Abstract
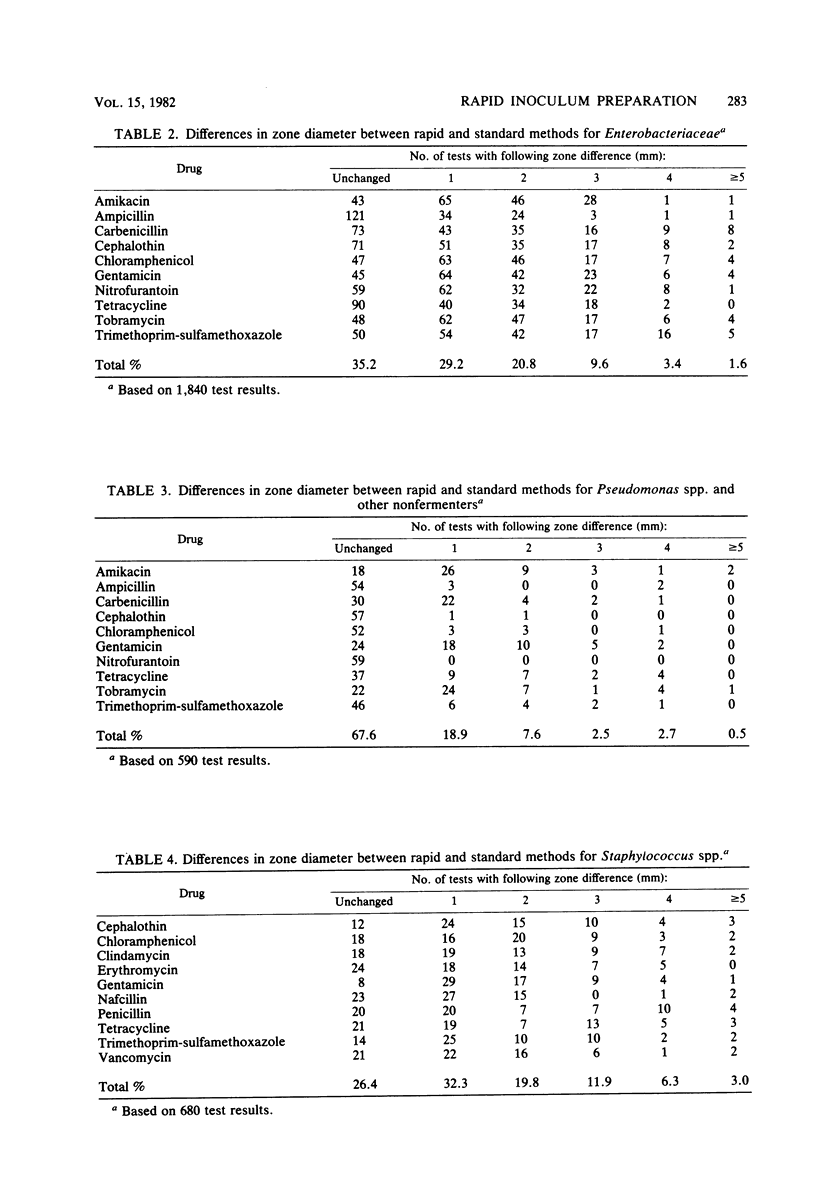
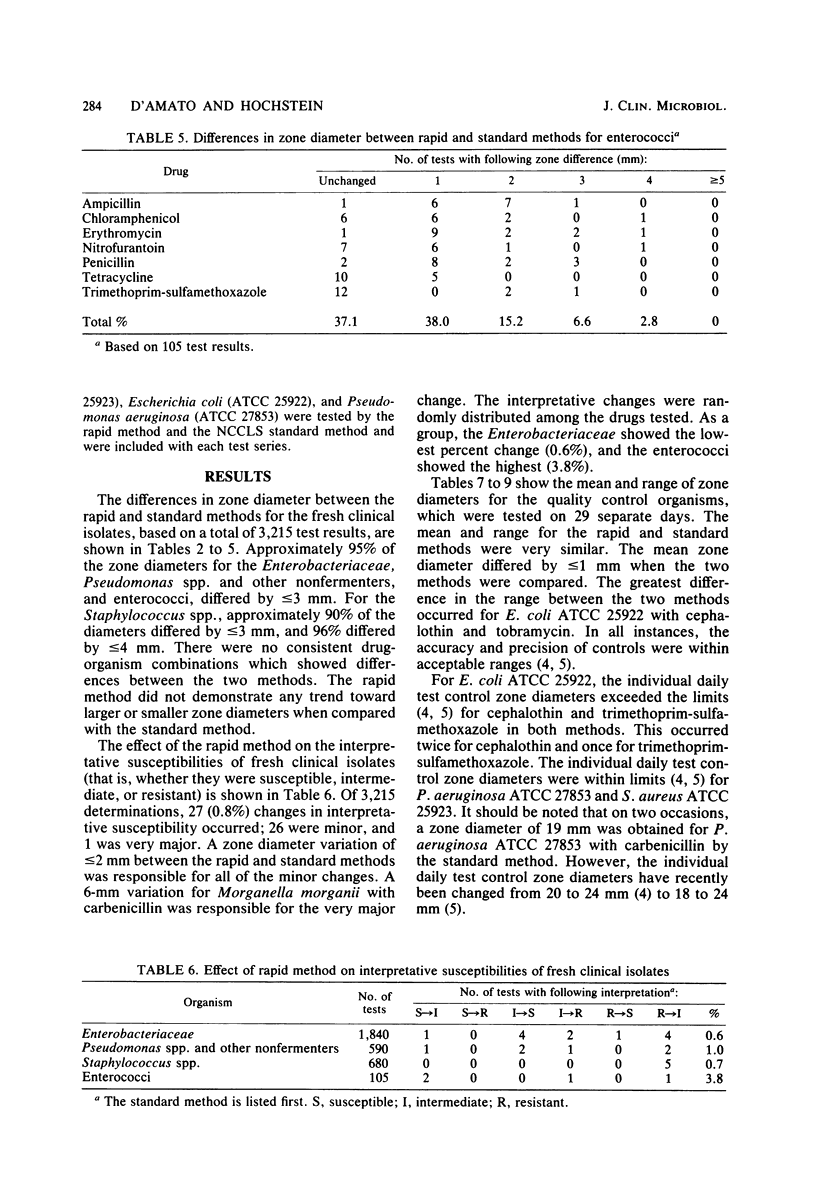
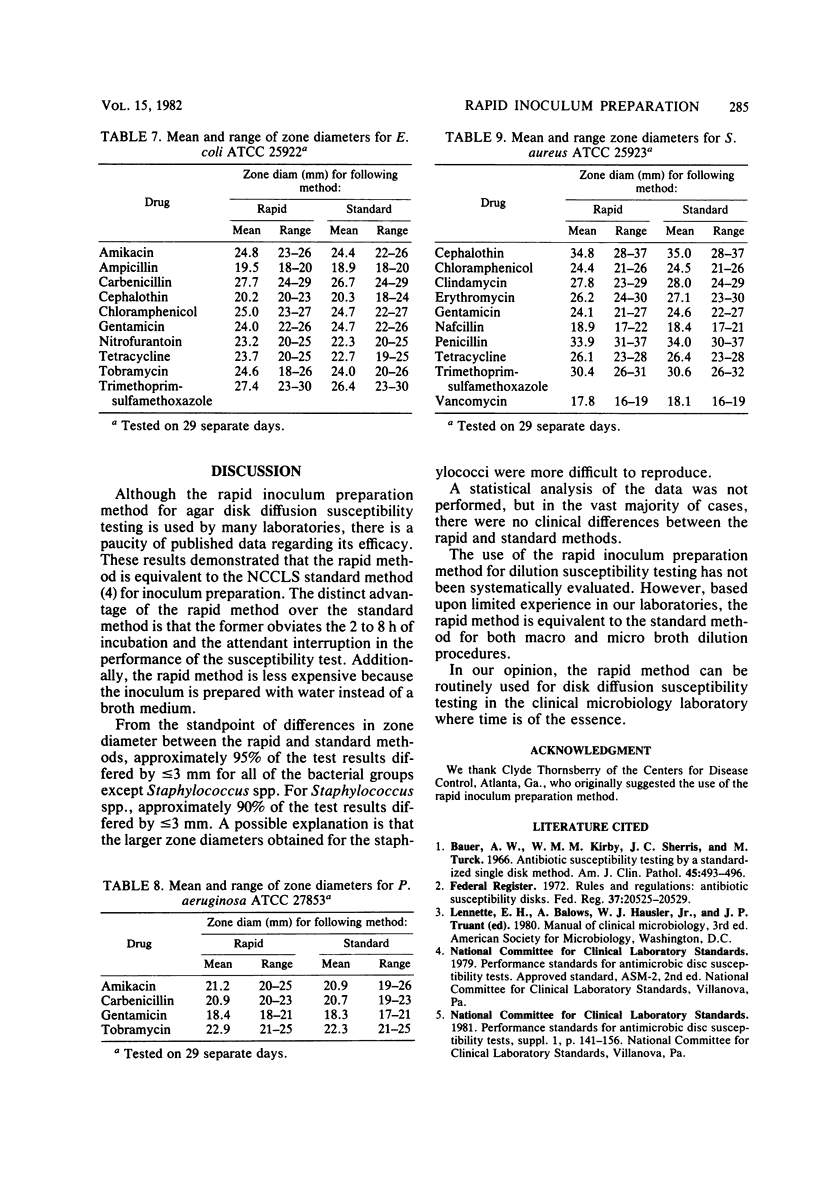
A rapid inoculum preparation method for agar disk diffusion susceptibility testing which does not require incubation before inoculation of Mueller-Hinton plates was compared with the National Committee for Clinical Laboratory Standards (NCCLS) method. A total of 326 fresh clinical isolates were tested, and the NCCLS-recommended quality control organisms were included with each test series. Randomly distributed interpretative changes occurred with 27 (0.8%) of the 3,215 test results for the clinical isolates. The quality control organisms were tested on 29 separate days, and results were consistently within tolerance limits. The rapid method was found to be equivalent to the standard NCCLS method and required less time and expense.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]


