Abstract
Background
Value-of-life methods are increasingly used in policy analyses of the economic burden of disease. The purpose of this study was to estimate and project the value of life lost from cancer deaths in the United States.
Methods
We estimated and projected US age-specific mortality rates for all cancers and for 16 types of cancer in men and 18 cancers in women in the years 2000–2020 and applied them to US population projections to estimate the number of deaths in each year. Cohort life tables were used to calculate the remaining life expectancy in the absence of cancer deaths—the person-years of life lost (PYLL). We used a willingness-to-pay approach in which the value of life lost due to cancer death was calculated by multiplying PYLL by an estimate of the value of 1 year of life ($150 000). We performed sensitivity analyses for female breast, colorectal, lung, and prostate cancers using varying assumptions about future cancer mortality rates through the year 2020.
Results
The value of life lost from all cancer deaths in the year 2000 was $960.6 billion; lung cancer alone represented more than 25% of this value. Projections for the year 2020 with current cancer mortality rates showed a 53% increase in the total value of life lost ($1472.5 billion). Projected annual decreases of cancer mortality rates of 2% reduced the expected value of life lost in the year 2020 from $121.0 billion to $80.7 billion for breast cancer, $140.1 billion to $93.5 billion for colorectal cancer, from $433.4 billion to $289.4 billion for lung cancer, and from $58.4 billion to $39.0 billion for prostate cancer.
Conclusions
Estimated value of life lost due to cancer deaths in the United States is substantial and expected to increase dramatically, even if mortality rates remain constant, because of expected population changes. These estimates and projections may help target investments in cancer control strategies to tumor sites that are likely to result in the greatest burden of disease and to interventions that are the most cost-effective.
CONTEXT AND CAVEATS
Prior knowledge
Estimates of value of life lost due to premature death are useful in determining the economic burden of disease.
Study design
Person-years of life lost (PYLL) due to cancer deaths for the years 2000–2020 from 16 types of cancer in men and 18 types in women were estimated and projected and used to calculate the total value of life lost due to cancer deaths during this period and the reductions in this amount that would occur if cancer mortality rates declined during the period. The value of a year of life was based on previous estimates.
Contribution
Based on a value of $150 000 for 1 year of life, the value of life lost from all cancer deaths was $960.6 billion in 2000 and was projected to be $1472.5 billion in 2020. Compared with projections of current mortality rates, projected annual decreases of cancer mortality rates of 2% reduced the expected value of life lost in the year 2020 from $121.0 billion to $80.7 billion for breast cancer, from $140.1 billion to $93.5 billion for colorectal cancer, from $433.4 billion to $289.4 billion for lung cancer, and from $58.4 billion to $39.0 billion for prostate cancer.
Implications
The value of life lost due to death from cancer is large and is expected to increase if mortality rates do not change. Such estimates of the value of life lost due to cancer death estimates such as these may be useful in targeting interventions to cancers that are likely to result in the greatest burden of disease.
Limitations
Misclassification of which type of tumor was the cause of death (eg, lung metastasis may be coded as lung cancer rather than cancer of the primary tumor) may have led to overestimation for some cancer sites. Use of all causes of death (including cancer) to approximate non-cancer deaths in estimation of PYLL may have led to underestimation.
From the Editors
Common measures of the burden of cancer include incidence and mortality rates, morbidity- or health-adjusted life years, and the costs of medical care (1,2). These measures do not incorporate the value of lost productivity or the value of life lost due to cancer deaths. Economists refer to the costs associated with premature deaths as mortality costs and generally use one of two methods to estimate their value—the human capital approach or the willingness-to-pay (WTP) approach. In the human-capital approach, sex- and age-specific average earnings are combined with expected productivity trends and years of life lost to estimate unrealized lifetime earnings. This approach explicitly values the years of life lost of individuals with greater earnings (eg, men aged 35–55 years) as higher than those of individuals with fewer earnings (eg, women aged ≥75 years). The WTP approach, in contrast, incorporates both lost productivity due to death and the intrinsic value of life (2,3) by estimating the amount an average individual would be willing to pay for an additional year of life.
Both approaches are relevant for informing health policy. The human-capital approach estimates the impact of premature deaths on the economy, whereas the WTP approach offers a more global estimate of the value of economic loss due to premature deaths. Because incidence and mortality rates for most tumor sites are highest in the elderly, a population that is less likely to be in the workforce than their younger counterparts, comparison of the results of these two approaches is particularly relevant for evaluating the burden of cancer.
Several studies have used the human-capital approach to estimate mortality costs of all cancers (4,5) or of cancers at specific sites (6,7). In the studies that measure both mortality costs and direct costs of medical care, estimated mortality of cost estimated are generally at least as high as the direct costs of medical care (5–7). To our knowledge, the WTP approach has not been used previously to systematically estimate the value of life lost from cancer deaths by tumor site. In this study, we estimated and projected person-years of life lost (PYLL) from cancer deaths and the corresponding value of life lost in women and men in the United States in the years 2000–2020, using previously published WTP estimates of the value of life (8,9). We also conducted sensitivity analyses of the value of life lost due to breast, colorectal, lung, and prostate cancer deaths through the year 2020, using various assumptions about changes in cancer mortality rates during this period.
Methods
Overview
We estimated US age-specific cancer mortality rates for all cancers combined and separately for 16 tumor sites in men and 18 tumor sites in women using the most recent available data. These mortality rates were then applied to age- and sex-specific population projections through the year 2020 to estimate and project the number of cancer deaths in each year. For each death, cohort life tables were used to compute the remaining life expectancy if the person had not died from cancer—the PYLL due to cancer. PYLLs were then multiplied by a previously published value of a year of life of $150 000 (8,9). The value of life lost due to cancer deaths was estimated for the year 2000 and projected through 2020 for the most prevalent tumor sites separately and for all tumor sites combined. We conducted sensitivity analyses for the four most prevalent tumor sites (ie, breast, colorectal, lung, and prostate) using varying assumptions about future mortality rates that highlight the impacts of a growing and aging population as well as the potential results of cancer control strategies. All estimates were grouped separately for men and women younger than 65 and 65 and older to allow evaluation of the relative impact of age-specific life expectancy and mortality rates on PYLL and value of life estimates.
Current Cancer Mortality Rates
We calculated sex- and age-specific cancer mortality rates using the underlying cause of death from death certificates in the United States for the most recent years of data (1999–2003) and US population estimates during the same period for all cancers combined and separately for the 20 most prevalent cancers. The use of 5 years of data allowed us to calculate relatively stable mortality rates, particularly for tumor sites with relatively few deaths in a single year. Age- and sex-specific mortality rates were calculated for the following tumor sites: brain and other neurologic sites, female breast, cervix, colorectal, corpus uteri, esophagus, gastric, head and neck, Hodgkin lymphoma, kidney, leukemia, liver, lung, melanoma of the skin, non-Hodgkin lymphoma, ovary, pancreas, prostate, and urinary bladder. Nineteen age groups were used to calculate age-specific mortality rates. We also calculated age-adjusted mortality rates for all cancers combined and separately by tumor site and by age groups younger than 65 and 65 and older using the 2000 US Standard Population.
US Age- and Sex-specific Population Projections
We used the National Interim Population Projections from the 2000 US Census that were released in March 2004 to estimate and project annual age- and sex-specific populations from 2000 through 2020. These data and related documentation are available at http://www.census.gov/ipc/www/usinterimproj/.
Estimates and Projections of Other Causes of Death
Cohort life tables from the Berkeley Mortality Database (http://www.demog.berkeley.edu/∼bmd/states.html) for birth years 1900–2000 were used to estimate and project sex-specific life expectancy in the years 2000–2020. The Berkeley Mortality Database was developed from historical series of national vital statistics (ie, births, deaths, and census populations). It is part of the Human Mortality Database project, whose aim is to construct high-quality national cohort life tables. Projections incorporate observed trends in life expectancy during the past century. Because these life tables contain years of birth only through 2000, we assumed that individuals born after 2000 (ie, 2001–2020) would have the same life expectancy as individuals born in 2000.
Estimates and PYLL
Sex- and age-specific mortality rates were multiplied by US population estimates for the year 2000 to calculate the number of deaths for each of the tumor sites and for all cancers combined. Sex- and age-specific estimates of other causes of death in each year were used to calculate the PYLL had individuals not died from cancer. We also used current mortality rates (1999–2003) and US population projections to project deaths and PYLL by tumor site and all cancers combined through the year 2020.
Sensitivity Analysis of Breast, Colorectal, Lung, and Prostate Cancer Mortality Rates
We evaluated several scenarios for projecting mortality rates for breast, colorectal, lung, and prostate cancers in sensitivity analyses. Cancers at these four sites are the most prevalent, are among the leading causes of cancer deaths (10), and have effective primary prevention, early detection, or treatment strategies (11–14). We used the most recent 5-year tumor site-specific mortality rates and assumed they were constant through the year 2020 as a baseline comparison. Yearly changes in PYLL under this base case scenario reflect changes in the population size and age composition and increases in life expectancy but not changes in cancer mortality rates. In sensitivity analyses, we selected changes in mortality rates that were consistent with recent declines in tumor site–specific mortality rates (10), which reflect greater use of cancer control strategies in the United States, including risk factor reduction (eg, smoking cessation) (15), screening (eg, mammography, colonoscopy) (16), and effective treatments (eg, chemotherapy) (17). Between 1996 and 2005, breast, colorectal, lung, and prostate cancer mortality rates declined annually by an average of 2.2%, 2.6%, 1%, and 4.0%, respectively (10). Thus, we evaluated the effect of annual declines in mortality rates of 1%, 2%, and 4% on PYLL for each tumor site, which allowed us to make direct comparisons across tumor sites.
Estimated Value of a Year of Life
We used a previously published value of a year of life, $150 000 (8,9), for men and women in all age groups This estimate is based on prior research that describes the willingness to pay for an additional year of life (8,9,18). A yearly estimate of $150 000 is also consistent with the value derived from other approaches for identifying a standard value of a year of life. For example, the World Health Organization Commission on Macroeconomics and Health suggested that, as a benchmark for evaluating the cost-effectiveness of health interventions, a cost-effectiveness ratio of less than three times per capita gross domestic product (GDP) should be considered as favorable (18). The per capita GDP in 2002 in the United States was about $36 000 (19), and three times the 2002 per capita GDP would be about $108 000. Although there is no consensus on the appropriate value of a year of life (20), the value we chose is consistent with other estimates (3), and our methods are sufficiently transparent to allow application of other dollar amounts that are based on other approaches.
All value-of-life estimates were discounted by 3% annually. This discount rate is consistent with recommendations for reporting the present value of future expenditures (21).
Results
Age-adjusted mortality rates for all cancers were dramatically higher for men and women aged 65 and older than for men and women younger than age 65 (Table 1). Among those aged 65 and older, cancer mortality rates were also higher for men than women—1446.5 per 100 000 men and 883.7 per 100 000 women. Among men and women younger than 65, cancer mortality rates were more similar—69.6 per 100 000 and 60.2 per 100 000, respectively. Within most tumor sites, mortality rates were much higher in men and women aged 65 and older than in men and women younger than 65. The only exception was testicular cancer, for which mortality rates were similar in the two age groups.
Table 1.
Age-adjusted mortality rates (per 100 000) in the United States by sex and tumor site, 1999–2003*
| Mortality rate (per 100 000) |
||
| Sex and tumor site | <65 years | ≥65 years |
| Men | ||
| Lung | 21.9 | 440.5 |
| Prostate | 2.0 | 216.6 |
| Colorectal | 6.6 | 146.7 |
| Pancreas | 3.9 | 69.3 |
| Leukemia | 2.9 | 60.0 |
| Lymphoma (non-Hodgkin) | 3.0 | 57.5 |
| Esophagus | 3.0 | 40.6 |
| Urinary bladder | 1.1 | 51.7 |
| Liver | 2.9 | 34.9 |
| Kidney | 2.3 | 32.5 |
| Gastric | 1.9 | 35.4 |
| Head and neck | 2.5 | 28.1 |
| Brain and ONS | 3.2 | 21.7 |
| Melanoma of the skin | 1.8 | 18.2 |
| Lymphoma (Hodgkin) | 0.3 | 2.2 |
| Testis | 0.3 | 0.3 |
| All cancers | 69.6 | 1446.5 |
| Women | ||
| Lung | 13.9 | 228.6 |
| Breast | 13.3 | 113.4 |
| Colorectal | 4.6 | 102.4 |
| Pancreas | 2.5 | 56.1 |
| Ovary | 3.7 | 44.8 |
| Lymphoma (non-Hodgkin) | 1.8 | 38.3 |
| Leukemia | 2.0 | 32.0 |
| Corpus uteri | 1.4 | 22.9 |
| Brain and ONS | 2.1 | 14.4 |
| Gastric | 1.0 | 17.8 |
| Liver | 1.0 | 17.2 |
| Kidney | 0.9 | 15.5 |
| Cervix | 2.0 | 7.1 |
| Urinary bladder | 0.4 | 15.4 |
| Esophagus | 0.5 | 10.4 |
| Head and neck | 0.6 | 9.7 |
| Melanoma of the skin | 0.9 | 7.4 |
| Lymphoma (Hodgkin) | 0.2 | 1.4 |
| All cancers | 60.2 | 883.7 |
Rates are age adjusted to the 2000 US Standard Population (19 age groups: Census P25-1130).
Tumor sites are listed from highest to lowest sex-specific age-adjusted mortality rate. ONS = other neurologic sites.
PYLL estimates varied by sex, age, and tumor site (Table 2), reflecting age-specific mortality rates, population size, and the years of life lost compared with life expectancy in the relevant birth cohort. PYLL estimates from all cancers combined were higher in men and women younger than age 65 than in those aged 65 and older. PYLL estimates were higher in women than men in both age groups despite the lower mortality rates in women, suggesting that life expectancy, population size, and age composition influence PYLL more than mortality rates.
Table 2.
Person-years of life lost (PYLL) due to cancer deaths in the year 2000 by sex and tumor site*
| Tumor site | Men | Women | ||
| <65 years | ≥65 years | <65 years | ≥65 years | |
| Lung | 610 855 | 635 080 | 488 915 | 576 102 |
| Breast | — | — | 526 508 | 267 769 |
| Prostate | 49 602 | 219 714 | — | — |
| Colorectal | 196 931 | 184 506 | 172 303 | 224 298 |
| Pancreas | 113 170 | 94 461 | 87 697 | 130 226 |
| Ovary | — | — | 140 152 | 109 080 |
| Leukemia | 118 013 | 74 698 | 97 195 | 70 818 |
| Lymphoma (non-Hodgkin) | 99 986 | 73 154 | 70 133 | 86 679 |
| Esophagus | 87 829 | 59 460 | 18 211 | 24 476 |
| Urinary bladder | 31 591 | 58 293 | 13 539 | 32 320 |
| Liver | 92 689 | 49 053 | 36 485 | 40 056 |
| Kidney | 68 986 | 44 248 | 35 353 | 36 315 |
| Gastric | 58 741 | 45 301 | 39 245 | 39 282 |
| Head and neck | 73 641 | 40 617 | 23 830 | 22 982 |
| Brain and ONS | 123 302 | 32 733 | 97 110 | 36 459 |
| Cervix | — | — | 88 979 | 17 692 |
| Corpus uteri | — | — | 50 962 | 54 898 |
| Melanoma of the skin | 59 723 | 24 394 | 39 860 | 17 343 |
| Lymphoma (Hodgkin) | 15 346 | 2991 | 12 575 | 3241 |
| Testis | 12 660 | 411 | — | — |
| All cancers | 2 148 725 | 1 883 620 | 2 331 853 | 2 084 256 |
Tumor sites are listed from highest to lowest sex-specific age-adjusted mortality rate. — = not available or not applicable to this population; ONS = other neurologic sites. To estimate PYLL, the number of deaths for each tumor site was calculated from age- and sex-specific mortality rates and age- and sex-specific population projections. For each death, cohort life tables were used to compute the remaining life expectancy had the person not died from cancer.
Among all men and women, lung cancer was the single largest contributor to PYLL due to early death from cancer. In men, PYLL estimates for colorectal, pancreas, and prostate cancers and leukemia were the next largest. Among women, PYLL estimates for cancers of the breast, colorectum, ovary, and pancreas were the largest. In men, PYLL estimates for most tumor sites were similar between age groups or higher in the younger than the older group. Among women, by contrast, this pattern held for fewer tumor sites; indeed, PYLL estimates were higher in the older than the younger age group for non-Hodgkin lymphoma and for lung, colorectal, pancreas, esophagus, and urinary bladder cancer.
Value of life lost that was associated with cancer deaths in 2000 for men younger than 65 and 65 and older was $222.4 billion and $245.8 billion (Table 3), respectively. Estimates for women younger than 65 and 65 and older were $227.9 billion and $264.5 billion, respectively. Lung cancer alone represented more than 25% of the value of life lost due to cancer deaths, in all years. Female breast cancer represented approximately 17% of the value of life lost due to cancer deaths in women. Each of the other tumor sites was less than 10% of the total value of life lost due to cancer deaths in men and women.
Table 3.
Value of life lost due to cancer deaths in the year 2000 by sex and tumor site in billions of dollars*
| Tumor site | Men | Women | ||
| <65 years (billion $) | ≥65 years (billion $) | <65 years (billion $) | ≥65 years (billion $) | |
| Lung | 66.1 | 82.3 | 50.1 | 72.3 |
| Breast | 51.3 | 33.9 | ||
| Prostate | 5.5 | 29.3 | ||
| Colorectal | 20.7 | 24.1 | 17.2 | 28.8 |
| Pancreas | 12.1 | 12.3 | 8.9 | 16.6 |
| Ovary | — | — | 13.9 | 13.8 |
| Leukemia | 10.7 | 9.8 | 8.4 | 9.1 |
| Lymphoma (non-Hodgkin) | 10.0 | 9.6 | 6.8 | 11.1 |
| Esophagus | 9.4 | 7.7 | 1.9 | 3.1 |
| Urinary bladder | 3.4 | 7.7 | 1.4 | 4.2 |
| Liver | 9.6 | 6.4 | 3.6 | 5.1 |
| Kidney | 7.2 | 5.8 | 3.4 | 4.6 |
| Gastric | 6.1 | 5.9 | 3.8 | 5.0 |
| Head and neck | 7.8 | 5.3 | 2.4 | 2.9 |
| Brain and ONS | 11.5 | 4.2 | 8.6 | 4.6 |
| Cervix | — | — | 8.2 | 2.2 |
| Corpus uteri | — | — | 5.2 | 6.9 |
| Melanoma of the skin | 6.0 | 3.2 | 3.8 | 2.2 |
| Lymphoma (Hodgkin) | 1.4 | 0.4 | 1.1 | 0.4 |
| Testis | 1.1 | 0.1 | — | — |
| All cancers | 222.4 | 245.8 | 227.9 | 264.5 |
Tumor sites are listed from highest to lowest sex-specific age-adjusted mortality rate. — = not available or not applicable to this population; ONS = other neurologic sites. Value of life lost was estimated using a previously published value of 1 year of life ($150 000) applied to the person-years of life lost estimate for each tumor site. All value of life lost estimates were discounted by 3% annually and reported in real dollars.
Overall, the value of life lost from cancer deaths in the year 2000 was $960.6 billion; under assumptions of constant mortality rates, this value would increase 53% to $1472.5 billion in 2020 (Table 4). Although we used the same process to project the underlying population to 2020, population changes have different impacts across tumor sites, because the age- and sex-specific mortality rates are different for each tumor site. For example, the value of life lost due to death from prostate cancer increased by 68% between 2000 and 2020, but the value of life lost due to death from cervical cancer increased by about 29% during the same period.
Table 4.
Value of life lost due to cancer deaths in the years 2000 and 2020 by tumor site in billions of dollars*
| Tumor site | Value of life lost | % increase in value of life lost | |
| 2000 (billion $) | 2020 (billion $) | ||
| Lung | 270.8 | 433.4 | 60.1 |
| Female breast | 85.3 | 121.0 | 41.8 |
| Prostate | 34.8 | 58.4 | 67.6 |
| Colorectal | 90.9 | 140.1 | 54.3 |
| Pancreas | 49.9 | 77.9 | 56.2 |
| Ovary | 27.7 | 41.0 | 48.1 |
| Leukemia | 38.0 | 55.4 | 45.9 |
| Lymphoma (non-Hodgkin) | 37.4 | 56.5 | 51.0 |
| Esophagus | 22.0 | 34.9 | 58.6 |
| Urinary bladder | 16.7 | 26.7 | 60.2 |
| Liver | 24.6 | 37.2 | 51.4 |
| Kidney | 21.0 | 32.6 | 54.9 |
| Gastric | 20.8 | 31.6 | 51.5 |
| Head and neck | 18.4 | 28.7 | 56.3 |
| Brain and ONS | 28.9 | 40.5 | 40.1 |
| Cervix | 10.5 | 13.5 | 28.7 |
| Corpus uteri | 12.1 | 18.5 | 52.4 |
| Melanoma of the skin | 15.1 | 21.6 | 42.8 |
| Lymphoma (Hodgkin) | 3.2 | 4.3 | 31.0 |
| Testis | 1.2 | 1.3 | 13.6 |
| All cancers | 960.7 | 1472.5 | 53.3 |
Tumor sites are listed from highest to lowest sex-specific age-adjusted mortality rate. ONS = other neurologic sites. Value of life lost was estimated using a previously published value of 1 year of life ($150 000) applied to the person-years of life lost estimate for each tumor site. All value of life lost estimates were discounted by 3% annually and reported in real dollars.
Sensitivity Analysis of Cancer Mortality Rates
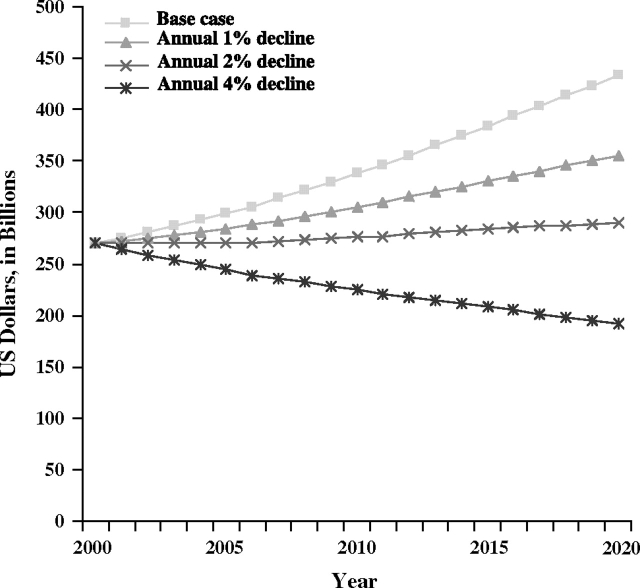
Compared with the base case scenario, in which constant lung cancer mortality rates are assumed, a projected 1% annual decline in lung cancer mortality rates would reduce the value of life lost due to lung cancer to $354.5 billion in 2020 from the expected $433.4 billion, and an annual 2% decline in lung cancer mortality rates would cause the value of life lost to be decreased to $289.4 billion in 2020 (Figure 1). Under a projected 4% annual decline in lung cancer mortality rates, the value of life lost would decrease to $191.6 billion in 2020. Projected value of life lost due to lung cancer deaths in 2020 under the 4% annual decline in mortality rates scenario was less than that estimated for the year 2000. The 4% annual decline scenario was the only scenario to result in a decline in value of life lost projections in 2020 from 2000 levels.
Figure 1.
Projected value of life lost due to lung cancer deaths in the United States. The most recent years of data (ie, from 1999 to 2003) were used to calculate sex- and age-specific lung cancer mortality rates for the base case mortality rate projections. Sensitivity analysis scenarios included annual 1%, 2%, and 4% declines in lung cancer mortality.
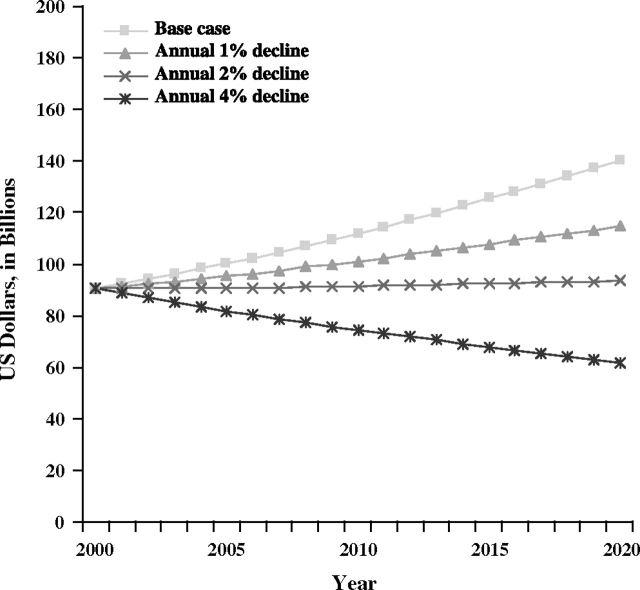
The value of life lost due to colorectal cancer deaths in the year 2020 would be reduced to $114.6 billion with a projected annual 1% decline in colorectal cancer mortality rates, compared with $140.1 billion in the base case scenario, and to $93.5 billion with the projected annual 2% decline in colorectal cancer mortality rates (Figure 2). As with lung cancer, the only scenario to lead to a reduction in value of life lost in 2020 compared with estimates in 2000 was the projection with a 4% annual decline in mortality rates, resulting in $61.9 billion estimate in 2020.
Figure 2.
Projected value of life lost due to colorectal cancer deaths in the United States. The most recent years of data (ie, from 1999 to 2003) were used to calculate sex- and age-specific colorectal cancer mortality rates for the base case mortality rate projections. Sensitivity analysis scenarios included annual 1%, 2%, and 4% declines in colorectal cancer mortality.
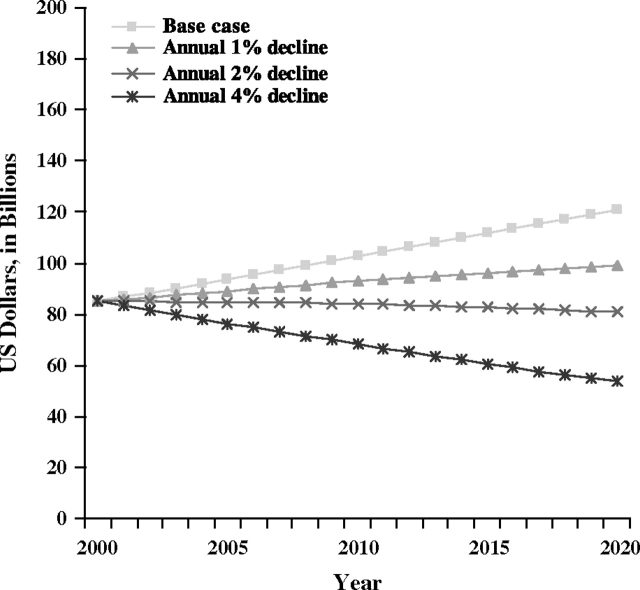
Compared with the base case scenario for breast cancer mortality in 2020, the value of life lost with the projected annual 1% decline in breast cancer mortality rates scenario declined from $121.0 billion to $98.9 billion in 2020, and to $80.7 billion with the projected annual 2% decline (Figure 3). A projected 4% annual decline in mortality rates was associated with a projected $53.5 billion value of life lost. Both the projected 2% and 4% annual declines in breast cancer mortality rate scenarios resulted in lower value of life estimates in the year 2020 than in 2000.
Figure 3.
Projected value of life lost due to breast cancer deaths in the United States. The most recent years of data (ie, from 1999 to 2003) were used to calculate sex- and age-specific breast cancer mortality rates for the base case mortality rate projections. Sensitivity analysis scenarios included annual 1%, 2%, and 4% declines in breast cancer mortality.
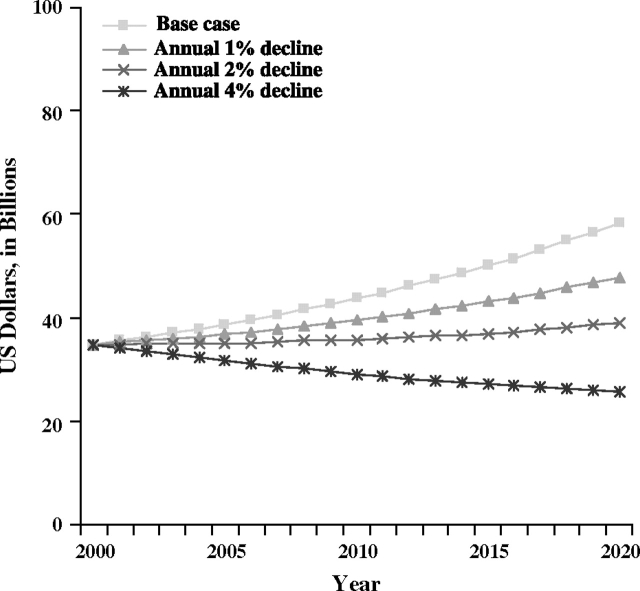
Reductions in value of life lost with changes in prostate cancer mortality rates were smaller than for the other three cancer sites examined. Compared with the base case scenario for prostate cancer mortality in 2020, expected value of life lost declined from $58.4 billion to $39.0 billion with the 2% annual decline in mortality rates, and to $25.8 billion with the 4% annual decline (Figure 4).
Figure 4.
Projected value of life lost due to prostate cancer deaths in the United States. The most recent years of data (ie, from 1999 to 2003) were used to calculate sex- and age-specific prostate cancer mortality rates for the base case mortality rate projections. Sensitivity analysis scenarios included annual 1%, 2%, and 4% declines in prostate cancer mortality.
To allow researchers to estimate value of life lost due to breast, colorectal, lung, or prostate cancer death using other values for a year of life, we created supplementary tables containing PYLL and average years of life lost (AYLL) estimates and projections by age group and sex. These tables contain the PYLL and AYLL estimates under different assumptions about changes in mortality rates between 2000 and 2020 and are available at htttp://healthservices.cancer.gov/publications/value_of_life_lost_tables/.
Discussion
In this study, we estimated and projected PYLL and the value of life lost due to cancer deaths in men and women in the United States. Using a standard value of life for men and women of all ages (ie, $150 000), we found that in the year 2000, the value of life lost due to early deaths from cancer was $960.6 billion. Despite higher cancer mortality rates in the population aged 65 and older than in the population younger than 65, value of life lost in the two age groups in the year 2000 was similar—$450.3 billion and $510.3 billion, respectively. To our knowledge, this is the only study to quantify value of life lost from premature death separately for multiple tumor sites and for all cancers combined. Our estimates reflect the most recent cancer mortality rates and can be combined with other components of the burden of cancer, including the direct medical costs of care, morbidity, patient time costs associated with medical care, and caregiver burden, to inform understanding of the burden of cancer in the United States.
Under assumptions of current constant cancer mortality rates in our base case scenario, we projected the value of life lost due to cancer deaths in 2020 to be $1472.5 billion, which is a 53% increase from the year 2000. This increase reflects the aging and growth of the US population and the expected increases in life expectancy and does not take into account the impact of effective cancer control strategies on cancer mortality that are currently disseminating and diffusing. We conducted sensitivity analysis using varying assumptions about trends in mortality rates for breast, colorectal, lung, and prostate cancer. Even with small annual declines in cancer mortality rates, the reductions in the total value of life lost were substantial. The hypothetical scenarios of 1%, 2%, and 4% annual declines in mortality rates allow comparisons of differences in value of life lost with the expected effects of cancer control strategies, including risk factor reduction, screening, and improvements in effective treatment. The relative contributions of these factors can be explored with more detailed simulation models, such as the Cancer Intervention and Surveillance Modeling Network models (22), and the resulting projections may be used to target investments to cancer control strategies that are likely to result in the greatest reduction in burden of illness and to interventions that are the most cost-effective. Some cancer control strategies, such as smoking cessation, reduce deaths from multiple chronic diseases, whereas others, such as chemotherapy, can only reduce deaths from cancer. In this study, we estimated the burden of deaths from cancer only, not from multiple chronic diseases. Thus, the potential overall impact of strategies that may reduce deaths for multiple chronic diseases on value of life lost will be understated.
A companion study (23) used the human capital method with these PYLL estimates in adults aged 20 and older with age- and sex-specific wage rates and employment levels to estimate productivity losses from cancer deaths. As already noted, this method assumes that earnings reflect underlying productivity and, as a result, estimates reflect lower wages and less employment among women and the elderly than among working age men. In 2000, the estimate of lost productivity from all cancer deaths using the human capital method was approximately $115.8 billion (23). Our value of life lost estimates were about eight times this amount, with the greatest differences in estimates for the population aged 65 and older. Differences in the two estimates reflect the inclusion of the intrinsic value of life in the WTP approach and the different methods for measuring productivity. The human capital approach estimates the impact of premature deaths on the economy, whereas the WTP approach yields a more global estimate. Regardless of the method used to estimate the societal value of premature deaths, these mortality costs are an important component of the burden of illness.
Others (5–7) have reported that mortality costs estimated using the human capital approach are at least as large as the estimated direct medical costs of care for cancer patients during the same period. Our estimates of the value of life lost are also substantially greater than estimates of direct medical costs of cancer care. For example, a recent study estimated that the aggregate prevalence costs of colorectal cancer care for all patients aged 65 and older in the United States in 2000 were approximately $7.4 billion (24). Our estimate of the value of life lost due to early death from colorectal cancer in the population aged 65 and older was more than seven times this amount in the same year. This ratio between value of life lost due to premature deaths and aggregate direct medical costs of care is likely to be even larger in the population younger than age 65, among whom incidence rates are smaller but PYLL due to cancer death are much greater.
The mortality rates presented here are similar to those reported routinely by the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program (10), but we report rates for different age groups (ie, younger than age 65 and 65 and older). Reporting the value of life lost for individuals younger than age 65 and aged 65 and older allows us to highlight the relative impact of age-specific mortality rates and life expectancies. It also allows us to compare value of life lost estimates with cost of care estimates in elderly populations from SEER-Medicare and provides a contrast to estimates calculated with the human capital approach.
Despite the strengths of using the best available national data on deaths and population projections, evaluating several assumptions about future mortality rates in sensitivity analyses, and using transparent methods, there are some limitations associated with this study. First, the validity of death certificate data varies by tumor site (24), and in some situations, the underlying cause of death may reflect a metastatic site rather than the original site (eg, prostate cancer metastasized to the lungs coded as lung cancer). As a result, our value of life lost estimates may be overstated for common metastatic sites, such as lung and liver. Our overall estimates of value of life lost for all cancers are unlikely to be impacted by the validity of death certificates for specific cancers, however. Second, we used all-cause mortality to approximate other-cause mortality in estimating PYLL. Because all-cause mortality includes cancer deaths, the hazards of death are overstated, and as a result, the PYLL and resulting value of life lost estimates are understated. The understatement is greatest when using all-cause mortality to approximate other-cause mortality for all cancers.
In summary, estimated value of life lost due to cancer death in the United States is substantial and is projected to increase dramatically even if mortality rates remain constant because of expected changes in population size, age composition, and life expectancy. Small decreases in mortality rates may lead to large reductions in the value of life lost. These estimates and projections may be used to target investments to cancer control strategies for tumor sites that are likely to result in the greatest reductions in burden of illness and those that are the most cost-effective.
Footnotes
We thank Danielle Melbert and Martin Krapcho of Information Management Services, Inc for assistance in calculating and projecting cancer mortality rates, other causes of death, and person-years of life lost.
The authors were responsible for the study design, data analysis, interpretation of the results, manuscript preparation, and the decision to submit the manuscript for publication.
References
- 1.Brown ML, Lipscomb J, Snyder C. The burden of illness in cancer: economic cost and quality of life. Ann Rev Public Health. 2001;22:91–113. doi: 10.1146/annurev.publhealth.22.1.91. [DOI] [PubMed] [Google Scholar]
- 2.Brown ML, Yabroff KR. Economic impact of cancer in the United States. In: Schottenfeld David, Joseph F Faumeni J., editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. pp. 202–214. [Google Scholar]
- 3.Nordhaus WD. The health of nations: the contribution of improved health to living standards. In: Murphy K, Topel R, editors. Measuring the Gains From Medical Research: An Economic Approach. Chicago, IL: University of Chicago Press; 2003. pp. 9–40. [Google Scholar]
- 4.Brown ML, Hodgson TA, Rice DP. Economic impact of cancer in United States. In: Schottenfeld D, Faumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 1996. pp. 255–266. [Google Scholar]
- 5.Rice DP, Hodgson TA. Social and Economic Implications of Cancer in the United States. DHHS Publication No. PHS81-1404. Washington, DC: US Government Printing Office; 1981. Vital and Health Statistics, Series 3, No. 20. [Google Scholar]
- 6.Max W, Rice DP, Sung H-Y, Michel M, Breuer W, Zhang X. The economic burden of prostate cancer, California, 1998. Cancer. 2002;94(11):2906–2913. doi: 10.1002/cncr.10532. [DOI] [PubMed] [Google Scholar]
- 7.Max W, Rice DP, Sung H-Y, Michel M, Breuer W, Zhang X. The economic burden of gynecologic cancers in California. Gynecol Oncol 1998. 2003;88(2):96–103. doi: 10.1016/s0090-8258(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 8.Cutler DM, Gruber J, Hartman RS, Landrum MB, Newhouse JP, Rosenthal MB. The Economic Impacts of the Tobacco Settlement. 2008 doi: 10.1002/pam.1037. NBER Working Paper No. W7760 http://ssrn.com/abstract=235716. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenberg FR. Sources of U.S. Longevity Increase, 1960–1997 (December 2000) 2008 CESifo Working Paper Series No. 405 http://ssrn.com/abstract=263113. [Google Scholar]
- 10.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistic Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 11.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Part 1):347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Cigarette smoking—attributable mortality and years of potential life lost—United States, 1990. MMWR Morb Mortal Wkly Rep. 1993;42(33):645–649. [PubMed] [Google Scholar]
- 14.Millikan R, Logothetis C. Update of the NCCN guidelines for treatment of prostate cancer. Oncology. 1997;11(11A):180–193. [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. 2nd ed. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 16.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 national health interview surveys. J Natl Cancer Inst. 2001;93(22):1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 17.Harlan LC, Clegg LX, Abrams J, Stevens JL, Ballard-Barbash R. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987–2000. J Clin Oncol. 2006;24(6):872–877. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Commission on Macroeconomics and Health. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization; 2001. [Google Scholar]
- 19.US Department of Labor Bureau of Labor Statistics, Office of Productivity and Technology. Comparative Real Gross Domestic Product Per Capita and Per Employed Person. Sixteen Countries 1960–2006. 2008. http://www.bls.gov/fls/flsgdp.pdf. [Google Scholar]
- 20.Hirth RA, Chernew ME, Miller E, Fendrick M, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 22.National Cancer Institute. Cancer Intervention and Surveillance Modeling Network (CISNET) http://cisnet cancer gov/index html 2007. Accessed December 20, 2007. [Google Scholar]
- 23.Bradley CJ, Yabroff KR, Dahman B, Feuer E, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States, 2000 to 2020. J Natl Cancer Inst. 2008;100(24):1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabroff KR, Mariotto AB, Feuer E, Brown ML. Projections of the costs associated with colorectal cancer care in the United States, 2000–2020. Health Econ. 2008;17(8):947–959. doi: 10.1002/hec.1307. [DOI] [PubMed] [Google Scholar]
- 25.Percy C, Stanek E, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]