Abstract
Increases in the incidence of postmenopausal breast cancers have been linked to screening and menopausal hormone use, but younger women have received less attention. Thus, we analyzed trends in breast cancer incidence (N = 387 231) using the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program 13-Registry database (1992–2004). Whites had higher incidence rates than blacks after age 40 years, but the reverse was true among younger women (black–white crossover). Among younger women, the rate per 100 000 woman-years was 16.8 for black vs 15.1 for white women; the highest black–white incidence rate ratio (IRR) was seen among women younger than 30 years (IRR = 1.52, 95% confidence interval = 1.34 to 1.73). This risk pattern was not observed in other ethnic groups. The black–white crossover among younger women was largely restricted to breast cancers with favorable tumor characteristics. The annual percentage change in the incidence of invasive breast cancers decreased modestly among older women but increased among younger (<40 years) white women. Continued surveillance of trends is needed, particularly for molecular subtypes that preferentially occur among young women.
CONTEXT AND CAVEATS
Prior knowledge
Incidence of postmenopausal breast cancer has been associated with screening and hormone therapy, but the mechanisms involved in premenopausal breast cancer incidence have not been studied as extensively.
Study design
The incidence of breast cancer during 1992–2004 (N = 387 231 women) was compared among racial and ethnic groups and among age groups using data from the National Cancer Institute's Surveillance, Epidemiology, and End Results Program 13-Registry database.
Contributions
White women who were 40 years or older had a higher rate of breast cancer than black women in this age group, but among younger women the reverse was true (black–white crossover). The annual percentage change in invasive breast cancer incidence increased only among white women younger than 40 years.
Implications
Trends in breast cancer incidence and the subtypes that occur among young women should continue to be monitored.
Limitations
The results reported in this study differ from those of a previous study, which may be due to differences in the study population and the methods used.
From the Editors
Recent analyses of breast cancer incidence trends have linked mammography and menopausal hormone usage to increases in incidence among postmenopausal women; however, analyses of data for younger women have received less attention. Given that mammography is not recommended for women younger than 40 years and menopausal hormone therapy is low in this group, incidence rates among such women may be associated with exposures occurring in early reproductive life. Breast cancers that occur in younger women are of concern because these cancers are often hormone receptor negative (estrogen receptor [ER]– and progesterone receptor [PR]–), are high grade, and are diagnosed at advanced stages (1).
We used the National Cancer Institute's Surveillance, Epidemiology, End Results Program (SEER, http://seer.cancer.gov/) and SEER*Stat 6.3.6 for the period 1992–2004 to analyze trends in breast cancer incidence using the 13-Registry database, which includes approximately 14% of the US population (2). Incidence data (N = 387 231) were stratified by age at diagnosis (all ages, <30, 30–39, 40–49, and ≥50 years), year of diagnosis (1992–1995, 1996–1999, and 2000–2004), racial and ethnic categories (non-Hispanic whites, non-Hispanic blacks, Hispanics, Asian or Pacific Islanders [API], American Indians or Alaskan natives [AI/AN], others/unknown), and pathologic features.
Age-adjusted incidence rates (2000 US standard population) were expressed per 100 000 woman-years (2). Incidence rate ratios (IRRs) were calculated to express relative risks compared with a referent. Temporal trends were assessed as annual percentage change (APC) in incidence rates from 1992 to 2004, with derivation of 95% confidence intervals to determine APCs that were statistically significantly different from a horizontal or flat trend line with a slope of zero. Poisson regression models were used to examine temporal trends with interaction terms for age and race. The null hypothesis of no trend interaction implied that the incidence rate curves for a given profile (eg, trends by blacks vs whites) had the same shapes and parallel slopes over time. The null hypothesis was rejected at the 95% confidence level, as previously described (3).
Although white women had higher incidence rates than black women after age 40 years, the reverse was true among younger women (black–white crossover). Among younger women, the rate per 100 000 woman-years was 16.8 for black vs 15.1 for white women; further, the highest black–white IRR was seen among women younger than 30 years (IRR = 1.52, 95% confidence interval = 1.34 to 1.73) (Table 1). Other racial and ethnic groups had lower incidence rates than non-Hispanic white women for all three age groups and did not exhibit the crossover pattern observed among black women, although IRRs were slightly higher among younger than older Hispanic, API, and AI/AN women.
Table 1.
Descriptive statistics for incident tumor characteristics among women with in situ or invasive breast cancer (n = 387 231), SEER-13 (1992–2004)*
| All ages combined | <30 y | 30–39 y | 40–49 y | ≥50 y | |||||||||||
| Sample size | 387 231 | 1851 | 19 641 | 71 650 | 294 089 | ||||||||||
| Percentage of total | 100.0 | 0.5 | 5.1 | 18.5 | 75.9 | ||||||||||
| Incidence rate | 157.9 | 1.6 | 49.2 | 193.7 | 434.2 | ||||||||||
| Characteristic | N | IR | IRR (95% CI) | N | IR | IRR (95% CI) | N | IR | IRR (95% CI) | N | IR | IRR (95% CI) | N | IR | IRR (95% CI) |
| Race/ethnicity | |||||||||||||||
| Non-Hispanic white | 292 342 | 173.2 | 1.0 (Referent) | 941 | 1.5 | 1.0 (Referent) | 12 035 | 52.5 | 1.0 (Referent) | 48 945 | 210.1 | 1.0 (Referent) | 230 421 | 478.6 | 1.0 (Referent) |
| Non-Hispanic black | 33 251 | 146.9 | 0.85 (0.84 to 0.86) | 328 | 2.3 | 1.52 (1.34 to 1.73) | 2650 | 56.5 | 1.08 (1.03 to 1.12) | 7296 | 183.5 | 0.87 (0.85 to 0.90) | 22 977 | 394.9 | 0.83 (0.81 to 0.84) |
| Hispanic | 27 694 | 105.0 | 0.61 (0.60 to 0.61) | 352 | 1.4 | 0.88 (0.78 to 1.00) | 2508 | 35.7 | 0.68 (0.65 to 0.71) | 6962 | 139.0 | 0.66 (0.64 to 0.68) | 17 872 | 280.9 | 0.59 (0.58 to 0.60) |
| API | 29 643 | 114.9 | 0.66 (0.66 to 0.67) | 190 | 1.3 | 0.84 (0.71 to 0.98) | 2121 | 45.4 | 0.87 (0.83 to 0.91) | 7374 | 174.5 | 0.83 (0.81 to 0.85) | 19 958 | 291.8 | 0.61 (0.60 to 0.62) |
| AI/AN | 1964 | 76.6 | 0.44 (0.42 to 0.46) | 19 | 1.0 | 0.66 (0.40 to 1.04) | 166 | 28.1 | 0.54 (0.46 to 0.62) | 506 | 100.8 | 0.48 (0.44 to 0.52) | 1273 | 204.0 | 0.43 (0.40 to 0.45) |
| Other or unknown | 2337 | — | — | 21 | — | — | 161 | — | — | 567 | — | — | 1588 | — | — |
| Size | |||||||||||||||
| ≤2.0 cm | 209 203 | 85.5 | 1.0 (Referent) | 673 | 0.6 | 1.0 (Referent) | 8420 | 21.2 | 1.0 (Referent) | 35 240 | 95.2 | 1.0 (Referent) | 164 870 | 244.0 | 1.0 (Referent) |
| >2.0 cm | 109 398 | 44.4 | 0.52 (0.52 to 0.52) | 865 | 0.7 | 1.29 (1.16 to 1.42) | 8050 | 20.1 | 0.95 (0.92 to 0.98) | 22 642 | 61.2 | 0.64 (0.63 to 0.65) | 77 841 | 114.4 | 0.47 (0.47 to 0.47) |
| Other or unknown | 68 630 | — | — | 313 | — | — | 3171 | — | — | 13 768 | — | — | 51 378 | — | — |
| Lymph nodes | |||||||||||||||
| Negative | 184 135 | 75.6 | 1.0 (Referent) | 737 | 0.6 | 1.0 (Referent) | 8606 | 21.6 | 1.0 (Referent) | 33 076 | 89.4 | 1.0 (Referent) | 141 716 | 210.9 | 1.0 (Referent) |
| Positive | 89 713 | 36.8 | 0.49 (0.48 to 0.49) | 712 | 0.6 | 0.96 (0.87 to 1.07) | 7167 | 17.9 | 0.83 (0.80 to 0.86) | 20 496 | 55.4 | 0.62 (0.61 to 0.63) | 61 338 | 91.4 | 0.43 (0.43 to 0.44) |
| Other or unknown | 113 383 | — | — | 402 | — | — | 3868 | — | — | 18 078 | — | — | 91 035 | — | — |
| Grade | |||||||||||||||
| Low | 182 928 | 74.7 | 1.0 (Referent) | 426 | 0.4 | 1.0 (Referent) | 6316 | 15.9 | 1.0 (Referent) | 29 959 | 81.0 | 1.0 (Referent) | 146 227 | 215.8 | 1.0 (Referent) |
| High | 122 285 | 50.0 | 0.67 (0.66 to 0.67) | 1107 | 0.9 | 2.57 (2.29 to 2.88) | 9932 | 24.8 | 1.56 (1.51 to 1.61) | 27 189 | 73.5 | 0.91 (0.89 to 0.92) | 84 057 | 124.8 | 0.58 (0.57 to 0.58) |
| Other or unknown | 82 018 | — | — | 318 | — | — | 3393 | — | — | 14 502 | — | — | 63 805 | — | — |
| AJCC stage | |||||||||||||||
| 0 | 66 296 | 27.4 | 1.0 (Referent) | 148 | 0.1 | 1.0 (Referent) | 2447 | 6.2 | 1.0 (Referent) | 15 148 | 40.9 | 1.0 (Referent) | 48 553 | 72.6 | 1.0 (Referent) |
| I | 140 232 | 57.3 | 2.10 (2.08 to 2.12) | 380 | 0.3 | 2.56 (2.11 to 3.12) | 4981 | 12.5 | 2.02 (1.93 to 2.12) | 21 584 | 58.3 | 1.42 (1.40 to 1.45) | 113 287 | 167.6 | 2.31 (2.28 to 2.33) |
| II | 109 317 | 44.6 | 1.63 (1.62 to 1.65) | 808 | 0.7 | 5.42 (4.54 to 6.50) | 8047 | 20.1 | 3.26 (3.11 to 3.41) | 23 296 | 63.0 | 1.54 (1.51 to 1.57) | 77 166 | 114.3 | 1.57 (1.56 to 1.59) |
| III | 21 684 | 8.8 | 0.32 (0.32 to 0.33) | 189 | 0.2 | 1.28 (1.02 to 1.59) | 1747 | 4.4 | 0.70 (0.66 to 0.75) | 4742 | 12.8 | 0.31 (0.30 to 0.32) | 15 006 | 22.1 | 0.30 (0.30 to 0.31) |
| IV | 13 816 | 5.6 | 0.20 (0.20 to 0.21) | 80 | 0.1 | 0.55 (0.41 to 0.72) | 743 | 1.9 | 0.30 (0.27 to 0.32) | 1997 | 5.4 | 0.13 (0.13 to 0.14) | 10 996 | 16.2 | 0.22 (0.22 to 0.23) |
| Other or unknown | 35 886 | — | 0.52 (0.51 to 0.53) | 246 | — | 1.71 (1.39 to 2.11) | 1676 | — | 0.68 (0.64 to 0.72) | 4883 | — | 0.32 (0.31 to 0.33) | 29 081 | — | 0.57 (0.56 to 0.58) |
| Behavior | |||||||||||||||
| In situ | 66 074 | 27.3 | 1.0 (Referent) | 148 | 0.1 | 1.0 (Referent) | 2438 | 6.2 | 1.0 (Referent) | 15 127 | 40.9 | 1.0 (Referent) | 48 361 | 72.3 | 1.0 (Referent) |
| Invasive | 321 157 | 130.7 | 4.79 (4.75 to 4.83) | 1703 | 1.4 | 11.51 (9.71 to 13.71) | 17 203 | 43.1 | 6.99 (6.70 to 7.29) | 56 523 | 152.8 | 3.74 (3.67 to 3.80) | 245 728 | 361.9 | 5.00 (4.96 to 5.05) |
| Histology subtype | |||||||||||||||
| Duct | 249 580 | 101.9 | 1.0 (Referent) | 1319 | 1.1 | 1.0 (Referent) | 14 147 | 35.4 | 1.0 (Referent) | 47 345 | 128.0 | 1.0 (Referent) | 186 769 | 276.3 | 1.0 (Referent) |
| Lobular | 33 239 | 13.6 | 0.13 (0.13 to 0.13) | 44 | 0.0 | 0.03 (0.02 to 0.05) | 720 | 1.8 | 0.05 (0.05 to 0.06) | 5851 | 15.8 | 0.12 (0.12 to 0.13) | 26 624 | 39.3 | 0.14 (0.14 to 0.14) |
| Ductal variants | 21 632 | 9 | 0.09 (0.08 to 0.09) | 97 | 0.1 | 0.07 (0.06 to 0.09) | 941 | 2.4 | 0.07 (0.06 to 0.07) | 3314 | 9 | 0.07 (0.07 to 0.07) | 17 280 | 25.3 | 0.09 (0.09 to 0.09) |
| Inflammatory | 3421 | 1.4 | 0.01 (0.01 to 0.01) | 41 | 0.0 | 0.03 (0.02 to 0.04) | 319 | 0.8 | 0.02 (0.02 to 0.03) | 737 | 2.0 | 0.02 (0.01 to 0.02) | 2324 | 3.5 | 0.01 (0.01 to 0.01) |
| Other or unknown | 79 359 | — | — | 350 | — | — | 3514 | — | — | 14 403 | — | — | 61 092 | — | — |
| ER status | |||||||||||||||
| Positive | 208 616 | 85.1 | 1.0 (Referent) | 682 | 0.6 | 1.0 (Referent) | 8626 | 21.7 | 1.0 (Referent) | 34 768 | 94.0 | 1.0 (Referent) | 164 540 | 242.7 | 1.0 (Referent) |
| Negative | 61 434 | 25.2 | 0.30 (0.29 to 0.30) | 626 | 0.5 | 0.92 (0.82 to 1.03) | 5842 | 14.6 | 0.67(0.65 to 0.70) | 14 358 | 38.8 | 0.41 (0.41 to 0.42) | 40 608 | 60.6 | 0.25 (0.25 to −0.25) |
| Other or unknown | 117 181 | — | — | 543 | — | — | 5173 | — | — | 22 524 | — | — | 88 941 | — | — |
Non-Hispanic whites and blacks are exclusive of Hispanics. Alaska registry was excluded in the calculation of estimates for Hispanics. Incidence rates for American Indians/Alaskan natives (AI/AN) are based on Contract Health Service Delivery Areas. API = Asian or Pacific Islander; IR = incidence rates, which were age adjusted (2000 US standard population) and expressed per 100 000 woman-years; IRR = incidence rate ratio, for which a given characteristic was compared with a referent characteristic with an assigned IRR of 1.0. IRRs were tested for statistical significance via 95% confidence intervals using SEER*Stat 6.2.4 (see text). CI = confidence interval; low grade = well differentiated grade I and moderately differentiated grade II; high grade = poorly differentiated grade III and undifferentiated anaplastic grade IV; ductal variants = tubular, medullary, mucinous, and papillary breast cancers; — = not calculated; lymph nodes = axillary lymph nodal status; AJCC = American Joint Committee on Cancer; ER = estrogen receptor.
Younger women had higher IRRs than older women for tumors with poor prognostic features, defined by tumor size (>2.0 vs ≤2.0 cm in diameter), lymph node status (positive vs negative), and nuclear grade (high [III–IV] vs low [I–II]). In addition, young women had higher IRRs for inflammatory breast cancers and ER tumors.
Overall, breast cancer incidence rates were higher for white women than black women, but this relationship was attributable to higher incidence rates for tumors with better prognostic features among older women. The black–white crossover among younger women was largely restricted to breast cancers with favorable tumor characteristics (Table 2). For poor prognosis tumors, there was little evidence of crossover. At all ages, black women fairly consistently showed higher rates than white women, with the largest black–white IRR observed among the youngest women.
Table 2.
Descriptive statistics for incident tumor characteristics among black and white women with in situ or invasive breast cancer (n = 325 593), SEER-13 (1992–2004)*
| All ages combined | <30 y | 30–39 y | 40–49 y | ≥50 y | |||||||||||
| Sample size blacks | 33 251 | 328 | 2650 | 7296 | 22 977 | ||||||||||
| Sample size whites | 292 342 | 941 | 12 035 | 48 945 | 230 421 | ||||||||||
| Incidence rate blacks | 146.9 | 2.3 | 56.5 | 183.5 | 394.9 | ||||||||||
| Incidence rate whites | 173.2 | 1.5 | 52.5 | 210.1 | 478.6 | ||||||||||
| Characteristic | Black IR | White IR | Black:white IRR (95% CI) | Black IR | White IR | Black:white IRR (95% CI) | Black IR | White IR | Black:white IRR (95% CI) | Black IR | White IR | Black:white IRR (95% CI) | Black IR | White IR | Black:white IRR (95% CI) |
| Size | |||||||||||||||
| ≤2.0 cm | 63.3 | 97.2 | 0.65 (0.64 to 0.66) | 0.7 | 0.7 | 1.03 (0.81 to 1.30) | 19.4 | 24.5 | 0.79 (0.73 to 0.85) | 72.4 | 108.3 | 0.67 (0.64 to 0.69) | 177.0 | 277.1 | 0.64 (0.63 to 0.65) |
| >2.0 cm | 53.7 | 45.3 | 1.18 (1.16 to 1.21) | 1.2 | 0.7 | 1.75 (1.45 to 2.11) | 27.5 | 19.3 | 1.43 (1.34 to 1.52) | 73.1 | 60.6 | 1.21 (1.16 to 1.26) | 136.7 | 118.7 | 1.15 (1.12 to 1.18) |
| Lymph nodes | |||||||||||||||
| Negative | 57.9 | 85.2 | 0.68 (0.67 to 0.69) | 0.8 | 0.7 | 1.11 (0.88 to 1.37) | 21.7 | 24.1 | 0.90 (0.84 to 0.96) | 73.2 | 99.8 | 0.73 (0.71 to 0.76) | 155.7 | 238.4 | 0.65 (0.64 to 0.67) |
| Positive | 38.7 | 39.3 | 0.99 (0.96 to 1.01) | 1.0 | 0.6 | 1.72 (1.40 to 2.11) | 21.6 | 18.4 | 1.17 (1.09 to 1.26) | 59.4 | 57.9 | 1.03 (0.98 to 1.07) | 93.6 | 98.9 | 0.95 (0.92 to 0.97) |
| Grade | |||||||||||||||
| Low | 55.1 | 83.9 | 0.66 (0.64 to 0.67) | 0.5 | 0.4 | 1.31 (0.98 to 1.74) | 13.7 | 17.7 | 0.77 (0.71 to 0.84) | 57.8 | 90.5 | 0.64 (0.61 to 0.67) | 158.8 | 242.9 | 0.65 (0.64 to 0.67) |
| High | 56.9 | 52.6 | 1.08 (1.06 to 1.10) | 1.4 | 0.9 | 1.55 (1.31 to 1.82) | 31.8 | 25.7 | 1.24 (1.17 to 1.31) | 86.7 | 76.0 | 1.14 (1.10 to 1.18) | 137.9 | 132.6 | 1.04 (1.02 to 1.06) |
| ER status | |||||||||||||||
| Positive | 61.6 | 96.6 | 0.64 (0.63 to 0.65) | 0.8 | 0.6 | 1.22 (0.98 to 1.51) | 20.1 | 24.2 | 0.83 (0.77 to 0.89) | 67.5 | 105.8 | 0.64 (0.61 to 0.66) | 172.9 | 276.3 | 0.63 (0.61 to 0.64) |
| Negative | 34.4 | 26.0 | 1.33 (1.29 to 1.36) | 0.8 | 0.5 | 1.60 (1.29 to 1.99) | 20.9 | 15.1 | 1.39 (1.29 to 1.49) | 54.6 | 39.8 | 1.37 (1.31 to 1.43) | 81.4 | 62.7 | 1.30 (1.26 to 1.34) |
Non-Hispanic whites and blacks are exclusive of Hispanics. IR = incidence rates, which were age adjusted (2000 US standard population) and expressed per 100 000 woman-years; IRR = incidence rate ratio, for which a given characteristic was compared with a referent characteristic with an assigned IRR of 1.0. IRRs were tested for statistical significance via 95% confidence intervals using SEER*Stat 6.2.4 (see text). CI = confidence interval; low grade = well differentiated grade I and moderately differentiated grade II; high grade = poorly differentiated grade III and undifferentiated anaplastic grade IV; ER = estrogen receptor.
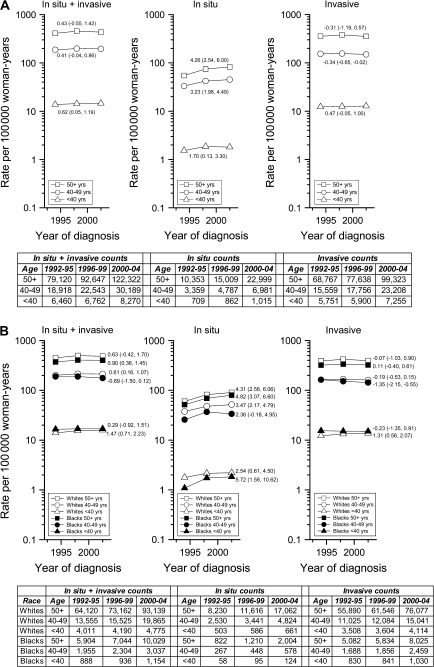
To derive stable secular trends for younger women, women who were younger than 30 years and 30–39 years were combined into a single age group (Figure 1). The absolute number (or counts) of breast cancers increased among younger women during 1992–2004, but this increase largely reflected population growth rather than rising rates of invasive disease (Figure 1, A). APC was 0.43 for older women (Figure 1, A, in situ + invasive). This increase was primarily due to increasing rates of in situ cancers, particularly among women aged 50 years and older (APC of 4.26 vs 1.70 for women <40 years, P < .001 for trend interaction by age). Invasive cancers decreased slightly over time among older women, but among younger women APC was 0.47 (P < .001 for trend interaction by age). This increase was restricted to younger white women (Figure 1, B).
Figure 1.
Annual percentage changes (APCs) for age-adjusted incidence rate trends and absolute numbers in the National Cancer Institute's Surveillance, Epidemiology, End Results Program 13-Registry database (1992–2004) for all breast cancers combined (in situ + invasive), in situ cancers only, and invasive cancers only. A) APCs and absolute numbers by age (<40, 40–49, ≥50 years). B) APCs and absolute numbers by age (<40, 40–49, ≥50 years) and race (white, black). APCs are recorded with point estimates; 95% confidence intervals are in parentheses. Under the null hypothesis, non–statistically significant APCs indicate that the trend line for a given age group was no different than a horizontal or flat trend line with a slope of zero. Using Poisson regression models we also assessed whether trends varied by age group (A) or by race within age group, see text for details.
Our results are somewhat discrepant with a previous analysis (4), which observed slight decreases in invasive cancer incidence over time among women who were younger than 40 years. This inconsistency may reflect differences in study areas, time periods, or analytic methods, or instability of rates involved. Although the previous analysis had postulated possible rate increases based on changes in many risk factors over time, trends are difficult to project given that a number of risk factors operate distinctively among young women (5). For example, unlike postmenopausal cancers, for which obesity is associated with increased risk and parity with decreased risk, for premenopausal breast cancers, obesity is associated with decreased risk and parity may be a risk factor. Therefore, increasing obesity among young women (6) and delays in childbirth (7) may be counteracting other risk factors that would increase incidence. Future surveillance is needed to monitor breast cancer incidence rates as cohorts of younger women advance to age groups with higher incidence.
The black–white crossover for overall breast cancer incidence rates at younger ages has been previously described (8–12), but the underlying mechanisms are unclear. Rates of mammography are reportedly higher among young black than white women (13). The explanation for this is unknown, but data suggest that fibrocystic changes are more commonly identified on physical examination among blacks, raising the possibility that these findings prompt mammography for further evaluation. However, generally low mammography rates among young women and our finding that young black women have higher incidence rates of tumors both with and without unfavorable characteristics argues against differential screening as a primary explanatory factor. If delayed detection was a factor, more black women should be diagnosed when they are older (not younger) (14). A more likely explanation for the black–white crossover is that black women have distinct risk factors (15). Differences in reproductive factors have been implicated (10), particularly younger ages at childbirth leading to short-term increases in postpartum breast cancer risk (11). Support for this hypothesis comes from reported associations between increased breast cancer risk and multiparity among young black women (15–17).
Similar to others (18,19), we found that younger women, especially black women, are often diagnosed with tumors that have poor prognostic features, which may partially reflect a detection bias favoring identification of faster-growing tumors among unscreened women. An impact of genetic factors, particularly on breast cancer incidence rates at younger ages, may also contribute to the poorer prognosis (20). For example, BRCA2 and other mutations that are more commonly detected in young black women might account for some racial disparities at young ages (21,22). Recent pregnancies have been shown to have a growth-promoting effect on breast tumors (23,24), which could have the greatest impact on black women, who have more children and shorter intervals since a last pregnancy than white women (25,26).
Further understanding of factors influencing breast cancer trends among younger women may benefit from a focus on breast cancer subtypes that occur preferentially among young women (particularly young black women), including basal-like (27) and the less specific category of “triple (ie, ER, PR, and HER2)-negative” (28) tumors. Genetic (29) and lifestyle factors (27) may contribute to the occurrence of these malignancies, but additional studies are needed to fully elucidate their etiology. Given that mammography is neither recommended for nor is sensitive in younger women because of high breast tissue density (30), additional efforts are needed to identify relevant primary and secondary preventive approaches, including the identification of early risk predictors and biomarkers.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
References
- 1.Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2005;23((1)):9–15. doi: 10.3233/bd-2006-23103. [DOI] [PubMed] [Google Scholar]
- 2. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Limited-Use, Nov 2006 sub (1992–2004) - Linked To County Attributes – Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission. [Google Scholar]
- 3.Anderson WF, Matsuno RK, Sherman ME, et al. Estimating age-specific breast cancer risks: a descriptive tool to identify age interactions. Cancer Causes Control. 2007;18(4):439–447. doi: 10.1007/s10552-006-0092-9. [DOI] [PubMed] [Google Scholar]
- 4.Tarone RE. Breast cancer trends among young women in the United States. Epidemiology. 2006;17(5):588–590. doi: 10.1097/01.ede.0000229195.98786.ee. [DOI] [PubMed] [Google Scholar]
- 5.Althuis MD, Brogan DD, Coates RJ, et al. Breast cancers among very young premenopausal women (United States) Cancer Causes Control. 2003;14(2):151–160. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 6.Blanck HM, Dietz WH, Galuska DA. State-specific prevalence of obesity among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 55(36):985–988. [PubMed] [Google Scholar]
- 7.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1–116. [PubMed] [Google Scholar]
- 8.Gray GE, Henderson BE, Pike MC. Changing ratio of breast cancer incidence rates with age of black females compared with white females in the United States. J Natl Cancer Inst. 1980;64(3):461–463. [PubMed] [Google Scholar]
- 9.Joslyn SA, Foote ML, Nasseri K, Coughlin SS, Howe HL. Racial and ethnic disparities in breast cancer rates by age: NAACCR Breast Cancer Project. Breast Cancer Res Treat. 2005;92(2):97–105. doi: 10.1007/s10549-005-2112-y. [DOI] [PubMed] [Google Scholar]
- 10.Krieger N. Social class and the black/white crossover in the age-specific incidence of breast cancer: a study linking census-derived data to population-based registry records. Am J Epidemiol. 1990;131(5):804–814. doi: 10.1093/oxfordjournals.aje.a115571. [DOI] [PubMed] [Google Scholar]
- 11.Pathak DR, Osuch JR, He J. Breast carcinoma etiology: current knowledge and new insights into the effects of reproductive and hormonal risk factors in black and white populations. Cancer. 2000;88(5 suppl):1230–1238. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1230::aid-cncr9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Yankaskas BC. Epidemiology of breast cancer in young women. Breast Dis. 2005;23((1)):3–8. doi: 10.3233/bd-2006-23102. [DOI] [PubMed] [Google Scholar]
- 13.Scharpf TP, Rimm AA. Mammography utilization rates among young white and black women in the USA. Public Health. 2006;120(10):937–941. doi: 10.1016/j.puhe.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Anderson WF, Reiner AS, Matsuno RK, Pfeiffer RM. Shifting breast cancer trends in the United States. J Clin Oncol. 2007;25((25)):3923–3929. doi: 10.1200/JCO.2007.11.6079. [DOI] [PubMed] [Google Scholar]
- 15.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and white women. Am J Epidemiol. 2005;161(1):40–51. doi: 10.1093/aje/kwh331. [DOI] [PubMed] [Google Scholar]
- 16.Brinton LA, Benichou J, Gammon MD, Brogan DR, Coates R, Schoenberg JB. Ethnicity and variation in breast cancer incidence. Int J Cancer. 1997;73(3):349–355. doi: 10.1002/(sici)1097-0215(19971104)73:3<349::aid-ijc8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Palmer JR, Wise LA, Horton NJ, Adams-Campbell LL, Rosenberg L. Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst. 2003;95(6):478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo JC, Ennis M, Knight JA, et al. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res Treat. 2006;105((1)):69–80. doi: 10.1007/s10549-006-9433-3. [DOI] [PubMed] [Google Scholar]
- 19.Zabicki K, Colbert JA, Dominguez FJ, et al. Breast cancer diagnosis in women < or = 40 versus 50 to 60 years: increasing size and stage disparity compared with older women over time. Ann Surg Oncol. 2006;13(8):1072–1077. doi: 10.1245/ASO.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 20.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298((24)):2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 21.Haffty BG, Silber A, Matloff E, Chung J, Lannin D. Racial differences in the incidence of BRCA1 and BRCA2 mutations in a cohort of early onset breast cancer patients: African American compared to white women. J Med Genet. 2006;43(2):133–137. doi: 10.1136/jmg.2005.034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaan Y, Kpenu E, Utley K, et al. Inherited BRCA2 mutations in African Americans with breast and/or ovarian cancer: a study of familial and early onset cases. Hum Genet. 2003;113(5):452–460. doi: 10.1007/s00439-003-0999-0. [DOI] [PubMed] [Google Scholar]
- 23.Kroman N, Mouridsen HT. Prognostic influence of pregnancy before, around, and after diagnosis of breast cancer. Breast. 2003;12(6):516–521. doi: 10.1016/s0960-9776(03)00159-0. [DOI] [PubMed] [Google Scholar]
- 24.Largent JA, Ziogas A, Anton-Culver H. Effect of reproductive factors on stage, grade and hormone receptor status in early-onset breast cancer. Breast Cancer Res. 2005;7(4):R541–R554. doi: 10.1186/bcr1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117(1):168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 26.Ursin G, Bernstein L, Wang Y, et al. Reproductive factors and risk of breast carcinoma in a study of white and African-American women. Cancer. 2004;101(2):353–362. doi: 10.1002/cncr.20373. [DOI] [PubMed] [Google Scholar]
- 27.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2007;109((1)):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 29.Tischowitz M, Foulkes W. The basal phenotype of BRCA1-related breast cancer: past, present and future. Cell Cycle. 2006;5((9)):963–967. doi: 10.4161/cc.5.9.2713. [DOI] [PubMed] [Google Scholar]
- 30.Houssami N, Irwig L, Simpson JM, McKessar M, Blome S, Noakes J. Sydney Breast Imaging Accuracy Study: Comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003;180(4):935–940. doi: 10.2214/ajr.180.4.1800935. [DOI] [PubMed] [Google Scholar]