Abstract
Coronary artery calcification (CAC) and common carotid artery intima-media thickness (CIMT) are measures of subclinical vascular disease. This 2000–2006 study aimed to characterize the associations among coronary artery disease risk factors, CAC quantity, and CIMT and to estimate shared genetic and environmental contributions to both CAC and CIMT among 478 asymptomatic Amish adults in Lancaster County, Pennsylvania. Heritability for CAC quantity and CIMT, adjusted for age and sex, was 0.42 (P = 0.0001) and 0.29 (P = 0.003), respectively. CAC quantity and CIMT were modestly correlated (adjusted r = 0.14, P = 0.003) but showed little evidence of shared genetic or environmental factors. However, significant genetic correlations were found for CAC quantity and total cholesterol (0.44 (standard error, 0.19); P = 0.03), for CAC quantity and low density lipoprotein cholesterol (0.55 (standard error, 0.17); P = 0.005), and for CIMT and waist circumference (0.58 (standard error, 0.25); P = 0.046), suggesting shared genes for these risk factors and measures of subclinical disease. Results suggest that some of the same genes influence variation in CAC and low density lipoprotein cholesterol, whereas a different set of genes influences variation in CIMT and waist circumference.
Keywords: atherosclerosis; calcification, physiologic; carotid arteries; coronary vessels; genetics; risk factors; vascular diseases
Coronary artery calcification (CAC) and common carotid artery intima-media thickness (CIMT) are noninvasive markers of subclinical vascular disease. Presence and quantity of CAC, as detected with computed tomography, are associated with coronary artery disease risk factors (1) and are predictive of future coronary artery disease events (2, 3). Likewise, in several prospective, population-based studies (4–6), CIMT, as measured by B-mode ultrasound, has been shown to predict coronary artery disease events.
Although CAC and CIMT both reflect presence of subclinical vascular disease, development of atherosclerosis is not uniform across different anatomic sites (7). Moreover, deposition of calcium in the coronary arteries and thickening of the carotid arterial wall may be influenced by overlapping sets of environmental and genetic risk factors. Understanding how these various factors differentially influence development of these 2 markers of subclinical atherosclerosis may provide important insights into interindividual variation in the development of coronary artery disease.
CAC quantity and CIMT have rarely been measured concurrently in the same individuals in a research setting, and the relation of these measures to each other and the degree to which they share risk factors are not clear (8, 9). The purposes of this study were to characterize the relation between CAC quantity and CIMT, to compare and contrast associations of both with coronary artery disease risk factors, and to estimate the shared genetic and environmental contributions to variation among CAC quantity, CIMT, and coronary artery disease risk factors. Thus, we measured CAC and CIMT in the same subjects from an Old Order Amish population from Lancaster County, Pennsylvania. This population is well suited for such a study because the Amish have a socially and culturally homogeneous lifestyle, use prescription medications relatively sparingly, and experience low rates of smoking.
MATERIALS AND METHODS
Subjects
Subjects were identified from 2 studies of cardiovascular health in the Old Order Amish community in Lancaster County: the Amish Family Calcification Study (2001–2006) and the Amish Longevity Study (2000–2006). The Amish Family Calcification Study was initiated in 2001 to identify determinants of vascular calcification and to evaluate the relation between calcification of bone and vascular tissue in the Old Order Amish community. Subjects were initially recruited into that study on the basis of their participation in an earlier family study of bone mineral density (10), although recruitment guidelines were later modified to allow other interested individuals in the community and their relatives to participate. Recruitment efforts were made without regard to coronary artery disease health status, and extensive analyses revealed no statistically significant correlations in Amish Family Calcification Study participants between bone mineral density and CAC or CIMT after accounting for age and sex ((11) and unpublished data). Women who were pregnant or lactating were not eligible to participate. The Amish Longevity Study was initiated in 2000 to identify the genetic factors associated with living to an old age (12). Subjects recruited into that study included Amish individuals living to age 92 years or older, their offspring, and the offspring's spouses. The individuals included in the present analysis were men aged 40 years or older and women aged 50 years or older who were examined to measure CAC quantity and CIMT in either the Amish Family Calcification Study (n = 216) or the Amish Longevity Study (n = 262).
Study protocols were approved by the institutional review boards of the University of Maryland and other participating institutions. Informed consent, including permission to contact relatives, was obtained before subjects participated.
Assessment of coronary artery disease risk factors
All study subjects underwent clinical examination at the Amish Research Clinic in Strasburg, Pennsylvania, including assessment of potential coronary artery disease risk factors and a medical history interview. Following an overnight fast, participants’ height and weight were measured with a stadiometer and calibrated scale with their shoes removed and wearing light clothing. Body mass index (weight (kg)/height (m)2) was calculated. Waist circumference (centimeters) was measured at the umbilicus. Systolic (first phase) blood pressure and diastolic (fifth phase) blood pressure were obtained in triplicate with a standard sphygmomanometer, and blood pressure was defined as the mean of the second and third measurements. Pulse pressure was defined as the difference between mean systolic blood pressure and diastolic blood pressure. Medication lists were obtained at the participants’ homes by a study nurse. Smoking habits (current smoker or not) were recorded by questionnaire.
Blood samples were obtained for determination of fasting glucose and lipid levels. Glucose concentrations were assayed with a YSI glucose analyzer (YSI Incorporated, Yellow Springs, Ohio) by using the glucose oxidase method. Lipid and high density lipoprotein cholesterol concentrations were assayed by Quest Diagnostics (Baltimore, Maryland). Low density lipoprotein cholesterol levels were calculated by using the Friedewald equation (13). Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL or use of antidiabetic medications (14).
Assessment of subclinical atherosclerosis
Electron beam computed tomography scans were performed with an Imatron C-150 Ultrafast CT scanner (Imatron Inc., South San Francisco, California) in Timonium, Maryland, as previously described (15). CAC was quantified by using the Agatston method (16). Presence of detectable CAC was defined as a density of >130 Hounsfield units in more than 3 contiguous pixels (>1 mm2). The sum of scores in the left main, left anterior descending, circumflex, and right coronary arteries was considered the CAC score. All scans were scored by an experienced cardiologist using AccuImage (AccuImage Diagnostic Corp., San Francisco, California) software. Interscan reproducibility for CAC quantity with this software ranges from 89% to 94%, with interreader and intrareader reproducibility each about 99% (17, 18).
High-resolution B-mode ultrasound was carried out to image the right and left common carotid arteries. CIMT was measured between lumen intima and media-adventitia interfaces of the far wall of the common carotid arteries (the 1-cm segment proximal to the bifurcation) by a single reader using an automated edge detection system. Mean CIMT of this 1-cm segment was measured on 2 separate images of the left and the right common carotid artery at the peak of the R wave on a simultaneous electrocardiogram tracing. The mean of these 4 measurements was used as the CIMT. Interscan reproducibility for CIMT was 89% with this software, and the interreader and intrareader reproducibilities were 97% and 98%, respectively.
Statistical analyses
The sample for this study was restricted to men aged 40 years or older and women aged 50 years or older because prevalence of detectable CAC is low in younger individuals. This restricted data set included 478 subjects with CAC measurements, 446 (93.3%) of whom also had CIMT measurements. Distributions of CAC score, CIMT, waist circumference, triglycerides, high density lipoprotein cholesterol, and low density lipoprotein cholesterol were positively skewed, so these measures were natural log-transformed. CAC score was log-transformed after adding 1. Hereafter, we refer to this transformed CAC quantity as ln(CAC score + 1).
We assessed the association between each coronary artery disease risk factor and high levels of CAC and CIMT by classifying subjects according to whether their CAC (or CIMT) measurements were in the upper 20th percentile of the distribution. The distributions of CAC quantity (and CIMT) were first residualized by regressing out effects of age, age2, sex, age × sex, and age2 × sex on each trait. This step enabled comparison of associations between each risk factor and having high CAC levels versus its association with having high CIMT. Associations between coronary artery disease risk factors and the presence of high CAC and CIMT levels (as defined above) were expressed in terms of odds ratios, constructed by using regression models that modeled the difference in levels of coronary artery disease risk factors as the independent variable and being in the top quintile of the CAC quantity (or CIMT) distribution as the dependent variable. These regression analyses, based on a threshold model for discrete traits, were carried out under a variance component framework (described below) in which the odds ratios were estimated while jointly considering correlations between related study subjects.
We exploited the fact that study subjects were related to one another to estimate the additive genetic effects (heritability) on both traits. We partitioned total variance in ln(CAC score + 1) and lnCIMT into effects attributable to measured risk factors (e.g., body mass index), additive genetic variance (estimated from the covariance among the relatives), and a residual environmental effect corresponding to unexplained phenotypic variation. Heritability corresponds to the proportion of the trait variance attributable to additive genetic effects after accounting for effects of measured risk factors. The residual variance unaccounted for by measured risk factors and (unmeasured) additive genetic factors corresponds to the residual environmental variance or the proportion of the variance attributable to unmeasured environmental factors, including measurement error. These analyses were simultaneously adjusted for effects of age, age2, and sex (and interactions of age terms with sex). We used maximum likelihood methods to estimate risk factor and genetic effects simultaneously, and we assessed significance of specific factors by comparing the likelihood of a model containing the factor of interest with that of a model in which the value of the factor of interest was constrained to zero (19). Full and restricted models were then compared by using a likelihood ratio test, producing a test statistic that is asymptotically distributed as a 1/2:1/2 mixture of a χ2 variable with 1 df and a point mass at zero (20). This step was performed by using the SOLAR software program (21).
We extended the analyses described above to a bivariate analysis to estimate potential shared, unmeasured genetic and environmental effects on the joint distribution of each risk factor and ln(CAC score + 1) or lnCIMT, and between ln(CAC score + 1) and lnCIMT. To improve computational analysis efficiency, we used age- and sex-adjusted residualized ln(CAC score + 1) and lnCIMT values. The joint trait variance was divided into components attributable to additive genetic effects and residual environmental effects (as previously described) and also to components corresponding to the degree to which shared genetic and environmental factors influence distribution of the traits (22, 23). For example, the genetic correlations can be interpreted as a measure of the degree of pleiotropy between 2 traits. The hypothesis of any pleiotropy was evaluated by a likelihood ratio test, calculated as the difference in −2 × ln likelihoods between a restricted model (where the genetic correlation value = 0, indicating no genetic correlation) and an unrestricted model (where all parameters are estimated).
RESULTS
The final sample of 478 study subjects included individuals from 279 sibships, of which 96 included multiple siblings (range: 2–9 siblings) and 183 included only a single individual. Additional relationship types were identified by linking study subjects into larger pedigrees through their unexamined (or examined) parents. Doing so resulted in 88 larger pedigrees with multiple examined individuals (range: 2–11 individuals) and representing 421 sibpairs, 39 parent-offspring pairs, 60 avuncular pairs, and 26 first-cousin pairs.
Characteristics of study subjects are shown in Table 1. Thirty-four (7.1%) subjects had a history of a prior cardiovascular disease event. Use of antidiabetic (1.1%), cholesterol-lowering (4.5%), and blood-pressure-lowering (4.3%) medications was low in this study. Men were younger than women, presumably because of the differential study inclusion criteria. Mean body mass index was higher in women than in men. Despite their younger age, men were more likely than women to have detectable CAC (67.0% vs. 57.0%) and had higher median CAC scores (37.9 vs. 4.1). After adjusting for age and age2, we found that men also had higher median lnCIMT than women did.
Table 1.
Clinical Characteristics of the Study Population, Lancaster County, Pennsylvania, 2000–2006a
| Characteristic | Men (n = 251) | Women (n = 227) | Total (n = 478) |
| Age, years | 59.1 (11.5) | 63.1 (8.6) | 61.1 (10.1) |
| Body mass index, kg/m2 | 26.8 (3.7) | 28.5 (5.6) | 27.7 (4.7) |
| Waist circumference, cmb | 94.2 (86.6, 103.0) | 88.0 (80.0, 94.9) | 91.1 (83.3, 98.9) |
| Systolic blood pressure, mm Hg | 117.4 (14.3) | 121.9 (19.0) | 119.7 (16.7) |
| Diastolic blood pressure, mm Hg | 74.1 (9.8) | 72.0 (10.7) | 73.1 (10.3) |
| Pulse pressure, mm Hg | 43.3 (10.5) | 49.5 (12.6) | 46.4 (11.6) |
| Total cholesterol, mg/dL | 213.9 (42.5) | 232.6 (46.6) | 223.3 (44.6) |
| High density lipoprotein cholesterol, mg/dLb | 51.0 (13.0) | 61.3 (17.2) | 56.2 (15.1) |
| Low density lipoprotein cholesterol, mg/dLb | 145.9 (40.3) | 152.0 (43.2) | 148.9 (41.8) |
| Triglycerides, mg/dLb | 71.0 (52, 104) | 76.0 (55, 111) | 73.5 (53.5, 107.5) |
| Diabetes | 3.6 | 5.3 | 4.5 |
| Current smokerc | 17.5 | 0 | 9.2 |
| Cholesterol-lowering medications | 4.0 | 4.9 | 4.5 |
| Antidiabetic medications | 1.2 | 0.9 | 1.1 |
| Blood-pressure-lowering medications | 2.4 | 6.2 | 4.3 |
| Prior cardiovascular disease event | 8.4 | 5.7 | 7.1 |
| CIMT, mmb | 0.70 (0.60, 0.83) | 0.71 (0.61, 0.81) | 0.71 (0.61, 0.82) |
| CAC presence | 67.0 | 57.0 | 62.0 |
| CAC scoreb | 37.9 (0, 339.5) | 4.1 (0, 89.3) | 21.0 (0, 214.2) |
Abbreviations: CAC, coronary artery calcification; CIMT, carotid artery intima-media thickness.
Values are expressed as mean (standard deviation), median (25th, 75th percentile), or percentage.
Natural log-transformed prior to analyses (after adding 1 for CAC score).
Smoking includes cigarettes, pipes, and cigars.
Table 2 summarizes associations between coronary artery disease risk factors and high levels of CAC or CIMT. The odds ratios shown reflect the percentage increase (or decrease) in the odds of being in the upper quintile of the CAC or CIMT distribution (after adjustment for age and sex) for a specified change in a given coronary artery disease risk factor. As indicated in this table, a 3-kg/m2 increase in body mass index and a 5-cm increase in waist circumference were associated with 16%–20% increases in the odds of having high CIMT (16% increase for waist, 20% increase for body mass index; P ≤ 0.0001 for both risk factors) but only a 5%–6% increase in the odds of having high CAC (P > 0.15 for both risk factors). Similarly, increases of 10 mm Hg in systolic blood pressure and diastolic blood pressure and of 5 mm Hg in pulse pressure were associated with 11%–12% increases in the odds of having high CIMT (P ≤ 0.001 for systolic blood pressure and pulse pressure) but only 7%–8% increases in the odds of having high CAC (P = 0.03–0.33). In contrast, higher total cholesterol and low density lipoprotein cholesterol were strongly associated with high CAC but not high CIMT. For example, 10-mg/dL increases in low density lipoprotein cholesterol and total cholesterol were associated with 6%–8% higher odds of having high CAC (6% for total cholesterol and 8% for low density lipoprotein cholesterol; P < 0.001 for both) but only a 1%–2% increase in the odds of having high CIMT (P > 0.25 for both). The odds associated with a 5-mg/dL increase in high density lipoprotein cholesterol were nearly equivalent for both high CAC (5% decreased odds; P = 0.02) and high CIMT (6% decreased odds; P = 0.008).
Table 2.
Increased Risk of Study Subjects Being in the Upper 20th Percentile of the Distribution of CAC Quantity or CIMT Associated With a Specified Difference in a Coronary Artery Disease Risk Factor, Lancaster County, Pennsylvania, 2000–2006a
| Risk Factor | Unit Increase in Risk Factor | CAC |
CIMT |
||||
| Odds Ratiob | 95% CI | P value | Odds Ratiob | 95% CI | P value | ||
| Body mass index | 3 kg/m2 | 1.06 | 0.98, 1.15 | 0.17 | 1.20 | 1.10, 1.32 | 0.0001 |
| Waist circumference | 5 cm | 1.05 | 0.96, 1.16 | 0.30 | 1.16 | 1.09, 1.24 | <0.0001 |
| Systolic blood pressure | 10 mm Hg | 1.08 | 1.00, 1.17 | 0.06 | 1.12 | 1.04, 1.22 | <0.001 |
| Diastolic blood pressure | 10 mm Hg | 1.07 | 0.93, 1.23 | 0.33 | 1.11 | 0.96, 1.28 | 0.14 |
| Pulse pressure | 5 mm Hg | 1.07 | 1.01, 1.14 | 0.03 | 1.12 | 1.05, 1.20 | 0.001 |
| Total cholesterol | 10 mg/dL | 1.06 | 1.02, 1.09 | <0.001 | 1.01 | 0.98, 1.04 | 0.54 |
| Triglycerides | 10 mg/dL | 0.99 | 0.97, 1.02 | 0.65 | 1.03 | 1.00, 1.05 | 0.04 |
| High density lipoprotein cholesterol | 5 mg/dL | 0.95 | 0.90, 0.99 | 0.02 | 0.94 | 0.89, 0.98 | 0.008 |
| Low density lipoprotein cholesterol | 10 mg/dL | 1.08 | 1.04, 1.11 | <0.0001 | 1.02 | 0.98, 1.05 | 0.28 |
Abbreviations: CAC, coronary artery calcification; CI, confidence interval; CIMT, carotid artery intima-media thickness.
Top 20th percentile of the CAC (and CIMT) distribution was defined on the basis of residualized ln(CAC score + 1) and lnCIMT values following adjustment for age and sex effects (refer to the text for further information).
Interpreted as the percentage change in the odds of being in the highest quintile (20%) of the CAC or CIMT distribution associated with a change in risk factor of the specified size. For example, a 3-kg/m2 increase in body mass index is associated with a 6% increase in the odds of being in the top quintile of CAC and a 20% increase in the odds of being in the top quintile of CIMT. Odds ratios can be compared between CAC and CIMT for the same risk factor but cannot be compared across different risk factors.
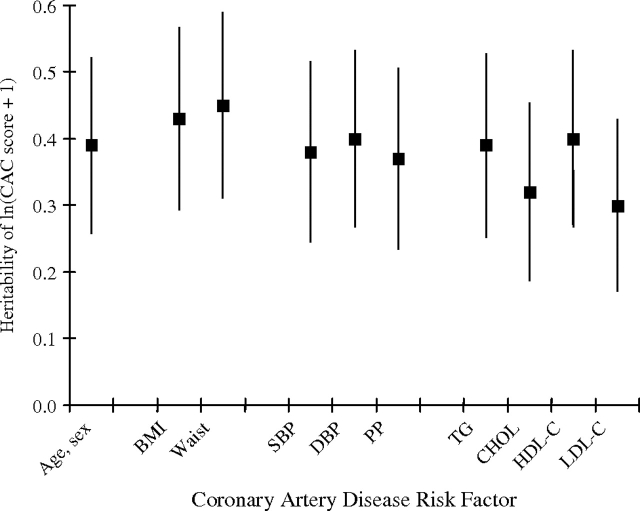
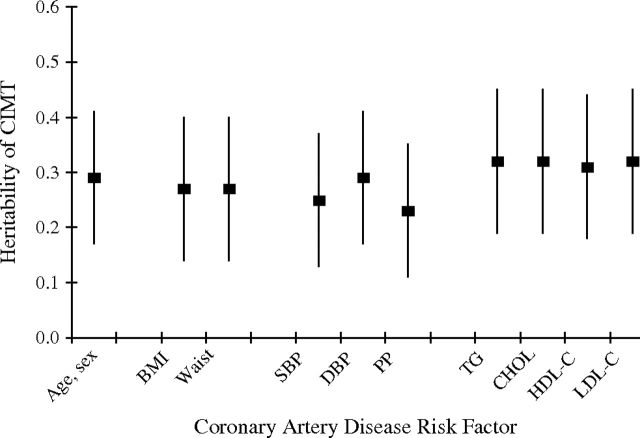
Age and sex (including age, age2, and their interactions with sex) accounted for 31% and 19% of the total variation in ln(CAC score + 1) and lnCIMT, respectively. Heritability of ln(CAC score + 1), adjusted for age, age2, sex, and their interactions, was 0.42 (standard error (SE), 0.13) (P = 0.0001). Adding body mass index, waist circumference, or any blood pressure measurements altered this estimate only slightly (adjusted h2 = 0.39–0.46) (Figure 1). In contrast, heritability of ln(CAC score + 1) was reduced by adjustment for either total cholesterol (h2 = 0.34) or low density lipoprotein cholesterol (h2 = 0.32) (Figure 1). Heritability of lnCIMT, adjusted for age, age2, sex, and their interactions, was 0.29 (SE, 0.12) (P = 0.003) (Figure 2). This estimate changed little with additional adjustment for any of the body size or lipid measures (h2 = 0.27–0.32) or additional adjustment for blood pressure measures (e.g., h2 = 0.25 with additional adjustment for systolic blood pressure).
Figure 1.
Heritability of quantity of coronary artery calcification (ln(CAC score + 1)), adjusted for age, age2, age × sex, and age2 × sex, with further adjustment for other coronary artery disease risk factors, among Old Order Amish study subjects from Lancaster County, Pennsylvania, 2000–2006. BMI, body mass index; CHOL, total cholesterol; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; PP, pulse pressure; SBP, systolic blood pressure; TG, triglycerides; Waist, waist circumference. Each vertical line and centered box represents heritability (standard error).
Figure 2.
Heritability of common carotid artery intima-media thickness (lnCIMT), adjusted for age, age2, age × sex, and age2 × sex, with further adjustment for other coronary artery disease risk factors, among Old Order Amish study subjects from Lancaster County, Pennsylvania, 2000–2006. BMI, body mass index; CHOL, total cholesterol; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; PP, pulse pressure; SBP, systolic blood pressure; TG, triglycerides; Waist, waist circumference. Each vertical line and centered box represents heritability (standard error).
The genetic correlations were high between ln(CAC score + 1) and both total cholesterol (ρgene = 0.44 (SE, 0.19); P = 0.028) and low density lipoprotein cholesterol (ρgene = 0.55 (SE, 0.17); P = 0.005) (Table 3). The genetic correlation between lnCIMT and waist circumference was high (ρgene = 0.58 (SE, 0.25); P = 0.046), whereas that between lnCIMT and systolic blood pressure was of borderline statistical significance (ρgene = 0.38 (SE, 0.20); P = 0.081). No other coronary artery disease risk factors showed evidence of a genetic correlation with either ln(CAC score + 1) or lnCIMT. There was little evidence for shared environmental factors influencing variation in coronary artery disease risk factors and ln(CAC score + 1) or coronary artery disease risk factors and lnCIMT, with the exception of ln(CAC score + 1) and body mass index (ρenv = 0.40 (SE, 0.17); P = 0.013).
Table 3.
Genetic and Environmental Correlations Between CAC Quantity and CIMT With Coronary Artery Disease Risk Factors for Study Subjects, Lancaster County, Pennsylvania, 2000–2006a
| Risk Factor | CAC (n = 478) |
CIMT (n = 446) |
||||||
| ρgene (SE) | P Value | ρenv (SE) | P Value | ρgene (SE) | P Value | ρenv (SE) | P Value | |
| Body mass index | −0.27 (0.21) | 0.2 | 0.40 (0.17)b | 0.013b | 0.36 (0.24) | 0.14 | 0.16 (0.14) | 0.26 |
| Waist circumferencec | −0.09 (0.27) | 0.75 | 0.22 (0.14) | 0.11 | 0.58 (0.25)b | 0.046b | 0.08 (0.12) | 0.53 |
| Systolic blood pressure | 0.15 (0.20) | 0.45 | 0.08 (0.19) | 0.67 | 0.38 (0.20) | 0.081 | 0.12 (0.17) | 0.5 |
| Diastolic blood pressure | 0.01 (0.19) | 0.96 | 0.15 (0.21) | 0.48 | 0.20 (0.21) | 0.36 | −0.03 (0.20) | 0.88 |
| Pulse pressure | 0.26 (0.23) | 0.25 | −0.04 (0.17) | 0.82 | 0.32 (0.23) | 0.19 | 0.20 (0.15) | 0.2 |
| Total cholesterol | 0.44 (0.19)b | 0.028b | −0.04 (0.21) | 0.86 | 0.26 (0.24) | 0.25 | −0.18 (0.19) | 0.35 |
| Triglyceridesc | −0.10 (0.18) | 0.6 | 0.22 (0.18) | 0.21 | 0.14 (0.21) | 0.5 | 0.13 (0.15) | 0.41 |
| High density lipoprotein cholesterolc | −0.16 (0.25) | 0.54 | −0.12 (0.13) | 0.38 | −0.37 (0.26) | 0.17 | −0.02 (0.12) | 0.86 |
| Low density lipoprotein cholesterolc | 0.55 (0.17)b | 0.005b | −0.08 (0.20) | 0.69 | 0.33 (0.24) | 0.16 | −0.17 (0.19) | 0.35 |
Abbreviations: CAC, coronary artery calcification; CIMT, carotid artery intima-media thickness; ρgene, genetic correlation between two traits; ρenv, environmental correlation between two traits; SE, standard error.
CAC (and CIMT) distribution was defined on the basis of residualized ln(CAC score + 1) and lnCIMT values following adjustments for age and sex effects (refer to the text for further information).
Correlation achieving statistical significance (P < 0.05).
Natural log-transformed prior to analyses.
Ln(CAC score + 1) and lnCIMT were modestly correlated in this sample (age-, age2-, and sex-adjusted r = 0.14 (SE, 0.05); P = 0.003). Further analyses revealed that neither genetic nor environmental correlations between these 2 traits differed significantly from zero (ρgene = 0.19 (SE, 0.27); ρenv = 0.12 (SE, 0.13); P > 0.30 for both).
DISCUSSION
This study is one of the few to examine determinants of CAC quantity and CIMT in the same adult population, thus permitting a more direct comparison of the relative impact of coronary artery disease risk factors on these 2 measures. In agreement with other studies (24–27), we found that both higher CAC quantity and CIMT were associated with older age and male sex. Despite both being well-established markers of subclinical atherosclerosis and predictors of coronary artery disease events, correlation between CAC quantity and CIMT was only modest. Strikingly, we found that increased total cholesterol and low density lipoprotein cholesterol were strongly associated with high CAC, whereas higher blood pressure and body size measures were strongly associated with high CIMT. These findings strongly suggest distinct pathophysiologic mechanisms driving these 2 measures.
The association we observed between CAC quantity and low density lipoprotein cholesterol has been previously reported in many (15, 18, 24, 25, 28, 29), but not all (30, 31), studies. In perhaps the largest study so far, Allison and Wright (31) reported only a modest correlation between CAC quantity and low density lipoprotein cholesterol (r = 0.055, P < 0.001). The associations observed between CIMT and measures of body size and blood pressure have also been reported by others (32, 33).
Our study extends what was previously reported by suggesting that correlations observed between CAC quantity and low density lipoprotein cholesterol and between CIMT and body size and blood pressure may be partly due to effects of genes that jointly influence variation in both sets of traits. Evidence for some shared genes influencing variation in both CAC quantity and low density lipoprotein cholesterol levels comes from 2 sources. First, the residual heritability of CAC quantity was 0.42 in this sample but decreased by 24% after adjustment for low density lipoprotein cholesterol (and by 19% after adjustment for total cholesterol); in contrast, there was little decrease with adjustment for any other coronary artery disease risk factors. Second, the formal analysis of pleiotropy provided evidence for a statistically significant genetic correlation between CAC quantity and low density lipoprotein cholesterol (as well as total cholesterol), implying shared genetic effects on both traits.
We also observed significant evidence for an environmental correlation between CAC quantity and body mass index (ρenv = 0.40, P = 0.013), suggesting that some shared, unmeasured environmental factors jointly influence variation in these traits. Physical activity and dietary preferences are possible joint contributors, as suggested by Cassidy et al. (34), who showed that low-risk individuals have greater progression of CAC quantity if they are overweight versus underweight or of normal weight (34). Others have similarly reported that CAC quantity in adults was significantly associated with childhood weight and body mass index (33).
Our analyses provided evidence for some shared genes influencing variation in both CIMT and waist circumference and, to a lesser extent, both CIMT and systolic blood pressure. First, the residual heritability of CIMT, initially estimated at 0.29, was modestly reduced when adjusted for blood pressure. Second, modest evidence for pleiotropy was detected by the estimates of genetic correlations between CIMT and waist circumference and between CIMT and systolic blood pressure. Our results are similar to those from previous studies of middle-aged or elderly individuals showing a direct, positive relation between increased body mass index and increased CIMT (34–37).
Heritability of CAC quantity in the Amish (h2 = 0.40) is similar to that reported in asymptomatic US Caucasians from Rochester, Minnesota (h2 = 0.40) (24) and in families ascertained regarding hypertension (h2 = 0.40) (38) or diabetes (h2 = 0.50) (39). Heritability of CIMT observed in the Amish (0.27–0.32) is similar to that reported in other populations (e.g., Zannad et al. (40), Xiang et al. (41), Swan et al. (42)), although slightly lower (43, 44) and higher (45–47) estimates have also been reported from other populations. Whether the genetic contribution to variation in CAC quantity and/or CIMT is stronger in families enriched with early coronary artery disease or coronary artery disease risk factors is an interesting and important question but one that we were unable to address in our study.
As reported in previous studies (2–4), we also found a modest correlation between CAC quantity and CIMT (r = 0.14; P = 0.003) after adjustment for age and sex variation, which remained after adjustment for additional risk factors (data not shown). In the context of this relatively weak correlation, there was little evidence for joint genetic effects influencing variation in both traits.
A primary strength of our study is that the Old Order Amish share a socially and environmentally homogeneous lifestyle, and they tend to have large families, which allowed for estimation of pleiotropy. In addition, their lower medication and tobacco usage compared with that of the general US population enabled us to estimate associations among CAC quantity, CIMT, and coronary artery disease risk factors with less confounding or effect modification due to these behaviors. A limitation of our study was that we measured only common CIMT. Assessment of the intima-media thickness of the internal carotid artery and/or the carotid bifurcation might provide additional useful information. Our study was underpowered to examine the relation of CAC quantity and CIMT with coronary artery disease risk factors separately in men and women. Finally, generalization of our findings to other European, American, and ethnic populations with more diverse lifestyles should be attempted cautiously.
Although the differential pattern of risk factor associations with CAC quantity and CIMT observed in our study and others provides important insights into the pathogenesis of these 2 processes, these differences also have important clinical implications in terms of monitoring of disease progression. Specifically, these results emphasize that choice of outcome to be used for monitoring (e.g., CAC quantity or CIMT) should be tied to the particular coronary artery disease risk factors to be targeted by the intervention or treatment.
In summary, CAC quantity and CIMT represent noninvasive measures of subclinical disease in different vascular beds and different pathologic processes involved in atherosclerotic disease. Although both are predictive of coronary artery disease events, they are associated with different risk factors and do not appear to share common genes. Furthermore, our results suggest that genes influencing lipid metabolism might be good candidates for CAC quantity and that genes influencing body size and blood pressure variation might be good candidates for CIMT. Additional studies are being pursued to identify the specific genes that influence CAC quantity and CIMT in the Old Order Amish.
Acknowledgments
Author affiliations: Division of Endocrinology, Diabetes and Nutrition, Department of Medicine, University of Maryland, Baltimore, Maryland (Evadnie Rampersaud, Afshin Parsa, Haiqing Shen, Kathleen A. Ryan, Patrick Donnelly, Alan R. Shuldiner, Braxton D. Mitchell); Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, Maryland (Wendy Post); Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan (Lawrence F. Bielak, Patricia A. Peyser); Department of Cardiovascular Diseases, The Ohio State University, Columbus, Ohio (John A. Rumberger); Department of Diagnostic Radiology, Mayo Clinic and Foundation, Rochester, Minnesota (Patrick F. Sheedy II); and Geriatric Research and Education Clinical Center, Baltimore Veterans Administration Medical Center, Baltimore, Maryland (Alan R. Shuldiner)
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute–sponsored NRSA Institutional Training Grant in Cardiac and Vascular Cell Biology T32HL072751; NIH Clinical Scholars Research Training grant K12RR023250; and NIH research grants U01 HL72515 and R01 HL088119. Support was also received from University of Maryland General Clinical Research Center grant M01 RR 16500, The Johns Hopkins University General Clinical Research Center grant M01 RR 000052, the General Clinical Research Centers Program, the National Center for Research Resources (NCRR), NIH, and the Paul Beeson Physician Faculty Scholars in Aging Program of the American Federation of Aging Research. Partial funding was also provided by the Clinical Nutrition Research Unit of Maryland (grant P30 DK072488).
All authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the contents of the manuscript as written.
Conflict of interest: none declared.
Glossary
Abbreviations
- CAC
coronary artery calcification
- CIMT
carotid artery intima-media thickness
- SE
standard error
References
- 1.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94(5):1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 3.Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104(4):412–417. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 4.Bots ML, Hoes AW, Koudstaal PJ, et al. Association between the intima-media thickness of the common carotid and subsequent cardiovascular events in subjects, 55 years and older, in the Rotterdam study (ERGO) Ned Tijdschr Geneeskd. 1998;142(19):1100–1103. [PubMed] [Google Scholar]
- 5.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146(6):483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 6.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults in the Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 7.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24(1):12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 8.Megnien JL, Simon A, Valensi P, et al. Comparative effects of diabetes mellitus and hypertension on physical properties of human large arteries. J Am Coll Cardiol. 1992;20(7):1562–1568. doi: 10.1016/0735-1097(92)90451-r. [DOI] [PubMed] [Google Scholar]
- 9.Megnien JL, Gariepy J, Saudubray JM, et al. Evidence of carotid artery wall hypertrophy in homozygous homocystinuria. Circulation. 1998;98(21):2276–2281. doi: 10.1161/01.cir.98.21.2276. [DOI] [PubMed] [Google Scholar]
- 10.Streeten EA, Ryan KA, McBride DJ, et al. The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity. J Clin Endocrinol Metab. 2005;90(8):4536–4541. doi: 10.1210/jc.2004-1924. [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Bielak LF, Streeten EA, et al. Relationship between vascular calcification and bone mineral density in the Old-Order Amish. Calcif Tissue Int. 2007;80(4):244–250. doi: 10.1007/s00223-007-9006-4. [DOI] [PubMed] [Google Scholar]
- 12.Sorkin J, Post W, Pollin TI, et al. Exploring the genetics of longevity in the Old Order Amish. Mech Ageing Dev. 2005;126(2):347–350. doi: 10.1016/j.mad.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 14.American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27(suppl 1):11–14. [Google Scholar]
- 15.Post W, Bielak LF, Ryan KA, et al. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115(6):717–724. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Budoff MJ, Lu B, et al. Reproducibility of three different scoring systems for measurement of coronary calcium. Int J Cardiovasc Imaging. 2002;18(5):391–397. doi: 10.1023/a:1016051606758. [DOI] [PubMed] [Google Scholar]
- 18.Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208(3):807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 19.Edwards AWF. Likelihood, Expanded Edition. Baltimore, MD: The Johns Hopkins University Press; 1992. [Google Scholar]
- 20.Self SG, Liang KY. Asymptotic properties of maximum likelihood ratio tests under nonstandard conditions. J Am Stat Assoc. 1987;82(398):605–610. [Google Scholar]
- 21.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell BD, Kammerer CM, Blangero J, et al. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans: the San Antonio Family Heart Study. Circulation. 1996;94(9):2159–2170. doi: 10.1161/01.cir.94.9.2159. [DOI] [PubMed] [Google Scholar]
- 23.Lange K, Boehnke M. Extensions to pedigree analysis. IV. Covariance components models for multivariate traits. Am J Med Genet. 1983;14(3):513–524. doi: 10.1002/ajmg.1320140315. [DOI] [PubMed] [Google Scholar]
- 24.Peyser PA, Bielak LF, Chu JS, et al. Heritability of coronary artery calcium quantity measured by electron beam computed tomography in asymptomatic adults. Circulation. 2002;106(3):304–308. doi: 10.1161/01.cir.0000022664.21832.5d. [DOI] [PubMed] [Google Scholar]
- 25.Wong ND, Sciammarella M, Arad Y, et al. Relation of thoracic aortic and aortic valve calcium to coronary artery calcium and risk assessment. Am J Cardiol. 2003;92(8):951–955. doi: 10.1016/s0002-9149(03)00976-7. [DOI] [PubMed] [Google Scholar]
- 26.Maher JE, Raz JA, Bielak LF, et al. Potential of quantity of coronary artery calcification to identify new risk factors for asymptomatic atherosclerosis. Am J Epidemiol. 1996;144(10):943–953. doi: 10.1093/oxfordjournals.aje.a008864. [DOI] [PubMed] [Google Scholar]
- 27.Valdes AM, Wolfe ML, Tate HC, et al. Association of traditional risk factors with coronary calcification in persons with a family history of premature coronary heart disease: the study of the inherited risk of coronary atherosclerosis. J Investig Med. 2001;49(4):353–361. doi: 10.2310/6650.2001.33901. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Feuerstein I, Wong H, et al. Do conventional risk factors predict subclinical coronary artery disease? Results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141(3):463–468. doi: 10.1067/mhj.2001.113069. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86(1):8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 30.Hecht HS, Superko HR, Smith LK, et al. Relation of coronary artery calcium identified by electron beam tomography to serum lipoprotein levels and implications for treatment. Am J Cardiol. 2001;87(4):406–412. doi: 10.1016/s0002-9149(00)01392-8. [DOI] [PubMed] [Google Scholar]
- 31.Allison MA, Wright CM. A comparison of HDL and LDL cholesterol for prevalent coronary calcification. Int J Cardiol. 2004;95(1):55–60. doi: 10.1016/j.ijcard.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Salonen R, Salonen JT. Determinants of carotid intima-media thickness: a population-based ultrasonography study in eastern Finnish men. J Intern Med. 1991;229(3):225–231. doi: 10.1111/j.1365-2796.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine study. J Am Coll Cardiol. 1996;27(2):277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 34.Cassidy AE, Bielak LF, Zhou Y, et al. Progression of subclinical coronary atherosclerosis: does obesity make a difference? Circulation. 2005;111(15):1877–1882. doi: 10.1161/01.CIR.0000161820.40494.5D. [DOI] [PubMed] [Google Scholar]
- 35.Lakka TA, Lakka HM, Salonen R, et al. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154(2):497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 36.De Michele M, Panico S, Iannuzzi A, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33(12):2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 37.Hassinen M, Lakka TA, Komulainen P, et al. Association of waist and hip circumference with 12-year progression of carotid intima-media thickness in elderly women. Int J Obes (Lond) 2007;31(9):1406–1411. doi: 10.1038/sj.ijo.0803613. [DOI] [PubMed] [Google Scholar]
- 38.Turner ST, Peyser PA, Kardia SL, et al. Genomic loci with pleiotropic effects on coronary artery calcification. Atherosclerosis. 2006;185(2):340–346. doi: 10.1016/j.atherosclerosis.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Wagenknecht LE, Bowden DW, Carr JJ, et al. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes. 2001;50(4):861–866. doi: 10.2337/diabetes.50.4.861. [DOI] [PubMed] [Google Scholar]
- 40.Zannad F, Visvikis S, Gueguen R, et al. Genetics strongly determines the wall thickness of the left and right carotid arteries. Hum Genet. 1998;103(2):183–188. doi: 10.1007/s004390050804. [DOI] [PubMed] [Google Scholar]
- 41.Xiang AH, Azen SP, Buchanan TA, et al. Heritability of subclinical atherosclerosis in Latino families ascertained through a hypertensive parent. Arterioscler Thromb Vasc Biol. 2002;22(5):843–848. doi: 10.1161/01.atv.0000015329.15481.e8. [DOI] [PubMed] [Google Scholar]
- 42.Swan L, Birnie DH, Inglis G, et al. The determination of carotid intima medial thickness in adults—a population-based twin study. Atherosclerosis. 2003;166(1):137–141. doi: 10.1016/s0021-9150(02)00317-9. [DOI] [PubMed] [Google Scholar]
- 43.North KE, MacCluer JW, Devereux RB, et al. Heritability of carotid artery structure and function: the Strong Heart Family Study. Arterioscler Thromb Vasc Biol. 2002;22(1):1698–1703. doi: 10.1161/01.atv.0000032656.91352.5e. [DOI] [PubMed] [Google Scholar]
- 44.Mayosi BM, Avery PJ, Baker M, et al. Genotype at the -174G/C polymorphism of the interleukin-6 gene is associated with common carotid artery intimal-medial thickness: family study and meta-analysis. Stroke. 2005;36(10):2215–2219. doi: 10.1161/01.STR.0000182254.47941.96. [DOI] [PubMed] [Google Scholar]
- 45.Fox CS, Polak JF, Chazaro I, et al. Genetic and environmental contributions to atherosclerosis phenotypes in men and women: heritability of carotid intima-media thickness in the Framingham Heart Study. Stroke. 2003;34(2):397–401. doi: 10.1161/01.str.0000048214.56981.6f. [DOI] [PubMed] [Google Scholar]
- 46.Wang AY, Ho SS, Liu EK, et al. Differential associations of traditional and non-traditional risk factors with carotid intima-media thickening and plaque in peritoneal dialysis patients. Am J Nephrol. 2007;27(5):458–465. doi: 10.1159/000106457. [DOI] [PubMed] [Google Scholar]
- 47.Zhao J, Cheema FA, Bremner JD, et al. Heritability of carotid intima-media thickness: a twin study. Atherosclerosis. 2008;197(2):814–820. doi: 10.1016/j.atherosclerosis.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]