Abstract
Background
Lysophosphatidic acid (LPA) acts through the cell surface G protein–coupled receptors, LPA1, LPA2, or LPA3, to elicit a wide range of cellular responses. It is present at high levels in intraperitoneal effusions of human ovarian cancer increasing cell survival, proliferation, and motility as well as stimulating production of neovascularizing factors. LPA2 and LPA3 and enzymes regulating the production and degradation of LPA are aberrantly expressed by ovarian cancer cells, but the consequences of these expression changes in ovarian cancer cells were unknown.
Methods
Expression of LPA1, LPA2, or LPA3 was inhibited or increased in ovarian cancer cells using small interfering RNAs (siRNAs) and lentivirus constructs, respectively. We measured the effects of changes in LPA receptor expression on cell proliferation (by crystal violet staining), cell motility and invasion (using Boyden chambers), and cytokines (interleukin 6 [IL-6], interleukin 8 [IL-8], and vascular endothelial growth factor [VEGF]) production by enzyme-linked immunosorbent assay. The role of LPA receptors in tumor growth, ascites formation, and cytokine production was assessed in a mouse xenograft model. All statistical tests were two-sided.
Results
SKOV-3 cells with increased expression of LPA receptors showed increased invasiveness, whereas siRNA knockdown inhibited both migration (P < .001, Student t test) and invasion. Knockdown of the LPA2 or LPA3 receptors inhibited the production of IL-6, IL-8, and VEGF in SKOV-3 and OVCAR-3 cells. SKOV-3 xenografts expressing LPA receptors formed primary tumors of increased size and increased ascites volume. Invasive tumors in the peritoneal cavity occurred in 75% (n = 4) of mice injected with LPA1 expressing SKOV-3 and 80% (n = 5) of mice injected with LPA2 or LPA3 expressing SKOV-3 cells. Metastatic tumors expressing LPA1, LPA2, and LPA3 were identified in the liver, kidney, and pancreas; tumors expressing LPA2 and LPA3 were detected in skeletal muscle; and tumors expressing LPA2 were also found in the cervical lymph node and heart. The percent survival of mice with tumors expressing LPA2 or LPA3 was reduced in comparison with animals with tumors expressing β-galactosidase.
Conclusions
Expression of LPA2 or LPA3 during ovarian carcinogenesis contributes to ovarian cancer aggressiveness, suggesting that the targeting of LPA production and action may have potential for the treatment of ovarian cancer.
CONTEXT AND CAVEATS
Prior knowledge
Signaling by lysophosphatidic acid (LPA) has effects on survival and proliferation of ovarian cancer cells as well as the production of neovascularizing factors. The extent to which LPA’s effects on ovarian cancer cells are mediated by G protein–coupled receptors LPA1, LPA2, and LPA3, the latter two of which are typically highly expressed in human ovarian cancer cell lines, was unknown.
Study design
Expression of individual LPA receptors was inhibited or increased using RNA interference or lentivirus constructs, respectively, in ovarian cancer cells. Effects of these expression changes were studied in vitro cell assays and in a mouse xenograft model.
Contribution
Based on the sensitivity of ovarian cancer cell proliferation and invasiveness and in vivo tumor growth to the levels of individual LPA receptors, this study suggests that signaling by LPA via LPA2 and LPA3 receptors may play a critical role in the progression of ovarian cancer.
Implications
The identification of individual LPA receptors that mediate LPA action in ovarian cancer raises the possibility that these receptors may be useful therapeutic targets.
Limitations
The role of LPA signaling in ovarian cancer was studied using cell lines that may not be adequate models for many human cancers.
From the Editors
Lysophosphatidic acid (LPA, 1-acyl-2-lyso-SN-glycero-3-phosphate), the simplest glycerophospholipid, mediates a wide range of biologic actions, including stimulation of DNA synthesis, cell proliferation, cytoskeleton reorganization, cell survival, drug resistance, cell adhesion, migration, cytokine production, and ion transport (1,2). The biologic functions of extracellular LPA are mediated through specific G protein–coupled receptors (GPCRs), including Edg-2/LPA1, Edg-4/LPA2, and Edg-7/LPA3, that belong to the endothelial differentiation gene (Edg) family (3–5). Other members of the Edg family, Edg-1/S1P1, Edg-3/S1P3, Edg-5/S1P2, Edg-6/S1P4, and Edg-8/S1P5, are high-affinity receptors for the structurally related lysophospholipid sphingosine 1-phosphate (S1P) (6,7). LPA receptors may heterodimerize, thereby increasing their selectivity and broadening their spectrum of activity (8). Recently, the GPCRs GPR23/p2y9 (LPA4) (9), GPR92/93 (LPA5) (10,11), GPR87/95 (LPA6) (12), p2y5 (13), and p2y10 (14) have been reported to be novel, non-Edg family LPA receptors that have little sequence homology to LPA1–3. The physiological roles and the relevance of these new receptors to LPA function have not yet been determined. LPA can also bind to and activate the intracellular receptor PPARγ, leading to physiological and pathophysiological effects, in particular aberrations in thrombosis and atherogenesis (15,16).
LPA also increases the expression and production of neovascularizing factors such as interleukin 6 (IL-6), interleukin 8 (IL-8), vascular endothelial growth factor (VEGF), growth-regulated oncogene alpha (Gro-α), urokinase plasminogen activator (uPA), and other oncogenesis-related proteins by ovarian cancer cells (17–21). It has been extensively studied for its roles in signal transduction and physiological responses in the context of wound healing and multiple pathophysiological processes such as ischemia reperfusion injury, fibrosis, autoimmune disease, and cancer (22).
LPA was first implicated in human oncogenesis by our observation that LPA is present at high levels in the ascitic fluid of ovarian cancer patients (23,24). This could be due to the increased number of ovarian cancer cells present in the peritoneal cavity, as ovarian cancer cells can produce LPA (25). It could also be due to irritation of the peritoneal mesothelium (mesothelial cells produce LPA) (26); aberrations in the production and action of LPA due to altered levels of autotaxin (27), the enzyme that produces LPA; or altered levels of lipid phosphate phosphohydrolases (LPPs), which metabolize LPA (28,29). Introduction of LPP3 into ovarian cancer cells decreases their growth both in vitro and in vivo (29).
Further evidence of a critical role for LPA in ovarian cancer was provided by the observation that LPA2 and LPA3 receptors are aberrantly overexpressed in the majority of ovarian cancer cells (30). We found that LPA1 mRNA levels are similar in normal and transformed ovarian epithelial cells (31,32); however, higher expression of LPA2 and LPA3 mRNA was detected in most ovarian cancer cell lines compared with normal ovarian epithelial cells (30,31). Using real-time reverse transcription–polymerase chain reaction (RT–PCR) we confirmed that LPA2 and LPA3 mRNAs are overexpressed in a substantial portion (15%–30% and 44%–49%, respectively) of ovarian cancers compared with tissues from normal ovaries, benign ovarian tumors, and other normal tissues of nonovarian origin (31). Aberrant expression of the LPA2 and/or LPA3 receptors, whether a cause or a consequence of cell transformation, has also been associated with other malignancies, including thyroid (33), colorectal (34) and breast (35) cancer. However, there is no evidence that the increased expression of LPA2 or LPA3 is causally linked to the malignant phenotype of cancer cells.
In this study, we investigated whether aberrant LPA receptor expression contributes to the malignant properties acquired by ovarian cancer cells, by exploring in vitro and in vivo systems of knockdown and overexpression of each Edg family LPA receptor in SKOV-3 cells, an ovarian cancer cell line exhibiting low to modest levels of endogenous LPA receptors (18).
Methods
Materials and Reagents
LPA (18:1) was obtained from Avanti Polar Lipids, Inc (Alabaster, AL). Before use, it was dissolved in phosphate-buffered saline (PBS) containing 0.5% fatty acid–free bovine serum albumin (BSA) (Roche Applied Science, Indianapolis, IN). Fetal bovine serum (FBS), anti-Flag M2 antibody, and anti–β-actin antibody were from Sigma (St Louis, MO). IL-6, IL-8, and VEGF enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (Minneapolis, MN). Anti–V-5 antibody was from Invitrogen (Carlsbad, CA). The cell death detection ELISA kit was from Roche Applied Science. SKOV-3 human ovarian cancer cells derived from ascitic fluid and OVCAR-3 ovarian epithelial adenocarcinoma cells were from American Type Culture Collection (ATCC) (Manassas, VA) and maintained in RPMI 1640 medium (CCSG Media Preparation Facility, The University of Texas M. D. Anderson Cancer Center, Houston, TX) supplemented with 10% FBS. For transfectants, SKOV-3 cell medium was also supplemented with blasticidin S HCl (10 μg/mL; Invitrogen) to maintain stable transfection. HT-29 human colon adenocarcinoma cells were from ATCC and maintained in Dulbecco's modified Eagle Medium (CCSG Media Preparation Facility) supplemented with 10% FBS.
Generation of Lentivirus Constructs Carrying LPA Receptors and Infection of Cells With Lentivirus
The ViralPower Lentiviral Expression System was obtained from Invitrogen (Carlsbad, CA). The system allows creation of a replication-incompetent, HIV-based lentivirus that can efficiently transduce mammalian cells, as we demonstrated previously (18). Restriction sites (NcoI (5′), Xho1 (3′)) and a consensus Kozak sequence were added to the cDNAs of LPA1, LPA2, and LPA3 by PCR amplification from their pcDNA3-based expression vectors (26). The PCR products were cloned into a Gateway entry vector (pENTR4) using NcoI and XhoI restriction sites. The viral constructs were made through homologous recombination between pENTR4-LPA1-3 and the lentiviral destination vector pLenti6/V-5-DEST. In the resultant viral constructs, each of the LPA receptors was tagged with V-5 at the C terminus. The downstream anti-blasticidin gene allows selection of stably transduced cells. The structures of these lentiviral constructs were verified by DNA sequencing.
High titers of viral stocks were generated by cotransfection of 293FT cells with the viral constructs and the packaging plasmids using Lipofectamine 2000 as we described previously (18). The ovarian cancer cell line SKOV-3 was routinely maintained in RPMI 1640 containing 10% FBS. SKOV-3 cells in six-well plates were infected with 1 mL of undiluted viral stocks in the presence of polybrene (7.5 μg/mL). Two days later, blasticidin (10 μg/mL) was added to the culture. Discrete colonies resistant to blasticidin appeared within 2–3 weeks. These colonies were then detached by trysinization and expanded to 60- and 100-mm dishes sequentially for further experiments.
Western Blotting
SKOV-3 cells were infected with recombinant lentivirus carrying LacZ, LPA1, LPA2, or LPA3 tagged with V-5. Cells were lysed in ice-cold Triton X-100 lysis buffer (18). Cellular protein concentrations were determined by BCA reaction kit (Pierce, Rockford, IL). Equal amounts of total cellular proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to Immobilon (polyvinylidene difluoride), and immunoblotted with antibodies following the protocols of manufacturers. Expression of the V-5–tagged LacZ and LPA1, LPA2, and LPA3 receptors were analyzed by immunoblotting with anti–V-5 antibody. The immunocomplexes were captured by horseradish peroxidase–conjugated secondary antibodies (Bio-Rad, Hercules, CA) and visualized with ECL (GE-Amersham Biosciences, Piscataway, NJ). The intensity of protein bands was quantified by densitometry. The blot was reprobed with anti–β-actin antibody to confirm equal amounts of protein loading among samples. Three repetitions of western blotting using SKOV-3 cells infected with recombinant lentivirus were performed to confirm data.
Cell Proliferation Assay
Cells were seeded in 96-well plates (5000–10 000 cells per well) and then transfected with small interfering RNAs (siRNAs) (100 nM) against LPA1, LPA2, or LPA3 as indicated. Nontargeting siRNA was included as a control. Twenty-four hours after siRNA delivery, cells were serum starved overnight prior to incubation with vehicle (0.5% BSA in PBS) or 10 μM LPA for an additional 48 hours. Triplicates were performed for each treatment. Cells were then washed with ice-cold PBS and stained with 0.5% crystal violet containing 20% methanol for 30 minutes at room temperature. After washing, the dye was extracted in Sorenson's buffer (36) for 1 hour and the absorbance was measured at 570 nm using a microplate reader. Results were presented as 95% confidence interval (CI) from triplicate samples. This set of experiments was performed twice with similar results.
Cell Motility Assay
Parental SKOV-3 or tumor cells recovered from nude mouse xenografts were cultured in RPMI 1640 containing 10% FBS as described above. After overnight starvation, cells expressing LacZ or one of the LPA receptors were detached by trypsinization, washed, and resuspended in serum-free RPMI 1640 medium. The ability of cells to migrate through an uncoated Boyden chamber (8 μm) or to invade through a Matrigel-coated Boyden chamber was assessed using the transwells from BD Biosciences (San Jose, CA). Briefly, cells were seeded in transwells at 25 000 cells in 500 μl serum-free medium (upper chamber) and placed in 24-well plates (lower chamber) containing 750 μl of serum-free medium with LPA (10 μM) or vehicle (0.5% BSA in PBS). Cells were cultured in the transwells for an additional 24 hours (for migration assay) or 48 hours (for invasion assay). Cells remaining in the upper surface of the transwells were removed with cotton swabs. Cells migrated through the transwell and those that attached on the surface underneath were stained with crystal violet for 15 minutes. The transwells were then rinsed with water and air dried before examination under a microscope. Cells were counted (two fields for each treatment), and the results are presented as number of cells observed in three microscopic fields with ×200 magnification. Experiments were repeated three to four times, all with similar results.
RNA Interference
The siRNAs targeting LPA1, LPA2, and LPA3 and nontarget control siRNA were obtained from Dharmacon in a format of SMARTpool. siRNAs were delivered into cells by electroporation with Nucleofector using Solution T according to the manufacturer's protocol (Amaxa Biosystems, Gaithersburg, MD). siRNA-induced gene knockdown was confirmed by RT–quantitative PCR (qPCR) 24 hours after siRNA delivery.
RT–qPCR
Total cellular RNA was isolated from siRNA-treated cells with Trizol reagent (Invitrogen) according to the manufacturer's protocol. qPCR was performed using One-Step RT–PCR Taqman master mix (Applied Biosystems, Foster City, CA) with primers and probe sets designed by the FileBuilder Software (Applied Biosystems, Assays by Design). Fluorogenic Taqman probes were obtained from Applied Biosystems. LPA receptor mRNA was quantified by real-time PCR (ABI PRISM 7700 Sequence Detection System, Applied Biosystems). Results were normalized to the levels of β-actin. Data were presented as % of control = mRNA with siRNA targeting LPA receptors/mRNA with nontargeting siRNA × 100%. Most RT–qPCR experiments were repeated six times with similar results. Representative experiments are presented.
Measurement of IL-6, IL-8, and VEGF Production
Parental SKOV-3 or tumor cells recovered from xenografts were cultured in serum-free medium in the presence or absence of LPA for 24 hours and the culture supernatants were collected. For in vivo studies, serum and ascites were collected from nude mice bearing SKOV-3 xenografts with or without exogenously expressed LPA receptors. Production of IL-6, IL-8, and VEGF in culture supernatants or in serum and ascites were quantified by ELISA using the QuantiGlo IL-6, QuantiGlo IL-8, or QuantiGlo VEGF ELISA kits (R&D Systems). Concentrations of these cytokines in samples were calculated based on the absorbance compared with standard curves (pg/mL) performed in the same assay. Results were repeated two to four times and are plotted against the LPA doses and presented as fold increase over control cells (treated with vehicle alone).
Mouse Xenograft Model
Four sets of mouse experiments were performed in this study, with each group including at least five mice. All animal studies were conducted in compliance with the policies and regulations of the University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committee (IACUC). To analyze the consequence of overexpression of LPA receptors in vivo, BALB/c Nu/Nu mice (female, 4–6 weeks old) were injected either subcutaneously on the left flank with 2 × 106 cells per injection or intraperitoneally with 10 × 106 cells per injection. The cells used for injection had contained vectors for overexpression of the LPA receptors as described above. Mice were monitored beginning at 8 days after injection and measured once every 3 days (days 11 and 14) until tumor burdens in experimental groups required euthanasia (day 17, LPA3-expressing tumors). The sizes of subcutaneous tumors were measured with a digital caliper. Development of ascites was monitored by the measurement of abdominal circumference and body weight, which was further confirmed by magnetic resonance imaging (MRI). The volume of ascites was estimated by MRI (measured from the femoral neck to the level of the upper pole of the right kidney) and is presented as the mean volume of three representative live mice in each group. Ascites formation was scored positive when the abdominal circumference increased at least 15%, and MRI of selected mice confirmed their presence in all cases. For the assessment of survival, per IACUC guidelines, mice were euthanized when the abdominal circumference increased 60% above normal controls. Animals were euthanized by carbon dioxide asphyxiation. Blood was collected from the tail vein of living mice, following the protocol recommended by the University of Texas M. D. Anderson IACUC.
Apoptosis Assay
SKOV-3 cells derived from xenograft tumors were cultured in 60-mm plates (1 × 104 cells) for 24 hours in the medium containing 10% FBS followed by another 48 hours in the medium containing 0.1% FBS. Cells were then exposed to UV radiation (0–200 × 100 J/cm3). Four hours later, both floating and attached cells were collected and washed with PBS. Cells were lysed in 200 μl of lysis buffer, and the cytoplasmic DNA fragments were captured by immobilized antihistone antibody and horseradish peroxidase–conjugated anti-DNA POD antibody. The immune complex was visualized and quantified using a colorimetric ELISA-based reaction (Roche Biomedicals, Indianapolis, IN) that detects fragmented DNA in the cytosol. The data are presented as mean absorbance of triplicate samples from a representative experiment that was repeated three times.
Histology and Histochemistry
Tumor-bearing mice were subjected to necropsy after carbon dioxide asphyxiation. A total of 19 mice were used in this study (LacZ = 5, LPA1 = 4, LPA2 = 5 and LPA3 = 5). Tumor tissues and organs were collected on a gross examination block. Each organ was examined by gross pathology before fixation in 3.7% formaldehyde and embedded in paraffin. Paraffin sections (5 μm) were processed for hematoxylin and eosin staining in order to determine organ lesion and tumor invasion. One slide per block was examined by a pathologist (C. Stephens) unless requested otherwise, to confirm the presence or absence of specific characteristics (ie, invasion or metastases).
Statistical Analysis
Results for cell counts to determine migration, invasion, proliferation, and cytokine measurement were assessed with the analysis of variance (ANOVA) and Student t test. Mouse survival time was defined as the time from injection with SKOV-3 cells transduced with LPA receptors or LacZ until the animals died (or were terminated per IACUC protocol, which specifically limits the tumor volumes allowed and abdominal circumference for ascites). The data on survival were estimated by week and plotted using the Kaplan–Meier method. Differences in survival between the groups of mice were assessed using the log-rank test. Statistical analyses were performed using Microsoft Excel extension files (Redmond, WA), SigmaPlot graphing software (San Jose, CA), and GraphPad Prism software (San Diego, CA). All statistical tests were two-sided. The threshold for statistical significance was .05.
Results
Effect of LPA and LPA Receptors on Cell Proliferation, Cytokine Production, and Cancer Cell Migration/Invasion
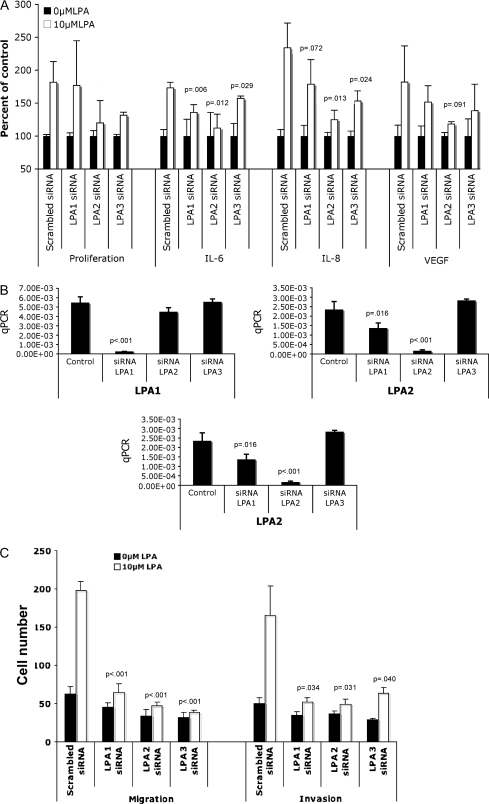
We previously reported that LPA stimulates cell proliferation and increases IL-6, IL-8, and VEGF production by ovarian cancer cells, including SKOV-3 cells (18). To determine whether these biologic effects are mediated through specific LPA receptors, we introduced siRNAs targeting LPA1, LPA2, and LPA3 into SKOV-3 cells. These cells express relatively low levels of these receptors (LPA2 > LPA3 > LPA1) as assessed by quantitative PCR (4), and LPA4 is not expressed at detectable levels in ovarian cancer cells (4). Consistent with our previous reports, proliferation as measured by crystal violet staining of cells that had been pretreated with nonspecific siRNA and with 10 μM LPA for 48 hours was 1.8-fold (95% CI = 1.53 to 2.1, P = .017) that of untreated control cells (Figure 1, A). Treatment of cells with LPA for 24 hours also caused marked increases in IL-6 (mean increase = 1.73-fold, 95% CI = 1.65 to 1.8, P < .001), IL-8 (mean increase = 2.34-fold, 95% CI = 1.99 to 2.68, P = .009), and VEGF (mean increase = 1.82, 95% CI = 1.31 to 2.33, P = .056) production as measured by ELISA relative to untreated control cells (Figure 1, A). Similar results were observed in OVCAR-3 human ovarian cancer cells and HT-29 human colon cancer cells (Supplementary Figure 1, available online).
Figure 1.
Assessment of the role of individual lysophosphatidic acid (LPA) receptors in LPA-induced cell proliferation; interleukin 6 (IL-6), interleukin 8 (IL-8), and vascular endothelial growth factor (VEGF) production; and cell migration and invasion in ovarian cancer cells. A) SKOV-3 cells were transfected with small interfering RNAs (siRNAs) against LPA1, LPA2, or LPA3, or with nontargeting (scrambled) siRNA control. After incubation with 10 μM LPA for 48 h, cells were stained with crystal violet and counted to determine cell proliferation. For the determination of IL-6, IL-8, and VEGF cytokine production, culture supernatants were collected, clarified, and analyzed by enzyme-linked immunosorbent assay. Results are presented as percent of control cells (treated with 0.5% bovine serum albumin in phosphate-buffered saline). Mean values are presented with 95% confidence intervals (CIs). Differences between groups were assessed using Student t test. B) Cells were transfected with siRNA, and after 24 h, mRNA was extracted and real-time PCR was performed to confirm the efficiency of gene knockdown by the specific siRNAs. The results are given in arbitrary units, and P values from Student t test comparing LPA receptor siRNA values with controls are indicated. C) SKOV-3 cells were transfected with siRNAs, and, 24 h after siRNA delivery, cells were seeded in the upper chamber of transwells coated with Matrigel (for invasion assay) or uncoated (for migration assay). Mean cell counts are given with error bars representing 95% CIs for the mean. Statistically significant P values are indicated. qPCR = quantitative polymerase chain reaction.
Treatment of cells with siRNA targeting LPA1 slightly decreased some LPA-induced responses in SKOV-3 cells (mean values for control and treated, IL-6 = 100 and 136, respectively, mean increase = 36, 95% CI = 25 to 46, P = .006; IL-8 = 100 and 178, respectively, mean increase = 78, 95% CI = 44 to 113, P = .072). Targeting LPA2 or LPA3 expression decreased the relative increase seen after LPA treatment of the cytokines IL-6, IL-8, and VEGF by a greater extent than LPA1 overall in OVCAR-3 and HT-29 cells (Figure 1, A, and Supplementary Figure 1, A and B, available online). In SKOV-3 cells in which LPA2 was targeted, the difference in IL-6 (mean values for control and treated = 100 and 112, respectively, mean increase = 12, 95% CI = 7 to 31, P = .012), IL-8 (mean increase = 24, 95% CI = 11 to 38, P = .013), and VEGF (mean values for control and treated = 100 and 118, mean increase = 18, 95% CI = 15 to 21, P = .091). In cells in which LPA3 was targeted, the difference in IL-6 mean values for control and treated were 100 and 157, respectively, mean increase = 57, 95% CI = 53 to 60, P = .029; the difference in LPA3 IL-8 mean values for control and treated were 100 and 153, respectively, mean increase = 53, 95% CI = 38 to 67, P = .024; the difference in LPA3 VEGF mean values for control and treated were 100 and 138, respectively, mean increase = 38, P = .167. Treatment of SKOV-3 with siRNAs targeting LPA2 and LPA3 also led to decreases in the stimulation of cell proliferation, although these were not statistically significant owing to the wide variation in proliferation of control cells. Similar results in terms of proliferation were observed in the OVCAR-3 cells that express moderate levels of LPA2 and higher levels of LPA3 (4) but not LPA1 receptors. We conducted similar experiments in HT-29 colon cancer cells, which exclusively express LPA2, and demonstrated that LPA2 siRNA effectively blocked each of these responses (Supplementary Figure 1, B, available online). RT–qPCR analysis confirmed that introducing these siRNAs into SKOV-3 cells markedly decreased expression of the specifically targeted LPA receptors (Figure 1, B). Taken together, these results suggest that elevated LPA receptor levels in cancer cells contribute to LPA receptor–mediated cell proliferation and cytokine production.
LPA1 has been hypothesized to be required for the effect of LPA on cell migration (37,38); however, whether LPA1 is the sole receptor mediating migration and invasion of ovarian cancer cells is unclear. To measure migration and invasion, cells were seeded in transwells (upper Boyden chamber, uncoated for migration and coated with Matrigel for invasion) and inserted into 24-well plates (lower chamber) containing 10 μM of LPA and incubated for 24 hours (migration) or 48 hours (invasion). LPA treatment increased chemotaxis and invasion of SKOV-3 cells (number of migrated in LPA-treated and untreated cells = 198 and 62, respectively, difference = 136, 95% CI = 125 to 146, P < .001; number of invaded LPA-treated and untreated cells = 165 and 49, respectively, difference = 116, 95% CI = 80 to 151 treated value, P = .031) (Figure 1, C). siRNA targeting of each LPA receptor markedly decreased LPA-induced chemotaxis and invasion (siLPA3 migration: number of migrated LPA-treated and untreated cells in the presence of random RNA sequence and siRNA targeting LPA3 = 32 and 38, difference = 6, 95% CI = 4 to 9, P < .001; siLPA3 invasion: number of invaded LPA-treated and untreated cells = 29 and 63, respectively, difference = 34, 95% CI = 27 to 41, P = .040). The results suggest that not only LPA1 but also LPA2 and LPA3 contribute to motility and invasion in vitro.
Effect of of LPA2 or LPA3 Receptor Expression on Tumorgenicity of SKOV-3 Cells in Nude Mice
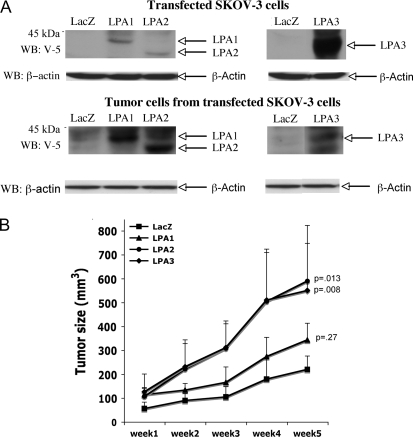
To directly assess the role of LPA receptors in ovarian carcinogenesis, we generated a lentivirus system to express each of the Edg LPA receptors in SKOV-3 cells and characterized their effects on tumorgenicity in nude mouse xenograft models. Although it has been difficult to ectopically overexpress the Edg LPA receptors, in particular the LPA2 receptor, in mammalian cells by most DNA transfection approaches (37), the lentivirus system provides highly efficient gene transfer and expression in ovarian cancer cell lines (18). SKOV-3 cells were infected with lentivirus containing the coding sequences of LPA1, LPA2, or LPA3 tagged with the V-5 epitope at their C terminus. LPA3 was highly expressed, whereas LPA1 and particularly LPA2 were only modestly expressed in the SKOV-3 cells as evidenced by western blot analysis (Figure 2, A).
Figure 2.
Tumorgenicity of SKOV-3 cells expressing exogenous lysophosphatidic acid (LPA) receptors. A) SKOV-3 cells were infected with recombinant lentivirus carrying LacZ, LPA1, LPA2, or LPA3 proteins tagged with the V-5 epitope. Expression of LacZ and LPA receptors was analyzed by immunoblotting with anti–V-5 antibody. The blot was reprobed with anti–β-actin antibody to confirm equal amounts of protein loading among samples. B) Female nude mice (4–6 weeks old, 8–9 mice for each group) were injected subcutaneously with 1 × 106 SKOV-3 cells expressing LacZ, LPA1, LPA2, or LPA3. The formation of subcutaneous tumors was monitored and measured with a digital caliper. Error bars represent 95% confidence intervals for the mean tumor volume and P values are from Student t test, comparing tumor sizes at week 5 with those in mice injected with cells expressing LacZ or LPA.
To determine the effect of LPA receptor overexpression on the behavior of SKOV-3 cells in vivo, female nude mice were injected subcutaneously with control cells expressing LacZ or with cells expressing LPA1, LPA2, or LPA3. The volumes of the subcutaneous tumors were monitored on a weekly basis. Overall, higher expression levels of the exogenous LPA1 and LPA2 receptors were confirmed in tumors removed from mice (after 5 weeks at the conclusion of the experiment) (Figure 2, A, and Supplementary Figure 2, available online) compared with the corresponding cells used for injection (Figure 2, A). Unfortunately, the lack of sensitive LPA receptor antibodies precluded direct comparison between the levels of endogenous and transfected receptors.
Expression of the LPA2 or LPA3 receptors led to statistically significantly enhanced tumor growth in nude mice (Figure 2, B, data are means of n = 8/9 mice per group, P < .001, two-way repeated-measures ANOVA test for tumor growth in LacZ control vs LPA receptor; the mean week 5 tumor volume in mice injected with control cells and cells expressing LPA2 receptor was 219 and 589 mm3, respectively, mean increase = 370 mm3, 95% CI = 404 to 774 mm3, P = .013). Cells expressing the LPA1 receptor showed a smaller but statistically significant increase in tumor growth rates compared with the control cells expressing LacZ. These results indicate that expression of LPA1, LPA2, or LPA3 increases the in vivo growth of (or confers a survival advantage on) ovarian cancer cells.
LPA Responsiveness in Tumor-Derived Cells Expressing LPA Receptors
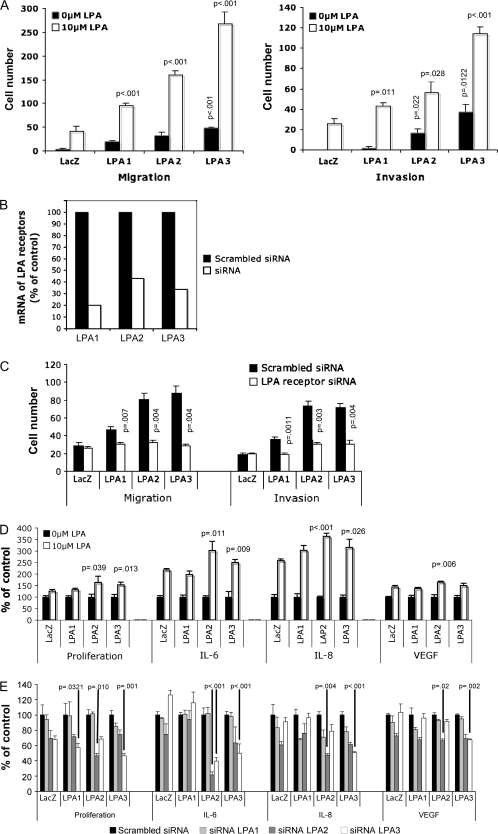
To determine whether the overexpressed LPA receptors mediated cellular responses to LPA, we isolated LacZ-, LPA1-, LPA2-, or LPA3-expressing tumor cells from xenografts in nude mice. After plating in culture, these tumor cells continued to express V-5–tagged LPA receptors at levels similar to those in tumor tissues (data not shown). We then performed motility assays to determine whether overexpression of the LPA receptors increased LPA-induced migration and invasion in SKOV-3 tumor cells. We observed increased cell motility in response to LPA stimulation in all of the LPA receptor-transduced tumor cells (Figure 3, A). Surprisingly, overexpression of LPA2 and LPA3 had greater effects on LPA-induced cell migration than LPA1 or LacZ and especially compared with LacZ for invasion. Taken together the data indicate that LPA1, and especially LPA2 and LPA3, may be involved in LPA regulation of tumor cell motility in ovarian cancer cells.
Figure 3.
Evaluation of migration/invasion, cell proliferation, and interleukin 8 (IL-8) production in response to lysophosphatidic acid (LPA) in SKOV-3 cells derived from tumors that expressed exogenous LPA receptors. A) Tumor cells derived from subcutaneous xenografts were seeded in uncoated (for migration) or Matrigel-coated (for invasion) transwells. Cells that migrated or invaded were stained, photographed, and counted. Mean cell numbers of triplicate samples and 95% confidence intervals (CIs) of a representative experiment are shown. P values are from comparisons with either untreated control (white bars) or treated control (black bars) using the Student t test. B) In a separate assay using the same tumor-derived cells, overexpression of the LPA receptors was blocked by specific small interfering RNAs (siRNAs). The expression of LPA receptor mRNA was measured by real-time PCR to verify the efficiency of gene knockdown. C) siRNA-treated tumor-derived cells were treated with 10 μM LPA for migration (24 h) and invasion (48 h) before comparison with control cells treated with scrambled siRNA (black bars) or siRNA targeting LPA receptors (white bars). Data are presented as cell numbers counted in three microscopic fields at ×200 magnification D) Tumor-derived cells overexpressing LacZ, LPA1, LPA2, or LPA3 were cultured, incubated with 10 μM LPA for 48 h, and stained with crystal violet, and the dye was extracted to determine proliferation. Data are presented as percent of control (cells not treated with LPA). For determination of cytokine production, tumor-derived cells overexpressing LacZ, LPA1, LPA2, or LPA3 were cultured and incubated with LPA prior to collection of culture supernatants and analysis for IL-8, interleukin 6 (IL-6), or vascular endothelial growth factor (VEGF) concentration by enzyme-linked immunosorbent assay. P values listed were obtained from Student t tests comparing LPA-treated LacZ controls with cells expressing LPA receptors. Data in graphs are means of a representative experiment, repeated (n = 2) with 95% CIs E) Expression of LPA receptors was blocked by the correspondent specific siRNAs in tumor-derived cells. LPA (10 μM)-induced cell proliferation and cytokine production in scrambled nontargeting siRNA–treated or LPA receptor siRNA–treated cells were measured as described in D. P values and 95% CIs are listed for comparisons of LPA receptor siRNA groups vs scrambled siRNA controls.
We then introduced siRNAs targeting the overexpressed LPA receptors to tumor-derived cells. Real-time PCR analysis indicated that mRNA of the receptors was decreased by 60%–80% (Figure 3, B). The decreases in LPA receptor expression led to statistically significant inhibition of LPA-mediated cell migration and invasion relative to that observed in the absence of siRNA targeting of the LPA receptors (Figure 3, C).
We also characterized LPA-mediated proliferation in the xenograft-derived SKOV-3 cells. As analyzed by crystal violet staining, the cells that overexpressed LPA2 or LPA3 grew faster than control cells in response to LPA (Figure 3, D). As expected from the studies with the primary cells, the effects of LPA1 on proliferation were modest. Similar to what was observed for LPA-induced cell migration and invasion, siRNA suppression of the LPA receptor expression inhibited LPA-induced proliferation in these cells (Figure 3, E).
We have shown previously that LPA induces the production of VEGF, IL-8, and IL-6 by ovarian cancer cells (21,31). We analyzed LPA-dependent cytokine production in culture supernatants of the LacZ-, LPA1-, LPA2-, and LPA3-expressing tumor cells. Expression of LPA2 and LPA3 receptors led to the production of more IL-6 and IL-8 in response to LPA stimulation (Figure 3, D). LPA2-expressing cells were the most responsive to LPA and the only cells in which we observed a statistically significant increase in VEGF production, consistent with our previous observations that LPA2 is the major receptor isotype driving LPA-mediated cytokine production (21,31). LPA-induced cytokine production in the LPA2- or LPA3-expressing cells was substantially reduced by siRNA targeting LPA receptors (Figure 3, E).
Effect of Exogenous Expression of LPA2 or LPA3 Receptors on UV-Induced Apoptosis
LPA is a potent survival factor protecting cells from serum withdrawal and chemotherapeutic drug–induced apoptosis (1,39,40). To determine whether overexpression of Edg LPA receptors in SKOV-3 cells would promote cell survival, we exposed the LacZ-, LPA1-, LPA2-, and LPA3-expressing tumor cells to UV light at 200 μJ/cm2. The treatment caused substantial cell death through apoptosis in LacZ- and LPA1-expressing cells as measured by DNA fragmentation with an ELISA-based assay (R&D Systems). In contrast, cells expressing LPA2 and LPA3 were less sensitive to UV irradiation, with UV-induced DNA fragmentation reduced by 50% as compared with LacZ- or LPA1-expressing cells (Supplementary Figure 3, A, available online). UV resistance was reversed by siRNA targeting LPA2 or LPA3 but not LPA1 (Supplementary Figure 3, B, available online), indicating that expression of LPA2 or LPA3 promotes SKOV-3 cell survival under stress conditions.
Intraperitoneal Tumor Growth and Ascites Formation in Nude Mice After LPA Receptor Expression
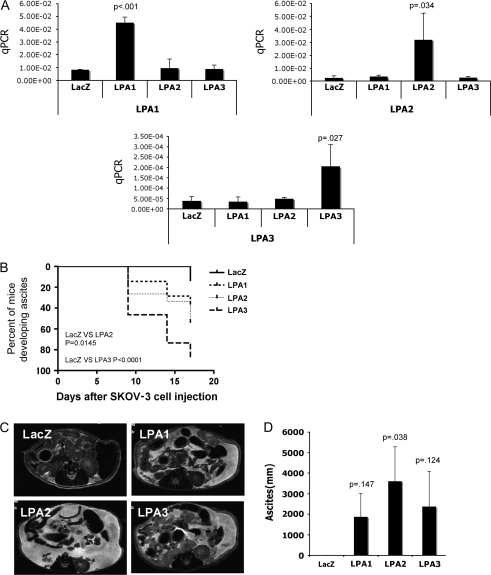
Most ovarian cancer cell lines are not sufficiently aggressive to support tumor formation following intraperitoneal injection in nude mice, although many do form tumors after subcutaneous injection. However, orthotopic intraperitoneal tumorgenicity is more relevant to human ovarian cancer. Large numbers of SKOV-3 cells (10 × 106 cells) must be injected in the peritoneal cavity of nude mice for palpable tumors and/or ascites to be detectable after a long latency period. To determine whether SKOV-3 cells would become more aggressive with overexpression of the Edg family LPA receptors, lentivirus-transduced SKOV-3 cells expressing LacZ, LPA1, LPA2, or LPA3 were injected intraperitoneally into nude mice. Before the injection of the cells, RT–PCR was used to demonstrate specific expression of individual LPA receptors (Figure 4, A). In each lentivirus-transduced cell line, we detected no compensatory changes in other nontransduced LPA receptors. Mice that developed ascites had at least a 15% increase in abdominal circumference and then exhibited lethargic behavior with continued abdominal growth. Representative mice were selected from each group for noninvasive MRI to confirm the presence of ascites. The percentage of mice showing signs of ascites formation was much higher in mice injected with LPA receptor–expressing cells than those injected with LacZ-expressing cells (Figure 4, B). Seventeen days after cell injection, only two mice (n = 15) injected with the LacZ-expressing cells showed signs of ascites. At this time point, ascites was detected in 5 of 14 mice injected with LPA1-, 8 of 15 mice injected with LPA2-, and 13 of 15 mice injected with LPA3-expressing cells. Seven of 15 mice injected with LPA3-expressing cells showed signs of ascites formation within 10 days.
Figure 4.
Intraperitoneal tumor formation and ascites formation by SKOV-3 cells expressing transduced lysophosphatidic acid (LPA) receptors. A) Lentivirus-transduced SKOV-3 cells were harvested and mRNA was extracted and amplified with real-time PCR to confirm the expression of LPA receptors. Mean values, with 95% confidence intervals, represent the amount of amplified product in arbitrary units. B) Female nude mice were injected intraperitoneally with the lentivirus-transduced SKOV-3 cells (1 × 106 per mouse) that expressed LacZ, LPA1, LPA2, or LPA3 (15 mice per group). Mice were monitored beginning 8 days after injection and abdominal circumference and body weight were measured every 3 days to determine the ascites formation. Results are presented as the percentage of mice with ascites. P values were derived from Student t test with comparisons of LPA receptor groups with LacZ control. C) Magnetic resonance imaging (MRI) was performed to further verify the development of ascites in these mice. Representative MRI images are demonstrating the formation of ascites, which appears as white-colored fluid throughout the cavity. D) Volumes of ascites were estimated by MRI and presented as mean volume from three representative live mice in each group. Measurement was performed from femoral neck to the level of upper pole of right kidney. Data in the bar graph represent the means of three mice for which MRI imaging was performed; P value was derived from Student t tests comparing LPA receptor expressing tumors with LacZ control. qPCR = quantitative polymerase chain reaction.
To demonstrate that the increase in abdominal circumference was due to the development of ascites, MRI was performed on the 17th day after cell injection to measure ascites volumes (and tumor sizes) in three mice (chosen randomly) from each group. MRI permits a noninvasive assessment of tumor volume and ascites formation in the peritoneal cavity (41). MRI images and MRI measurement of ascites volume, respectively (Figure 4, C), demonstrated that most of the mice injected with cells expressing LPA receptors developed considerable amounts of ascites, whereas mice injected with LacZ-expressing cells did not. Furthermore, we observed marked increases in tumor volume in mice with LPA receptor–expressing tumors, particularly those expressing LPA2 and LPA3 (Figure 4, D), and in all cases MRI assessment confirmed that a greater than 15% increase in abdominal circumference was a valid means of detecting ascites formation circumference measurements.
The Effect of Expression of LPA Receptors on IL-6 and IL-8 Production In Vivo
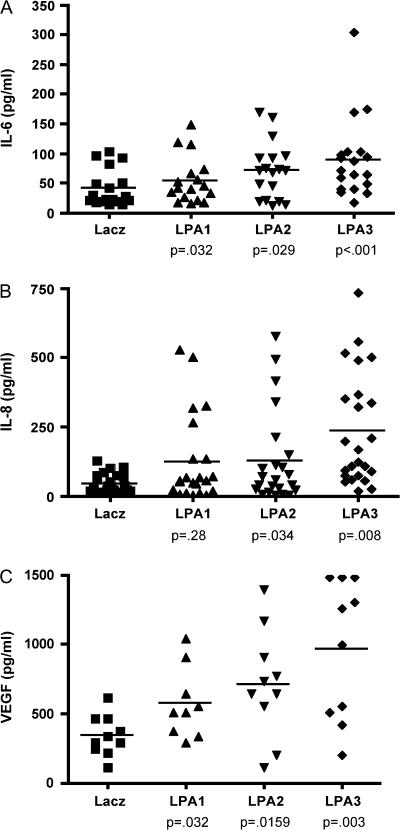
Previous reports demonstrated that serum levels of IL-6, IL-8, and VEGF are elevated in ovarian cancer patients and that these levels correlate with chemotherapy resistance and poor patient outcomes (42–44). To determine whether expression of LPA receptors alters the production of angiogenic factors by ovarian cancer cells in vivo, we measured the concentrations of IL-6, IL-8, and VEGF in serum from mice bearing intraperitoneal SKOV-3 tumors expressing LacZ, LPA1, LPA2, or LPA3 (Figure 5). Using ELISA kits specific for detection of human proteins and not cross-reactive with mouse homologs, we demonstrated statistically significantly higher levels of IL-6 (Figure 5, A), IL-8 (Figure 5, B), and VEGF (Figure 5, C) in mice bearing tumors expressing LPA1, LPA2, and LPA3. The highest level of expression of each angiogenic factor was observed in LPA3-expressing tumors (increase in IL-6 relative to cells expressing LacZ = 57 pg/mL, 95% CI = 28 to 90, P = .008; increase in IL-8 relative to cells expressing LacZ = 224 pg/mL, 95% CI = 110 to 267, P < .001; increase in VEGF relative to cells expressing LacZ = 627 pg/mL, 95% CI = 334 to 922, P = .003). When levels of these factors were assessed in relation to tumor volume, there was a strong correlation (eg, correlation coefficient for the volume of LPA2-expressing tumors and IL-6 levels = 0.73; correlation coefficient for the volume of LPA2-expressing tumors and IL-8 = 0.87; correlation coefficient for the volume of LPA3-expressing tumors and IL-6 levels = 0.78) suggesting that increased tumor volume could, in part, contribute to the relative levels of these factors present in serum. Much higher concentrations of IL-6, IL-8, and VEGF were detected in ascites than in serum (data not shown), suggesting that overexpression of LPA receptors by tumor cells stimulates these cells to synthesize and secrete angiogenic factors in ascites that then circulate to the bloodstream. Similar differences in IL-6 and IL-8 levels have been found in ascites and plasma of human patients with ovarian cancer (42–44).
Figure 5.
Serum levels of interleukin 6 (IL-6), interleukin 8 (IL-8), and vascular endothelial growth factor (VEGF) from mice intraperitoneally injected with SKOV-3 cells expressing lysophosphatidic acid (LPA) receptors. Female nude mice were injected intraperitoneally with the lentivirus-transduced SKOV-3 cells (10 × 106 per mouse) that express LPA1, LPA2, or LPA3 receptors. Three weeks later, blood was collected from the tail vein to determine concentrations of IL-6 (A), IL-8 (B), and VEGF (C) in serum. Each data point corresponds to IL-8 or IL-6 in individual mouse serum (LacZ [n = 23], LPA1 [n = 22], LPA2 [n = 23], or LPA3 [n = 24]) or VEGF in mouse serum (LacZ [n = 10], LPA1 [n = 9], LPA2 [n = 10], or LPA3 [n = 10]). Data points represent concentrations determined by enzyme-linked immunosorbent assay. P values are derived from comparisons with LacZ controls using Student t test, and horizontal lines represent the mean of values.
In Vivo Effect of Exogenous LPA Receptor Expression on Tumor Cell Invasion and Metastasis
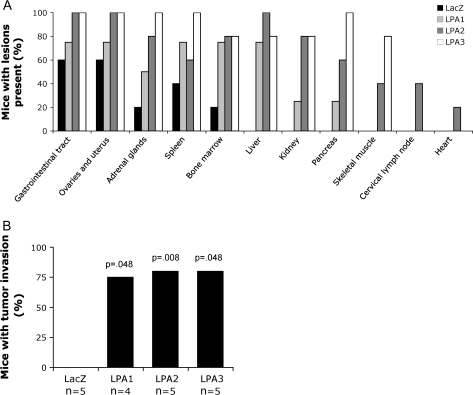
To investigate tumor invasion and metastasis in vivo, we performed necropsy in tumor-bearing mice on the 17th day after intraperitoneal injection of SKOV-3 cells expressing LacZ, LPA1, LPA2, or LPA3. Invasion was observed in multiple organs in the mice bearing SKOV-3 tumors expressing LPA receptors, particularly in those in which tumors expressed LPA2 or LPA3 (Figure 6, A). Eighty percent of the mice bearing LPA2 or LPA3 tumors displayed lesions in the liver, kidney, gastrointestinal tract, adrenal glands, and ovaries and uterus, and bone marrow. In contrast, although mice with LacZ-expressing tumors demonstrated seedings of intraperitoneal organs such as uterus, ovary, adrenal gland, and spleen, only one of five demonstrated evidence of metastasis to bone marrow. The LPA2 and LPA3 receptor–expressing tumors were found in other distant sites including skeletal muscle, while only LPA2 receptor–expressing tumors were found in the cervical lymph node and heart, a rare site of metastasis. In two of five of the LPA2 receptor–expressing tumors, cervical lymph nodes were partially replaced with neoplastic and malignant epithelial cells. This is an indication of distant metastases that was not seen in vivo using LPA1 or LPA3 receptor–expressing cells to establish tumors.
Figure 6.
Enhanced tumor invasion by cells that overexpress lysophosphatidic acid (LPA) receptors. Female nude mice were injected intraperitoneally with lentivirus-transduced SKOV-3 cells (10 × 106 per mouse) expressing LacZ (n = 5), LPA1 (n = 4), LPA2 (n = 5), or LPA3 (n = 5) receptor. Seventeen days after injection, tumor-bearing mice were sacrificed and subjected to necropsy, and the organs were processed for hematoxylin and eosin staining. Pathological sections were examined for the presence of tumor invasion. Data are presented as the percentage of mice with invaded lesions (A) and the overall percentage of mice with tumor invasion in the peritoneal cavity (B). LPA receptor groups and the LacZ control were compared using the Student t test.
In addition, invasive tumors were observed in the peritoneal cavity in three of four of mice injected with LPA1-expressing SKOV-3 and in four of five of mice injected with LPA2- or LPA3-expressing SKOV-3 cells (Figure 6, B). In contrast, five mice injected with LacZ-expressing SKOV-3 cells did not develop any signs of tumor invasion. Taken together, the histopathologic examination results indicate that LPA receptors are major contributors to invasion and metastases of ovarian cancer cells in vivo.
LPA2 or LPA3 Receptor Expression in Ovarian Cancer and Survival Rate
In an independent experiment, nude mice (four or five per group) were injected intraperitoneally with lentivirus-transduced SKOV-3 cells expressing LacZ, LPA1, LPA2, or LPA3 receptors. Mice bearing LPA3-expressing cells did not survive beyond 4 weeks, and all mice injected with LPA2-expressing cells exhibited morbidity requiring euthanasia within 5 weeks (Supplementary Figure 4, available online). However, all the mice injected with LacZ or LPA1-expressing cells lived for 6 weeks or longer. Thus, a substantial reduction occurred in the survival of mice injected with LPA2- or LPA3-expressing cells compared with mice injected with LacZ- or LPA1-expressing cells.
Discussion
Ovarian cancer leads to more fatalities than any other form of gynecological cancer in the developed world. The dismal prognosis results from an inability to detect the tumor at an early, curable stage and from a lack of effective therapies for advanced disease (45). We have previously found that LPA is present at high concentrations in ascites of ovarian cancer patients (23,24). As ascitic fluid of ovarian cancer represents the in vivo microenvironment of tumor cells, LPA's effects on tumor cells may contribute to the development and progression of ovarian cancer (30,31). The relevance of LPA to the pathogenesis of ovarian cancer is supported by numerous lines of evidence: 1) LPA promotes growth and survival of ovarian cancer cells (24,30,31,46); 2) LPA stimulates migration and invasion of ovarian cancer cells (47–49); 3) ovarian cancer cells can produce LPA, forming an autocrine loop mediating growth, survival, and motility of tumor cells; 4) LPA action increases expression of angiogenic and metastatic factors such as IL-6, IL-8, VEGF, uPA, and Cox-2 (17–20,50); 5) exogenous LPA can increase growth and metastasis by ovarian cancer cells (24); 6) ovarian cancer cells can induce LPA production by mesothelial cells, thereby increasing invasion and metastases (26); 7) expression of LPP3, an enzyme that dephosphorylates LPA, reduces the growth and survival of ovarian cancer cells in nude mice (29); 8) inhibitors of autotaxin, the main enzyme producing LPA, decrease metastatic potential in a number of tumor models (51,52).
Our previous studies indicated that the LPA2 and LPA3 receptors are overexpressed in primary ovarian cancers (31). It was unclear, however, whether increases in their expression are causally linked to ovarian oncogenesis or merely represent a consequence of cell transformation. In the current study, we ectopically overexpressed the Edg LPA receptors in the SKOV-3 ovarian cancer cells. In vitro and in vivo analysis of control cells and cells expressing each LPA receptor demonstrated that exogenous expression of Edg family LPA receptors enhanced cellular responses to LPA and led to a more malignant phenotype in vivo, especially in cells expressing LPA2 or LPA3 receptors. These results provide direct evidence that LPA receptor expression and LPA signaling regulate the behavior and aggressiveness of ovarian cancer cells. Therefore, the overexpression of the LPA2 or LPA3 receptor observed in ovarian cancer likely plays a role in the development and/or progression of this disease.
Under normal circumstances, LPA in the bloodstream and body fluids is at submicromolar concentrations or lower, likely reflecting its rapid clearance or degradation (30,53). The major pathway for inactivation of LPA in most cell types is dephosphorylation to form monoacylglycerol by LPP (53,54). In ovarian cancer, LPA levels are abnormally high in the ascitic fluid that bathes tumor cells. The concurrent increases in expression of LPA receptors observed in ovarian cancer suggests that increased LPA levels in the tumor microenvironment are not sufficient to amplify LPA signaling without overexpression of specific LPA receptors. This requirement for increased expression of LPA receptors could be due in part to the presence of LPP and other LPA-metabolizing enzymes in ovarian cancer cells that control the level of active, accessible LPA at the cell membrane (55).
The three Edg LPA receptors are not functionally equivalent. The LPA1 receptor is commonly expressed in normal tissues, and its expression pattern is not consistently altered in ovarian cancer (3,31). The LPA2 receptor is normally expressed in lung, kidney, spleen, thymus, and testes, and the LPA3 receptor, in the heart, lung, kidney, testes, and intestine (4,5,56). Acquisition of the LPA2 or LPA3 receptor during ovarian carcinogenesis may lead to novel cellular responses to LPA. We have demonstrated that the LPA2 receptor is more effective than LPA1 and LPA3 in LPA-mediated production of the cytokines IL-6, IL-8, and Gro-α (18,21,39). The study of LPA2 transgenic mice also suggests a critical role for LPA2 in the production of VEGF and uPA in the ovary (57). Unlike LPA1 and LPA2, the LPA3 receptor is expressed at low levels in normal tissues (12), with its expression largely confined to malignant cells (31). Previous studies showed that LPA3 has a preference for LPA with an unsaturated fatty acyl chain in SN-2 LPA (5,58). These unsaturated isoforms of LPA are abundant in ascites of ovarian cancer. The ligand selectivity of LPA3 may permit a unique set of cellular responses that are not triggered by the activation of other LPA receptors.
Considerable effort has focused on determining which receptors lead to specific functional responses. The demonstration that altering levels of LPA1, LPA2, and LPA3 all affected cellular proliferation, production of growth factors, motility, invasion, tumor growth, ascites formation, and metastases suggests that the three receptors are pleomorphic in function. Alternatively, specific Edg family LPA receptors may form heterodimers with other receptors (8), resulting in novel signaling and different functional outcomes. Nevertheless, our work suggests that certain receptors are more efficient in mediating particular functions. For example, LPA1 was much less efficient than either LPA2 or LPA3 in mediating cellular proliferation. Surprisingly, LPA2 and LPA3 when expressed in ovarian cancer cells were more efficient at increasing in vitro motility and invasion and in vivo tumor growth, in particular metastasis to distant organs such as skeletal muscle, cervical lymph node, and heart. Our results are in contrast to those of previous reports (59,60) implicating LPA1 in the metastasis of breast cancer cells to bone marrow. Whether this inconsistency represents differences between breast and ovarian cancer cells or a unique interaction between breast cancer cells and the environment in the bone marrow remains to be determined.
This study had several potential limitations. One is the use of a single cell line, SKOV-3, for most of the experiments to ascertain the role of Edg family LPA receptors in tumorgenicity. We observed similar effects using OVCAR-3 and HT-29 in vitro, but the effect of expression of the Edg family of receptors in other lines and lineages in vivo are not known. The SKOV-3 xenograft could be extended by studies of spontaneous or transgenic models of ovarian and other cancers. Second, we characterized the Edg family LPA receptors; however, additional LPA receptors have recently been discovered (13,14). It is not yet known whether these receptors play a role in ovarian carcinogenesis. Finally, it is not clear whether the effects of altered expression of the Edg family of LPA receptors are solely due to signaling through each receptor or whether there is cooperativity mediated by the formation of receptor heterodimers or through downstream signaling with endogenous receptors.
In summary, our results demonstrate that expression of exogenous LPA2 or LPA3 causes characteristic changes associated with ovarian cancer progression. The involvement of LPA in ovarian cancer and other human malignancies warrants thorough investigation of LPA production and action as potential targets of cancer therapy. Inhibition of LPA functionality could be achieved through controlling LPA production/metabolism or availability with immunoneutralizing antibodies or through blocking LPA receptor binding or signaling. The location of LPA receptors on the cell surface and previous success in designing drugs that target GPCRs suggest that LPA2 and LPA3 receptors are ideal candidates for therapeutic exploitation in ovarian cancer.
Funding
This work was supported by the National Institutes of Health (RO1 CA82716 to G.B.M. and R01 CA102196 to X.F.). LPATH provided salary support for M.M.M.
Supplementary Material
Footnotes
S. Yu and M. M. Murph contributed equally to this work.
The authors take full responsibility for the study design, data collection, analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
- 1.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev. 2003;3(8):582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 2.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 3.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135(4):1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273(14):7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 5.Bandoh K, Aoki J, Hosono H, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274(39):27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 6.Goetzl EJ, An S. A subfamily of G protein-coupled cellular receptors for lysophospholipids and lysosphingolipids. Adv Exp Med Biol. 2001;469:259–264. doi: 10.1007/978-1-4615-4793-8_38. [DOI] [PubMed] [Google Scholar]
- 7.Hla T. Sphingosine 1-phosphate receptors. Prostaglandins. 2001;64(1–4):135–142. doi: 10.1016/s0090-6980(01)00109-5. [DOI] [PubMed] [Google Scholar]
- 8.Zaslavsky A, Singh LS, Tan H, Ding H, Liang Z, Xu Y. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim Biophys Acta. 2006;1761(10):1200–1212. doi: 10.1016/j.bbalip.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278(28):25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 10.Kotarsky K, Boketoft A, Bristulf J, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318(2):619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 11.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281(33):23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 12.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363(3):861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Pasternack SM, von Kugelgen I, Aboud KA, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40(3):329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 14.Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371(4):707–712. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre TM, Pontsler AV, Silva AR, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci USA. 2003;100(1):131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Baker DL, Yasuda S, et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199(6):763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz BM, Hong G, Morrison BH, et al. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81(2):291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- 18.Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279(10):9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 19.Hu YL, Tee MK, Goetzl EJ, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93(10):762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 20.Pustilnik TB, EstrellaV, Wiener JR, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5(11):3704–3710. [PubMed] [Google Scholar]
- 21.Lee Z, Swaby RF, Liang Y, et al. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006;66(5):2740–2748. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 22.Murph M, Tanaka T, Liu S, Mills GB. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin Cancer Res. 2006;12(22):6598–6602. doi: 10.1158/1078-0432.CCR-06-1721. [DOI] [PubMed] [Google Scholar]
- 23.Mills GB, May C, Hill M, Campbell S, Shaw P, Marks A. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest. 1990;86(3):851–855. doi: 10.1172/JCI114784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309(pt 3):933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umezu-Goto M, Tanyi J, Lahad J, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92(6):1115–1140. doi: 10.1002/jcb.20113. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Xiao YJ, Singh LS, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66(6):3006–3014. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 27.Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158(2):227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanyi JL, Hasegawa Y, Lapushin R, et al. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin Cancer Res. 2003;9(10 pt 1):3534–3545. [PubMed] [Google Scholar]
- 29.Tanyi JL, Morris AJ, Wolf JK, et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003;63(5):1073–1082. [PubMed] [Google Scholar]
- 30.Fang X, Gaudette D, Furui T. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann NY Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 31.Fang X, Schummer M, Mao M, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582(1–3):257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 32.Furui T, LaPushin R, Mao M, et al. Overexpression of edg-2/vzg-1 induces apoptosis and anoikis in ovarian cancer cells in a lysophosphatidic acid-independent manner. Clin Cancer Res. 1999;5(12):4308–4318. [PubMed] [Google Scholar]
- 33.Schulte KM, Beyer A, Kohrer K, Oberhauser S, Roher HD. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: over-expression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int J Cancer. 2001;92(2):249–256. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1166>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Shida D, Watanabe T, Aoki J, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest J Tech Method Pathol. 2004;84(10):1352–1362. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 35.Kitayama J, Shida D, Sako A, et al. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Br Cancer Res. 2004;6(6):R640–R646. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KS, Sengupta S, Berk M, et al. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66(16):7983–7990. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 37.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J Biol Chem. 2003;278(1):400–406. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 38.Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279(17):17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 39.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90(6):447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 40.Murph MM, Hurst-Kennedy J, Newton V, Brindley DN, Radhakrishna H. Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells. Mol Cancer Res. 2007;5(11):1201–1211. doi: 10.1158/1541-7786.MCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Kawamoto S, Saga T, et al. Avidin-dendrimer-(1B4M-Gd)(254): a tumor-targeting therapeutic agent for gadolinium neutron capture therapy of intraperitoneal disseminated tumor which can be monitored by MRI. Bioconjug Chem. 2001;12(4):587–593. doi: 10.1021/bc010002o. [DOI] [PubMed] [Google Scholar]
- 42.Penson RT, Kronish K, Duan Z, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10(1):33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 43.Duan Z, Feller AJ, Penson RT, Chabner BA, Seiden MV. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res. 1999;5(11):3445–3453. [PubMed] [Google Scholar]
- 44.Harlozinska A, Sedlaczek P, Kulpa J, et al. Vascular endothelial growth factor (VEGF) concentration in sera and tumor effusions from patients with ovarian carcinoma. Anticancer Res. 2004;24(2C):1149–1157. [PubMed] [Google Scholar]
- 45.See HT, Kavanagh JJ, Hu W, Bast RC. Targeted therapy for epithelial ovarian cancer: current status and future prospects. Int J Gynecol Cancer. 2003;13(6):701–734. doi: 10.1111/j.1525-1438.2003.13601.x. [DOI] [PubMed] [Google Scholar]
- 46.Goetzl EJ, Dolezalova H, Kong Y, et al. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999;59(20):5370–5375. [PubMed] [Google Scholar]
- 47.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17(11):1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 48.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6(6):2482–2491. [PubMed] [Google Scholar]
- 49.Luquain C, Singh A, Wang L, Natarajan V, Morris AJ. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J Lipid Res. 2003;44(10):1963–1975. doi: 10.1194/jlr.M300188-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Symowicz J, Adley BP, Woo MM, Auersperg N, Hudson LG, Stack MS. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65(6):2234–2242. doi: 10.1158/0008.5472.CAN-04-2781. [DOI] [PubMed] [Google Scholar]
- 51.Baker DL, Fujiwara Y, Pigg KR, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281(32):22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchiyama A, Mukai M, Fujiwara Y, et al. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim Biophys Acta. 2007;1771(1):103–112. doi: 10.1016/j.bbalip.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15(5):477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92(5):900–912. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 55.Imai A, Furui T, Tamaya T, Mills GB. A gonadotropin-releasing hormone-responsive phosphatase hydrolyses lysophosphatidic acid within the plasma membrane of ovarian cancer cells. J Clin Endocrinol Metab. 2000;85(9):3370–3375. doi: 10.1210/jcem.85.9.6793. [DOI] [PubMed] [Google Scholar]
- 56.Contos JJ, Chun J. The mouse lp(A3)/Edg7 lysophosphatidic acid receptor gene: genomic structure, chromosomal localization, and expression pattern. Gene. 2001;267(2):243–253. doi: 10.1016/s0378-1119(01)00410-3. [DOI] [PubMed] [Google Scholar]
- 57.Huang MC, Lee HY, Yeh CC, Kong Y, Zaloudek CJ, Goetzl EJ. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2) Oncogene. 2004;23(1):122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- 58.Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478(1–2):159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- 59.Boucharaba A, Serre CM, Gres S, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114(12):1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci USA. 2006;103(25):9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.