were amplified in a single step of 35 PCR cycles. A nested PCR amplified specific TCR genes using TCR BV gene-specific oligonucleotides after an additional 33 cycles. PCR products were resolved on a 4% high resolution agarose gel.
Results
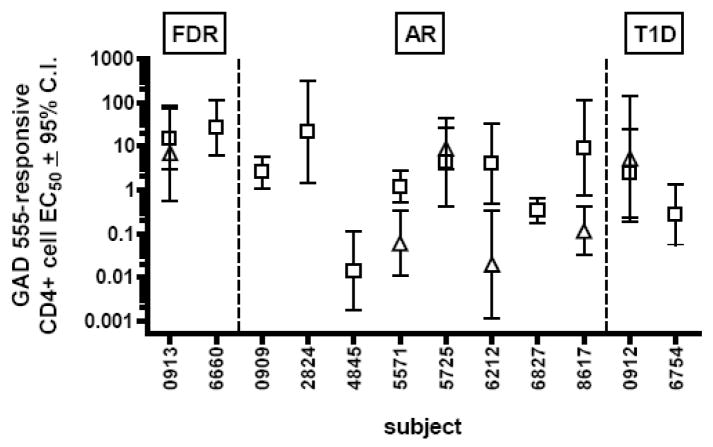
In order to assess autoreactive T cell avidity maturation over time in subjects at different stages of T1D development, we calculated EC50 values of GAD 555-reactive CD4+ cells from longitudinal PBMC samples drawn from autoantibody-positive at-risk subjects, autoantibody-negative first-degree relatives and T1D patients. The EC50 is the peptide dose that induces one-half maximal levels of stimulation and inversely correlates with T cell functional avidity. EC50 values were calculated from IFN-γ ELISPOT assays of CD4+ cells cultured with escalating doses of GAD 555. We also assessed the binding of HLA-DR4 tetramers presenting either GAD 555 or its analog peptide, GAD 557I, to quantify the frequencies of antigen-specific CD4+ cells in cultures.
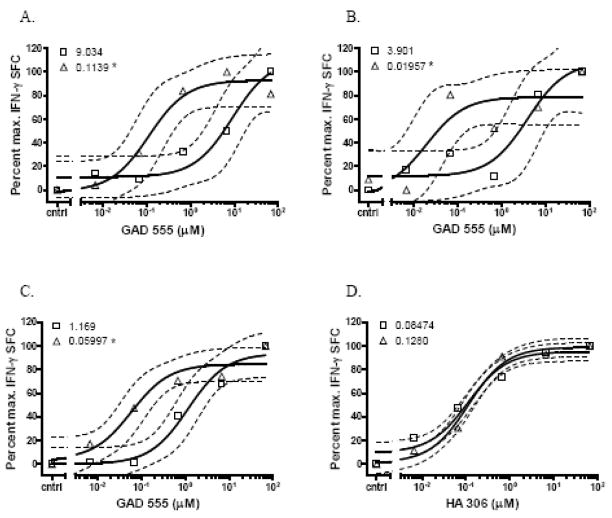
We observed that eight of twelve (67%) at-risk subjects and two of four (50%) first-degree relatives exhibited increasing numbers of IFN-γ secreting cells as a function of antigen dose. In contrast, only two of nine (22%) T1D patients exhibited dose-responses to GAD 555. All samples that harbored IFN-γ secreting cells also contained GAD 555 or GAD 557I tetramer-staining cells, but there was no correlation between the frequencies of tetramer-binding cells and disease status (Table 2). The EC50 magnitudes were relatively heterogeneous among responsive subjects regardless of disease status (Fig. 1). We observed that subjects 6660, 4845 and 6827 failed to demonstrate IFN-γ responses in the follow-up samples even though tetramer-staining cells were present (Table 2). We noted that follow-up samples from at-risk subjects 5571, 6212, and 8617 exhibited significantly lower EC50 values (p<0.05 by F test) than those of initial samples implying a shift from a low to a high avidity response over time (Fig 2A, B, and C). One of these subjects, 6212, was subsequently diagnosed with T1D; therefore, we decided to further investigate T cell avidity maturation using samples from this bona fide pre-diabetic patient.
Table 2.
Tetramer staining of GAD 555-cultured CD4+ cells
| % DR4/GAD 555 staining | % DR4/GAD 557I staining | ||||
|---|---|---|---|---|---|
| Subject | Interval between sample collection | Initial sample | Follow-up sample | Initial sample | Follow-up sample |
| 0913 | 2 years | 0 | 0 | 0.24 | 0 |
| 6660 | 3 years | 0.11 | 0.46 | 0 | 0.41 |
| 0909 | 1 year | 0 | 0 | 1.07 | 0 |
| 2824 | 2 years | 0 | 0 | 0.2 | 0.25 |
| 4845 | 1 year | 0 | 0.3 | 0.29 | 0.08 |
| 5571 | 1 year | 0.1 | n/d | 0.51 | n/d |
| 5725 | 2 months | 0.67 | 0.85 | 0.37 | 0.26 |
| 6212 | 4 years | 0.23 | 0.55 | n/d | n/d |
| 6827 | 2 years | 0.08 | 0.7 | 0.14 | 0.08 |
| 8617 | 3 years | 0 | 0.3 | 0.29 | 0.08 |
| 0912 | 1 year | 0.14 | 0.08 | 0.19 | 0 |
| 6754 | 2 years | 1.0 | 0 | 0 | 0 |
n/d: not determined
Fig. 1.
Avidities of GAD 555-reactive CD4+ T cells from autoantibody negative first-degree relatives (FDR), autoantibody positive, at-risk subjects (AR) and type 1 diabetic patients (T1D). EC50 values were calculated by culturing initial (open squares) and follow-up (open triangles) samples with escalating doses of GAD 555 and calculating EC50 values from dose-response curves.
Fig. 2.
GAD 555-reactive CD4+ T cell avidities of serial samples from at-risk subjects. Initial (open squares) and follow-up (open triangles) CD4+ samples were analyzed for responses to escalating doses of GAD 555. Dashed lines represent the 95% confidence intervals of the regression curves, and the EC50 values are indicated to the right of each legend symbol. Data are plotted as the percent of maximum spot forming cell frequencies for each sample. * denotes EC50 is significantly reduced (p < 0.05 by F test) compared to initial sample. A) At-risk subject 5571 responses to GAD 555. B) At-risk subject 6212 responses to GAD 555. C) At-risk subject 8617 responses to GAD 555. D) At-risk subject 6212 responses to HA 306.
We first wished to ensure that the avidity shift was specific for the GAD 555-responsive cells and not a global phenomenon. Therefore, separate aliquots of samples from pre-diabetic subject 6212 were cultured with the influenza hemagglutinin 306-318 (HA 306) peptide for 10 days prior to analyzing EC50 values by IFN-γ ELISPOT assay. We observed no significant differences between the HA 306-reactive T cell population EC50 values over time demonstrating that avidity maturation only occurred in the GAD 555-reactive T cell population (Fig. 2D).
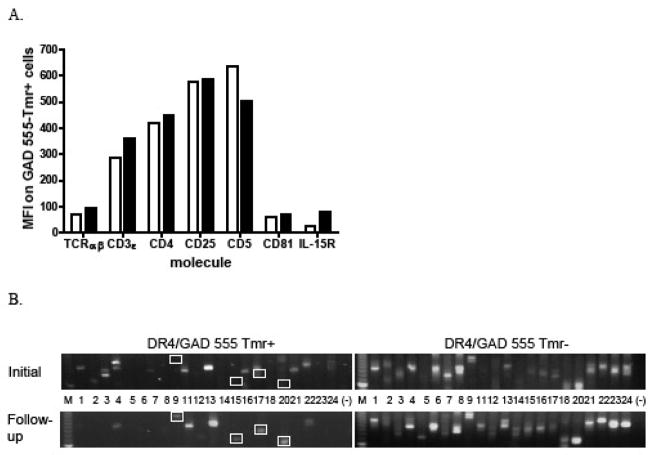
We next sought to identify the mechanism of avidity maturation operable in this subject. Since T cell avidity maturation has been demonstrated to result from the up-regulation of proteins that enhance TCR signaling strength [7], we analyzed expression levels of several cell-surface molecules associated with T cell activation on expanded, GAD 555 tetramer-labeled cells. We observed only modest increases (5–30%) in expression of TCR αβ, CD3ε, CD4, CD25 and CD81 on higher avidity cells from the follow-up sample while expression of CD5 was slightly increased on cells from the initial sample (Fig. 3A). The IL-15Rα chain was up-regulated by greater than 3-fold on the higher avidity cells compared to cells from the initial sample.
Fig. 3.
Expression levels of T cell signaling-associated molecules and TCR Vβ repertoires of GAD 555-reactive CD4+ cells from pre-diabetic subject 6212. A) GAD tetramer-binding cells from initial (open bars) or follow-up (closed bars) samples were stained with fluorochrome-labeled antibodies with specificities for the indicated cell surface proteins. MFI denotes mean fluorescence intensity. B) GAD 555 tetramer-binding (left panels) and non-binding cells (right panels) from initial (top) and follow-up (bottom) samples were sorted, and the expression patterns of TCR BV genes by each population were determined. White boxes denote TCR BV genes that are expressed at higher levels by GAD-reactive CD4+ cells from the follow-up sample.
Another putative mechanism of avidity maturation is the preferential expansion of T cells expressing high affinity TCR, which can be detected by the outgrowth of T cell clonotypes over time [12, 13]. To assess this as a possible avidity maturation mechanism, GAD 555 tetramer-binding and non-binding cells from the initial and follow-up samples from the pre-diabetic patient were sorted by flow cytometry, and the TCR Vβ repertoires of each population were analyzed using a nested PCR-based technique. TCR BV gene expression by GAD 555 tetramer-binding cells differed between the two time points. In general, a broader repertoire of TCR Vβ families was represented in the initial sample, while populations expressing TCR BV 9, 15, 17, and 20 were expanded in the follow-up sample (Fig. 3B upper and lower left, compare boxes). Importantly, only minimal changes in the TCR Vβ repertoires were observed in the non-tetramer binding populations (Fig. 3B, upper and lower right). These findings suggested that the avidity shift observed between samples correlates with the outgrowth of novel T cell clonotypes in the GAD 555-reactive population.
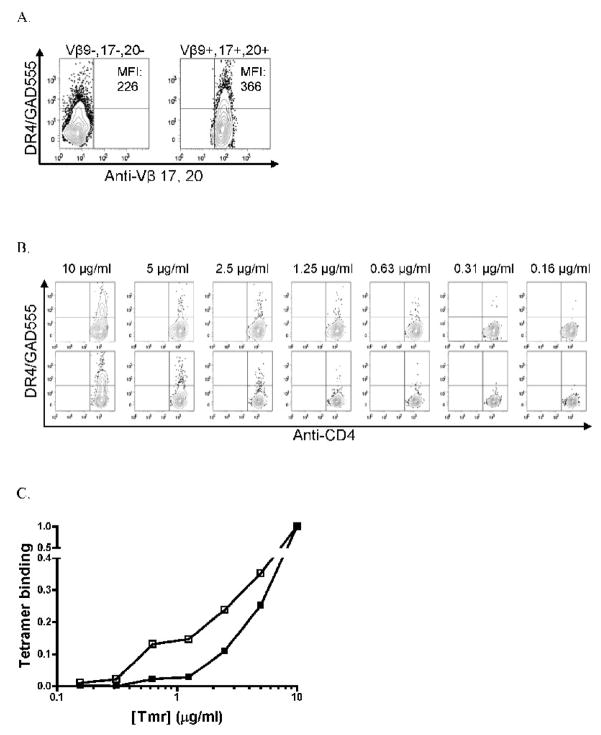
In an effort to further characterize the operable avidity maturation mechanism, we expanded CD4+ cells from the follow-up sample and isolated cells expressing TCR BV 9, 17, or 20 and those expressing other TCR BV genes. Both populations were expanded with peptide for an additional 10 days and then analyzed for the levels of tetramer-binding. The putative high avidity cells expressing TCR BV 9, 17, or 20 exhibited higher tetramer-binding levels as evinced by a higher mean fluorescence intensity (MFI) of tetramer staining compared to CD4+ cells expressing other TCR BV genes (Fig. 4A). There was no difference in TCRαβ or IL-15Rα expression levels between these two subsets (data not shown). Since avidity shifts can result from repetitive exposure to antigen, we did not perform ELISPOT assays on sorted, expanded cells. Instead, we assessed the ability of sorted cells to bind to decreasing quantities of GAD 555 tetramers, which has been shown to correspond with TCR affinity [19]. Each population demonstrated large decreases in the frequency of tetramer-staining cells over the dilutions tested (Fig. 4B). By plotting the frequency of tetramer-staining cells from each population as a percentage of the frequency of CD4+ cells stained by the highest tetramer concentration (10 μg/ml), we observed that an increased proportion of CD4+ cells expressing TCR BV 9, 17, or 20 were bound by low levels of tetramers compared to cells expressing other TCR BV genes (Fig. 4C). Taken together, these data demonstrate that a subset of GAD 555-reactive T cells expressing TCR BV 9, 17 or 20 is able to bind to lower quantities of tetramers implying a higher TCR affinity for GAD 555. Therefore, it is most likely that avidity maturation in this pre-diabetic subject results from the selective expansion of T cells expressing higher affinity TCR.
Fig. 4.
GAD 555 tetramer binding levels of Vβ9+, 17+, 20+ CD4+ cells compared to Vβ9-, 17-, 20- CD4+ cells sorted from high avidity sample. A) GAD 555 tetramer binding by Vβ9-, 17-, 20- (left) or Vβ9+, 17+, 20+ (right) CD4+ cells after sorting and culturing for 10 days. MFI denotes mean fluorescence intensity of PE-tetramer labeled populations in upper left or right quadrants. B) Binding of diluted GAD 555 tetramer by sorted Vβ9+, 17+, 20+ (top) or Vβ9-, 17-, 20- (bottom) CD4+ cells. Tetramer concentrations are denoted at top. C) Data in B. expressed as a fraction of maximum tetramer binding by sorted Vβ9+, 17+, 20+ or Vβ9-, 17-, 20- CD4+ cells. Open squares = Vβ 9+, 17+, 20+ CD4+ population; closed squares =Vβ 9-, 17-, 20- CD4+ population.
Discussion
The avidity of a T cell population for antigen dictates the ability to differentiate into memory cells and, ultimately, the effectiveness of an immune response [20]. In this study, we assessed autoreactive CD4+ T cell avidity maturation in subjects at different stages of T1D development. Since the frequency of autoreactive T cells in the peripheral circulation of T1D patients is very low [17], CD4+ T cells were expanded for 10 days with GAD 555 prior to analysis by ELISPOT. Antigen-limitation can result in the outgrowth of high avidity cells in cultures; therefore, we utilized a modest dose (10 μg/ml) of peptide that has been demonstrated to expand both high and low avidity populations in other systems [21]. In our hands, doses lower than 1 μg/ml of GAD 555 fail to expand GAD-reactive cells making it unlikely that a dose of 10 μg/ml is limiting and skews the response.
There were no significant differences in the proportions of subjects demonstrating IFN-γ responses in each group, which was not unexpected since autoreactive T cells have been shown to be prevalent in both healthy and T1D subjects [5, 6]. We had expected to observe a correlation between avidity and disease status such that samples from T1D patients would exhibit the highest avidities. However, the majority of T1D subject samples failed to secrete IFN-γ in response to GAD 555, and, of those that did, the avidities were no different from the at-risk subjects and first-degree relatives. These findings may indicate that there is no absolute T cell avidity threshold correlating with disease onset and that the range of GAD-reactive T cell avidities is unique to each subject. Non-responsive T1D patients may reflect the natural loss of GAD immunity due to reductions in the availability of islet antigens after diagnosis.
The observation of avidity maturation between samples from a pre-diabetic subject led us to investigate two possible mechanisms by which this may have occurred: up regulation of molecules associated with TCR signaling strength or outgrowth of T cells expressing high affinity TCR. Expression levels of TCRαβ, CD3ε, CD4, CD5, CD25 and CD81 differed very little between samples, but there was greater than 3-fold increase in IL-15Rα chain expression by the higher avidity GAD 555-responsive cells. Elevated serum levels of IL-15 have been observed in T1D patients and islet transplant recipients on immunosuppressive therapies [22, 23]. Interestingly, IL-15Rα signaling has been demonstrated to play a role in avidity maturation of mouse CD8+ T cells, in part, by inducing the up-regulation of Bcl-2, an anti-apoptotic transcription factor [24]. It is possible that the up-regulation of IL-15Rα on higher avidity GAD 555-reactive CD4+ cells serves to prolong their survival, particularly since cells of the same specificity isolated from T1D patients have been shown to be more susceptible to AICD compared to low avidity cells [16]. Since we did not assess levels of IL-15 in the T cell cultures or compare long-term viabilities of the high and low avidity populations, additional studies are needed to address potential mechanisms, if any, by which IL-15 signaling may promote CD4+ T cell avidity maturation.
T cells expressing TCR BV 9, 15, 17 and 20 were preferentially expanded in the high avidity pre-diagnostic sample. Subsequent analysis of these cells revealed that they exhibited higher tetramer binding strengths as revealed by increased MFI of staining and a higher frequency of stained cells at low tetramer concentrations. We conclude that expression of TCR BV 9, 17 or 20 is a marker of high affinity clonotypes that are selectively expanded during the pre-clinical stage, and this is the dominant mechanism of avidity maturation in this subject.
These findings are compelling in that the avidity maturation mechanism we identified is similar to that utilized by IGRP 206-reactive CD8+ T cells in pre-diabetic NOD mice; although, little is known about the factors responsible for the induction of avidity maturation [15]. Antigen dose has been reported to inversely correlate with the avidity of the responding T cell population [25]. Since the pre-clinical stage of diabetes in humans and NOD mice is characterized by progressive loss of islets, which are the presumed sources of GAD antigens, it is tempting to speculate that the preferential outgrowth of high avidity clonotypes is the result of gradual antigen limitation. However, Turner and colleagues have reported that reduced expression levels of a neo-self antigen limits, rather than enhances, avidity maturation of CD8+ memory T cells [26]. Also, reduced vaccine doses did not result in a statistically significant increase in the clonal selection of high avidity CD4+ T cells in an immunization model system [27]. While these data do not rule-out a role for progressive antigen reduction in T cell avidity maturation, other factors probably play important roles. The type and activation states of APC mediating T cell activation likely determine the magnitude of the response. Mature dendritic cells (DC) are known to be potent inducers of immunity and have been shown to selectively induce high avidity CD8+ T cells regardless of antigen dose [28]. Moreover, it was recently reported that plasmacytoid DC are expanded in recent-onset T1D patients and present higher levels of antigen in the presence of autoantibodies [29]. The increase in avidity we observed between pre-diabetic samples could be due to decreasing antigen doses or an increase in the prevalence of mature DC over time.
These findings have important implications for future studies of autoreactive T cell populations in T1D and other autoimmune diseases. Avidity maturation by selective expansion of a limited repertoire may represent the means by which benign self-reactive T cells develop into a pathological autoreactive population during type 1 diabetes development. Quantification of autoreactive T cell avidities from longitudinal samples may serve as a means of further identifying high-risk populations and staging patients for clinical trials of preventive or interventional therapies. Indeed, antigen-specific therapies designed to elicit peripheral deletional tolerance may be critically dependent on the avidity of the autoreactive T cells at specific stages of the disease.
Acknowledgments
This work was supported by grant 4-2007-1058 from the Juvenile Diabetes Research Foundation and AI50864 from the National Institutes of Health. N.E.S. is a recipient of a Juvenile Diabetes Research Foundation advanced post-doctoral fellowship grant (10-2008-587). The authors are grateful to Dr. K. Arumuganathan for expertise in flow cytometry. Portions of these data were presented in abstract form at the 2008 Federation of Clinical Immunology Societies annual meeting held in Boston, MA.
Footnotes
No potential conflicts of interest relevant to this article were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: Immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oling V, Marttila J, Ilonen J, Kwok WW, Nepom G, Knip M, Simell O, Reijonen H. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II teramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun. 2005;25:235–243. doi: 10.1016/j.jaut.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu AW, Kwok WW, Nepom GT. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 4.Standifer NE, Ouyang Q, Panagiotopoulos C, Verchere CB, Tan R, Greenbaum CJ, Pihoker C, Nepom GT. Identification of Novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes. 2006;55:3061–3067. doi: 10.2337/db06-0066. [DOI] [PubMed] [Google Scholar]
- 5.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 6.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects.m. J Autoimmun. 2005;25:303–311. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.van den Boorn JG, Le Poole IC, Luiten RM. T-cell avidity and tuning: the flexible connection between tolerance and autoimmunity. Int Rev Immunol. 2006;25:235–258. doi: 10.1080/08830180600743081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, Kwok WW, Nepom GT. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol. 2003;33:1409–1417. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 9.Tian J, Gregori S, Adorini L, Kaufman DL. The frequency of high avidity T cells determines the hierarchy of determinant spreading. J Immunol. 2001;166:7144–7150. doi: 10.4049/jimmunol.166.12.7144. [DOI] [PubMed] [Google Scholar]
- 10.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 11.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- 12.Fasso M, Anandasabapathy N, Crawford F, Kappler J, Fathman CG, Ridgway WM. T cell receptor (TCR)-mediated repertoire selection and loss of TCR vbeta diversity during the initiation of a CD4(+) T cell response in vivo. J Exp Med. 2000;192:1719–1730. doi: 10.1084/jem.192.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 14.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest. 2005;115:1879–1887. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallone R, Kochik SA, Laughlin EM, Gersuk VH, Reijonen H, Kwok WW, Nepom GT. Differential recognition and activation thresholds in human autoreactive GAD-specific T-cells. Diabetes. 2004;53:971–977. doi: 10.2337/diabetes.53.4.971. [DOI] [PubMed] [Google Scholar]
- 17.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, Kwok WW, Greenbaum C, Nepom GT. GAD65-specific CD4+ T-cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes. 2004;53:1987–1994. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 18.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichstetter S, Ettinger RA, Liu AW, Gebe JA, Nepom GT, Kwok WW. Distinct T cell interactions with HLA class II tetramers characterize a spectrum of TCR affinities in the human antigen-specific T cell response. J Immunol. 2000;165:6994–6998. doi: 10.4049/jimmunol.165.12.6994. [DOI] [PubMed] [Google Scholar]
- 20.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 22.Kuczynski S, Winiarska H, Abramczyk M, Szczawinska K, Wierusz-Wysocka B, Dworacka M. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:231–236. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118:1806–1814. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrancois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J Exp Med. 2008;205:1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28:698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroger CJ, Amoah S, Alexander-Miller MA. Cutting edge: Dendritic cells prime a high avidity CTL response independent of the level of presented antigen. J Immunol. 2008;180:5784–5788. doi: 10.4049/jimmunol.180.9.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen JS, Pang K, Skowera A, Ellis R, Rackham C, Lozanoska-Ochser B, Tree T, Leslie RD, Tremble JM, Dayan CM, Peakman M. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes. 2009;58:138–145. doi: 10.2337/db08-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]