Abstract
Hypothesis
Intratympanic (IT) application of dexamethasone will reduce ototoxicity associated with systemic cisplatin therapy.
Background
Cisplatin is a common chemotherapeutic drug often dose-limited by ototoxicity attributed to the formation of reactive oxygen and nitrogen species damaging critical inner ear structures. Steroids have been shown to reduce formation of reactive oxygen species, and thus may reduce ototoxicity. In the present pilot study, we test this hypothesis by IT administration of dexamethasone in a novel murine model of cisplatin ototoxicity.
Methods
Click and pure-tone evoked auditory brainstem responses (ABR) in young CBA/J mice were measured. The first phase consisted of a dosing study to identify the optimal cisplatin dose for ototoxicity. In the next phase, ABR thresholds were measured in cisplatin-treated mice after 5 days of IT injection of 24 mg/mL dexamethasone in one ear and normal saline in the opposite ear to serve as controls.
Results
Intraperitoneal injection of 14 mg/kg of cisplatin induces significant hearing loss (click-evoked ABR threshold elevation = 12±7 dB, μ±SEM) with acceptable mortality (20%). The ears that received IT dexamethasone in cisplatin-treated mice had minimal ABR threshold shifts with the click, 8 kHz and 16 kHz stimuli. There was no significant difference between IT dexamethasone and IT saline ears at 32 kHz.
Conclusions
IT dexamethasone protected the mouse ear against cisplatin-induced ototoxicity in a frequency-dependant manner The present results suggest that IT dexamethasone may be a safe, simple and effective intervention that minimizes cisplatin ototoxicity without interfering with the chemotherapeutic actions of cisplatin.
INTRODUCTION
Cisplatin is a common chemotherapeutic agent used to treat many different types of cancer including medulloblastoma, neuroblastoma, osteosarcoma, testicular, ovarian, cervical, bladder, lung, and head and neck cancers1. Cisplatin has several side effects stemming from its non-specific cytotoxic action. The most common dose limiting side effect is ototoxicity. Cisplatin-induced ototoxicity generally manifests as tinnitus and sensorineural hearing loss2 which starts in the high frequencies, but extends into lower frequencies that are important for speech perception3. This hearing impairment is dose related, cumulative, bilateral and usually permanent. Sixty-80% of patients treated show elevations of hearing thresholds and nearly 15% sustain significant hearing handicap4, 5. It has long been recognized that cisplatin results in the loss of cochlear outer hair cells (OHC), starting in the base of the cochlea6, 7. More recent evidence suggests that in addition to the OHCs, the stria vascularis and the spiral ganglion cells are also injured and that the latter injury occurs in a time course paralleling the loss of OHCs suggesting that cisplatin targets them directly8.
In current clinical practice, treatment of cancer with cisplatin is either interrupted when ototoxicity develops (by switching to another less potent anti-neoplastic agent such as carboplatin) or the resulting hearing impairment is tolerated as an acceptable side effect of cancer treatment. A desired goal is to find a safe therapy that protects the ear from cisplatin ototoxicity without affecting its chemotherapeutic actions.
At the molecular level, cisplatin induces the generation of reactive oxygen species (ROS)9, such as superoxide anion10. With increased ROS, glutathione and antioxidant enzymes are depleted11. As antioxidant enzymes are depleted, superoxide, hydrogen peroxide, and toxic lipids lead to calcium influx within cochlear cells, triggering apoptosis9. Dozens of experimental studies have attempted to find an ideal otoprotectant by administration of antioxidants against ROS at an early stage in the ototoxic pathways12. Unfortunately, many of these agents have been found to inhibit the tumoricidal effects of cisplatin and/or have toxicities or unknown effects in humans1. As a result, there are currently no clinical interventions that have been shown to prevent cisplatin ototoxicity in humans.
Glucocorticoids (prednisone, dexamethasone, methylprednisolone) are another promising class of drugs that have significant potential for otoprotection. In fact, systemic glucocorticoids are currently in use for treatment of hearing loss in a variety of cochlear disorders such as autoimmune inner ear, endolymphatic hydrops and Meniere's disease, tinnitus and cases of sudden or idiopathic rapidly progressing hearing loss when etiology is unclear13. Corticosteroids have been shown to limit the formation of reactive oxygen species in the inner ear14, 15. Animal studies have shown corticosteroids to have a protective benefit against aminoglycoside ototoxicity, which is believed to have a similar pathogenesis to cisplatin ototoxicity16, 17. The presence of corticosteroid receptors within critical mouse inner ear structures provides further evidence that steroids can exert an effect on the inner ear18. Unfortunately, corticosteroids also down-regulate apoptosis genes in tumor cells19. Therefore, their systemic application to protect against cisplatin-induced ototoxicity may result in decreased efficacy of cisplatin's tumoricidal properties.
Intratympanic administration of drugs is a contemporary method of locally treating inner ear disorders, allowing diffusion across the round window into the inner ear where it can exert its effects. Specifically, steroids placed into the middle ear have been shown to diffuse across the round window into the inner ear and bathe the inner ear structures18, 20, 21. This method allows concentration of steroid to much higher levels within the inner ear compared to oral or parenteral routes20, 21, 22. Also, local administration prevents systemic absorption avoiding the common systemic side effects of steroids including hyperglycemia, peptic ulcers, hypertension and osteoporosis13, 20 and more problematic, reduced efficacy of chemotherapeutic agents19. Intratympanic administration of steroids has been used to safely treat other inner ear disorders such as sudden sensorineural hearing loss and Méniere's disease for several years13, 23. We hypothesize that intratympanic steroids may also have a place for preventing cisplatin ototoxicity. In this study we use CBA/J mice as an animal model to determine if cisplatin ototoxicity can be prevented by intratympanic administration of corticosteroids. This also represents the first murine model of auditory function for cisplatin-induced ototoxicity.
METHODS
This study was approved by the University of Connecticut Health Center Animal Care Committee, Protocol # 2006-287.
Animal Subjects
Female CBA/J mice purchased from Jackson Laboratories (Bar Harbor, Maine) were used in this study. The mice were 1-2 months old with a weight ranging from 18 gm to 23 gm. Subjects were kept in standard housing until injected with cisplatin when they were single housed in an isolated area for chemotherapy-treated animals. Access to food and water was provided to all subjects ad libitum. Subjects were monitored daily for signs of distress, pain and weight loss > 20% of baseline. No subjects required euthanization due to excessive weight loss, distress or pain.
Study Design
This study was performed in three phases as follows:
Phase I
Establishing the optimal ototoxic dose of cisplatin. Twenty five mice were divided into groups of five. For each group, on Day 1, pre-treatment auditory brainstem evoked response (ABR) thresholds were obtained prior to injection of cisplatin. Each group of five was then injected with 8, 10, 12, 14, or 16 mg/kg of cisplatin. Post-treatment ABR thresholds were then recorded on Day 8. Pre- and post-treatment thresholds were compared to measure threshold change.
Phase II
Intratympanic dexamethasone control group. Pre-treatment ABR thresholds were obtained in five mice. On Days 1-5, one ear received a daily intratympanic injection of dexamethasone 24 mg/mL and the contralateral ear received a daily intratympanic injection of sterile 0.9% saline, as the control. Post-treatment ABR thresholds were recorded again on Day 8 allowing time for any effusion to clear.
Phase III
Determining the effect of intratympanic dexamethasone on cisplatin ototoxicity. Pre-treatment ABR thresholds were obtained in ten mice. On Day 1, each mouse received intratympanic dexamethasone in one ear and sterile saline in the contralateral ear. The mice were divided so that five mice received dexamethasone in the left ear and saline in the right ear, while the other five mice received saline in the left ear and dexamethasone in right ear. Each mouse then received an intraperitoneal injection of 14 mg/kg cisplatin, which was determined based on the results of Phase I experiments. On Days 2-5 daily intratympanic injections of either dexamethasone or saline were continued. Post-treatment ABR was obtained on Day 8.
ABR Testing
All recording was conducted under anesthesia in a soundproof chamber. Prior to recording, the animals were examined for evidence of tympanic membrane perforation, middle ear infection, effusion, and/or debris in the external auditory canals. ABRs were measured using a Tucker-Davis Technologies (Alachua, FL) recording system and recorded with transcutaneous sterile, stainless steel electrode needles. The active electrode was placed at the vertex, the reference electrode at the bullae and the ground electrode in the posterior neck. The recorded signal was amplified 100,000X and band-pass filtered between 0.1-5000 Hz. Signal duration recorded for analysis was 10 msec. Stimuli were presented through a Bose (Framingham, MA) speaker 5 cm above the level of the mouse's ears. The sound delivery system was calibrated by placing a 1/2″ Bruel and Kjaer microphone 5 cm from the Bose speaker. Signal averaging was carried out after 500 presentations of either 100 microsecond alternating polarity clicks or 5 msec pure-tone bursts (1 msec rise/fall) of 8, 16 and 32 kHz delivered at a rate of 21/sec. The stimuli were presented at 5 dB steps between 90 and 25 dB SPL. Monaural thresholds were obtained by occluding the contralateral ear with silicone ear molds to isolate the test ear. Averaged ABR waveforms were filtered between 300 and 3000 Hz for determining the threshold.
Anesthesia and Injections
Anesthesia for ABR recording and intratympanic injections was administered using a self-contained, closed circuit isoflurane delivery system. The mouse was first placed into an induction chamber where 4% isoflurane mixed with one liter/minute oxygen flow was administered. After induction of anesthesia, the mouse was transferred to a snout-mask for maintenance anesthesia which varied from 1% to 3% mixed with one liter/minute oxygen flow.
Dexamethasone solution (24 mg/mL) was created by mixing dexamethasone phosphate with 0.9% saline.
Intratympanic injections of dexamethasone or 0.9% saline were carried out under anesthesia using an operating microscope. After the tympanic membrane was visualized using an aural speculum, a sterile 28 gauge needle connected via a polyethylene tube to a 10 microliter Hamilton syringe was passed through the inferior portion of the tympanic membrane. Five to seven microliters (enough to fill the middle ear) of either solution was injected into the middle ear space.
Intraperitoneal cisplatin at a concentration of 1 mg/mL was injected with a 27 gauge needle on a tuberculin syringe as a bolus dose followed by an intraperitoneal bolus of one milliliter of 0.9% saline.
At the conclusion of the final ABR recording session, mice were deeply anesthetized with pentobarbital (45 mg/kg; IP) and transcardially perfused with paraformaldehyde and the heads were stored for future histological evaluation.
Statistical Analysis
Mean ABR thresholds and mean threshold shifts are expressed as mean ± SEM. Comparisons of the mean ABR thresholds using a two tailed Student's t-test were calculated to assess statistical significance. A p value less than or equal to 0.05 was considered significant.
RESULTS
Phase I
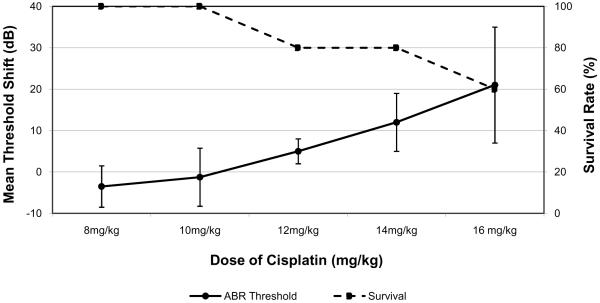
A dose-response curve was created comparing elevation of ABR thresholds and mortality as the dose of cisplatin increased (Figure 1). At a dose of 12 mg/kg there was a mortality rate of 20% but the click threshold shift was only 5±3 dB (p=0.02). At 14 mg/kg, the mean ABR threshold shift was found to be 12±7 dB (p<0.0001) with a mortality rate of 20%. Above this, at a dose of 16 mg/kg, the ABR threshold increased to 21±14 dB (p=0.02) but the mortality increased to 40%. Based on these results 14 mg/kg was selected as the optimal dosage for creating measurable auditory ototoxicity with acceptable mortality.
Figure 1.
Dose-response curve showing mean ABR threshold shift and corresponding survival rate after a single IP adminstration of cisplatin as a function of dosage. Error bars represent one standard error of the mean.
Phase II
Mice treated with only intratympanic dexamethasone or saline without IP cisplatin showed no significant ABR threshold shift. The five ears treated with IT dexamethasone showed a mean threshold shift of 1±2 dB (p=0.4). The five ears treated with IT saline had a mean threshold shift of 3±4 dB (p=0.2). All of the ears in this phase cleared any residual “effusion” between Day 5 (last IT injection) and Day 8 when the post-treatment ABR measurements took place.
Many of the ears had small tympanic membrane perforations at the injection site. Specifically, 3 of the 5 dexamethasone-treated ears and 4 of the 5 saline-treated ears had perforations on Day 8 for the ABR testing (three days after the last IT injection). Calculating the mean threshold shifts for only the ears with perforations, the 3 dexamethasone-treated ears had a mean threshold shift of 1.7 dB (p=0.4) and the 4 saline-treated ears had a mean threshold shift of 3.8 dB (p=0.2).
Phase III
Ten mice treated with 14 mg/kg cisplatin received intratympanic injections of dexamethasone in one ear, and saline in the contralateral ear for five days. Similar to the results from Phase I, there was a 20% mortality rate for this dose of cisplatin. Of the remaining 8 mice, one developed otitis media in the dexamethasone ear and this ear was excluded from analysis. As a result, ABR thresholds from 7 dexamethasone-treated ears were compared to 8 saline-treated ears.
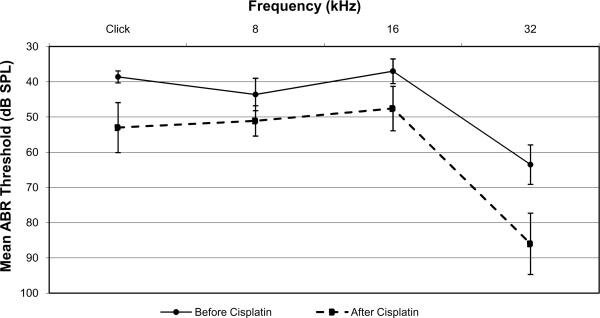
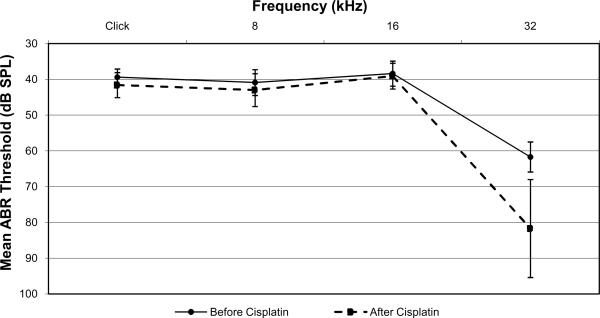
Ears treated with IT saline had an average click-evoked ABR threshold elevation of 14±6 dB, whereas ears treated with IT dexamethasone had a minimal average click-evoked ABR threshold shift of 2±2 dB (p=0.0005). A similar significant difference was measured with pure-tone evoked ABR thresholds at 8 kHz and 16 kHz (Figures 2 and 3). At 8 kHz, saline-treated ears had a mean threshold shift of 8 ± 4 dB while dexamethasone-treated ears had a mean threshold shift of 2 ± 4 dB (p=0.03). At 16 kHz, saline-treated ears had a mean threshold shift of 11 ± 6 dB while dexamethasone-treated ears had a mean threshold shift of 1 ± 2 dB (p=0.002). There was no significant difference between dexamethasone- and saline-treated ears at 32 kHz. Saline-treated ears at 32 kHz had a mean threshold shift of 23 ± 11 dB while dexamethasone-treated ears had a mean threshold shift of 20 ± 17 dB (p=0.8).
Figure 2.
Mean ABR thresholds for saline-treated ears before (solid line) and after (dashed line) cisplatin administration. Error bars represent one standard error of the mean.
Figure 3.
Mean ABR thresholds for dexamethasone-treated ears before (solid line) and after (dashed line) cisplatin administration. Error bars represent one standard error of the mean.
Similar to Phase II, there were several tympanic membrane perforations at the injection site. Specifically, 4 of the 7 dexamethasone-treated ears and all 8 of the saline-treated ears had perforations.
DISCUSSION
Ototoxicity from cisplatin therapy continues to be a common side effect that has been shown to afflict a majority of cancer patients receiving this chemotherapeutic agent. A recent evaluation of intravenous cisplatin ototoxicity in adults showed that 88% of patients had measurable hearing loss with 49% becoming candidates for hearing aids after the therapy24. A significant impact has also been demonstrated in the younger population. Another recent study investigating patients under 23 years of age showed 61% of patients receiving intravenous cisplatin experienced measurable hearing loss with 25% becoming candidates for hearing aids25.
Several studies have explored the possibility of administering an otoprotectant in an effort to reduce the negative impact of cisplatin on hearing. One area of focus has been on administering antioxidant compounds in an attempt to reduce the accumulation of reactive oxygen and nitrogen species before they induce apoptosis in the inner ear. Several thiol antioxidants (e.g., sodium thiosulfate, diethyldithiocarbamate, d- or l-methionine, methylthiobenzoic acid, lipoic acid, N-acetylcysteine, tiopronin, glutathione ester and amifostine) have been evaluated for protective effect against cisplatin ototoxicity. While thiols appear to have otoprotectant properties against cisplatin, some diminish the anti-neoplastic properties of cisplatin, thus are not suitable for clinical application1. In addition, promising experimental findings have not translated into clinical success. A recent phase III clinical trial performed involving sodium thiosulfate showed that 36% of patients treated with cisplatin still qualified for hearing aids26. In another clinical study, amifostine, a broad-spectrum cytoprotectant which reduces cisplatin associated neuro- and nephrotoxicity, did not protect against cisplatin ototoxicity27.
In the current study we investigated the otoprotective effect of dexamethasone administered intratympanically in cisplatin-treated mice. Our results show that intratympanic dexamethasone played a protective role against cisplatin-induced ototoxicity by reducing ABR threshold shifts. This finding extends the results of a recently published report from Turkey, where intratympanic dexamethasone at a concentration at 4 mg/mL protected outer hair cell function, as measured by DPOAE's at frequencies up to 6 kHz, in guinea pigs injected with cisplatin28. Our results evaluate ABR thresholds which estimate auditory function and are believed to reflect functional capacity of cochlear hair cells (both inner and outer), spiral ganglion cells and neurons in the brainstem auditory nuclei. Furthermore, our results provide additional information about ABR thresholds at relatively higher frequencies than the Daldal study. In the guinea pig, 6 kHz represents approximately 50% of the distance from the apex of the cochlea29. For comparison in the mouse, 32 kHz is approximately at 70% of the distance from the apex, 16 kHz approximately 43% of the distance from the apex and 8 kHz approximately 18% of the distance from the apex of the cochlea30. We believe higher frequencies, representing areas of the cochlea closer to the base, are very important to investigate, as they have been shown to be affected by cisplatin ototoxicity first. Our study showed good protection at 8 and 16 kHz but none at 32 kHz. Decreased efficacy of any antioxidant may be expected at the highest frequencies, as the basal turn of the cochlea is the most susceptible to cisplatin ototoxicity. In general, destruction of OHCs due to drug toxicity progresses in a base-to-apex gradient. This gradient may be related to a differential vulnerability of basal and apical OHCs demonstrated in guinea pig organotypic cultures arising from lower levels of antioxidant glutathione in the base and thus intrinsic susceptibility to free radicals31. In our study, the damage in this area may have been too extensive to be prevented by intratympanic steroids. This may reflect some limitations of dexamethasone to protect the highest frequencies, or more likely, this may be a reflection of the relatively large bolus dose of cisplatin given in this study that had a 20% mortality rate. It is possible a more clinically relevant administration of cisplatin (i.e. a more clinical administration with slow transfusion and multiple smaller doses over several days) would result in less extensive damage at the base of the cochlea which in turn could be protected by intratympanic application of dexamethasone.
The current study has significant translational potential since intratympanic steroids are already used in humans as a simple and safe therapy for other inner ear disorders. Intratympanic steroids provide the advantage of potentially fewer systemic side effects while concentrating the drug in the desired location within the inner ear. This is supported by a recent investigation that measured the perilymph levels of methylprednisolone in humans undergoing cochlear implantation after either intratympanic or parenteral injection of the steroid. It was found that intratympanic injection of methylprednisolone produced perilymph concentrations that were 33-fold higher and plasma concentrations that were 136 fold lower than the respective concentrations from parenteral dosing22. This study also supports the translational potential of animal studies investigating potential uses of intratympanic steroids.
Prior to this study, there were no investigations of cisplatin ototoxicity in mice available. A mouse model of cisplatin ototoxicity offers several advantages. For example, since the majority of cancer patients are older than 50, consideration has to be given to how age and age-related hearing loss interact with cisplatin-induced ototoxicity, and for that matter, whether a successful otoprotectant identified in the young animal model is effective in an animal model with pre-existing deficits. Mouse models of hearing loss, aging and age-related hearing loss are well established32. In addition, the mouse genome has been well defined and knock out models are increasingly available that facilitate gene targeting as a future strategy in ameliorating ototoxicity.
In the published cisplatin-induced ototoxicity literature two different dosing models have been utilized: a single large dose28,33 vs. multiple small doses 34,35. We chose the single, large dose model and study design, comparable to that of Drottar et al.33, because it is more practical to implement and the resulting data are easier to interpret. It is, however, recognized that in the clinical realm standard treatment protocols utilize a multiple dosing regimen. Dexamethasone as a successful otoprotectant can be examined in the multiple dosing regimens in future investigations.
The present study evaluated ototoxicity up to 8 days after cisplatin administration. Whether this amount of time is sufficient for full expression of cisplatin-induced ototoxicity in the mouse remains to be determined. However, other single-dose studies in the rat and guinea pig suggest that hearing loss has stabilized by 5-7 days after cisplatin injection36,37. The only other study of dexamethasone effect on cisplatin ototoxicity measured DPOAEs only three days after cisplatin administration28.
The limitations of this study include the small number of animal subjects, no inner ear sampling of dexamethasone concentration and no histologic correlation. Further studies will include histologic evaluation, lower concentrations of dexamethasone for intratympanic injection, and variations in dosing of cisplatin to more similarly match what is given clinically.
CONCLUSION
In this study we describe a murine model for cisplatin-induced ototoxicity. Using this model it is demonstrated that intratympanic administration of dexamethasone has a protective effect on hearing thresholds. This effect was frequency related, such that little protection was noted at the highest stimulus frequency (32 kHz) tested.
Acknowledgements
We would like to thank Dr. Shigeyuki Kuwada for his assistance throughout the conduct of this investigation. This study is supported by the New England Otolaryngologic Society Resident Grant, the Division of Otolaryngology at UCHC and NIH grant R01DC000127 to DKM.
LITERATURE CITED
- 1.Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–21. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 2.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biro K, Noszek L, Prekopp P, et al. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology. 2006;70:177–184. doi: 10.1159/000093776. [DOI] [PubMed] [Google Scholar]
- 4.Laurell G. High-dose cisplatin treatment: hearing loss and plasma concentrations. Laryngoscope. 1990;100:724–734. doi: 10.1288/00005537-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Blakley BW, Gupta AK, Myers SF, Schwan S. Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg. 1994;120:541–546. doi: 10.1001/archotol.1994.01880290051009. [DOI] [PubMed] [Google Scholar]
- 6.Komune S, Asakuma S, Snow JBJ. Pathophysiology of the ototoxicity of cis-diamminedichloroplatinum, Otolaryngol. Head Neck Surg. 1981;89:275–282. doi: 10.1177/019459988108900226. [DOI] [PubMed] [Google Scholar]
- 7.Nakai Y, Konishi K, Chang KC, et al. Ototoxicity of the anticancer drug cisplatin. An experimental study. Acta Otolaryngol. (Stockh.) 1982;93:227–232. doi: 10.3109/00016488209130876. [DOI] [PubMed] [Google Scholar]
- 8.Van Ruijven MWM, de Groot JCMJ, Klis SFL, Smoorenburg G. Cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205:241–248. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Clerici WJ, DiMartino DL, Prasad MR. Direct effect of reactive oxygen species on cochlear outer hair cells. Hear Res. 1995;84:30–40. doi: 10.1016/0378-5955(95)00010-2. [DOI] [PubMed] [Google Scholar]
- 10.Dehne N, Lautermann J, Petrat, et al. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2001;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- 11.Somani SM, Husain K, Jagannathan R, Rybak LP. Amelioration of cisplatin-induced oto- and nephrotoxicity by protective agents. Ann Neurosci. 2001;8:101–113. [Google Scholar]
- 12.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–67. doi: 10.1016/j.heares.2006.09.015. (2007) [DOI] [PubMed] [Google Scholar]
- 13.Doyle KJ, Bauch C, Battista R, et al. Intratympanic steroid treatment: a review. Otol Neurotol. 2004;25:1034–9. doi: 10.1097/00129492-200411000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Kolls J, Xie J, LeBlanc R, Malinski T, et al. Rapid induction of messenger RNA for nitric oxide synthase II in rat neutrophils in vivo by endotoxin and its suppression by prednisolone. Proc Soc Exp Biol Med. 1994;205:220–229. doi: 10.3181/00379727-205-43700. [DOI] [PubMed] [Google Scholar]
- 15.Nagura M, Iwasaki S, Wu R, Mizuta K, et al. Effects of corticosteroid, contrast medium and ATP on focal microcirculatory disorders of the cochlea. Eur J Pharmacol. 1999;366:47–53. doi: 10.1016/s0014-2999(98)00881-4. [DOI] [PubMed] [Google Scholar]
- 16.Himeno C, Komeda M, Izumikawa M, et al. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;67:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- 17.Park SK, Choi D, Russell P, et al. Protective effect of corticosteroid against the cytotoxicity of aminoglycoside otic drops on isolated cochlear outer hair cells. Laryngoscope. 2004;114:768–771. doi: 10.1097/00005537-200404000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Hargunani CA, Kempton JB, DeGagne JM, Trune DR. Intratympanic injection of dexamethasone: time course of inner ear distribution and conversion to its active form. Otol Neurotol. 2006;27:564–9. doi: 10.1097/01.mao.0000194814.07674.4f. (2006) [DOI] [PubMed] [Google Scholar]
- 19.Herr I, Ucur E, Herzer K, Okouoyo S, et al. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003;63:3112–20. [PubMed] [Google Scholar]
- 20.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekhar SS, Rubinstein RY, Kwartler JA, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- 22.Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic Versus Intravenous Delivery of Methylprednisolone to Cochlear Perilymph. Otol Neurotol. 2007;28(8):1124–1130. doi: 10.1097/MAO.0b013e31815aee21. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar SS. Intratympanic dexamethasone for sudden sensorineural hearing loss: clinical and laboratory evaluation. Otol Neurotol. 2001;22(1):18–23. doi: 10.1097/00129492-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Zuur CL, Simis YJ, Lansdaal PE, Hart AA, Schornagel JH, Dreschler WA, Rasch CR, Balm AJ. Ototoxicity in a randomized phase III trial of intraarterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2007;20;25(24):3759–65. doi: 10.1200/JCO.2006.08.9540. [DOI] [PubMed] [Google Scholar]
- 25.Gilmer Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23(34):8588–96. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 26.Zuur CL, Simis YJ, Lansdaal PE, Hart AA, et al. Risk factors of ototoxicity after cisplatin- based chemo-irradiation in patients with locally advanced head-and-neck cancer: a multivariate analysis. Int J Radiat Oncol Biol Phys. 2007;68:1320–1325. doi: 10.1016/j.ijrobp.2007.01.042. 2007. [DOI] [PubMed] [Google Scholar]
- 27.Marina N, Chang KW, Malogolowkin M, London WB, et al. Children's Oncology Group Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children's Oncology Group study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- 28.Daldal A, Odabasi O, Serbetcioglu B. The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg. 2007;137:747–752. doi: 10.1016/j.otohns.2007.05.068. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202(12):63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Sha S-H, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 32.Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. CRC Press; London: 2001. [Google Scholar]
- 33.Drottar M, Liberman MC, Ratan RR, Roberson DW. The histone deacetylase inhibitor sodium butyrate protects against cisplatin-induced hearing loss in guinea pigs. Laryngoscope. 2006;116:292–296. doi: 10.1097/01.mlg.0000197630.85208.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyppolito MA, de Oliveira JA, Rossato M. Cisplatin ototoxicity and otoprotection with sodium salicylate. European Archives of Oto-Rhino-Laryngology. 2006;263:798–803. doi: 10.1007/s00405-006-0070-6. [DOI] [PubMed] [Google Scholar]
- 35.Chan DK, Lieberman DM, Musatov S, et al. Protection against cisplatin-induced ototoxicity by adeno-associated virus-mediated delivery of the X-linked inhibitor of apoptosis protein is not dependent on caspase inhibition. Otology & Neurotology. 2007;28:417–425. doi: 10.1097/01.mao.0000247826.28893.7a. [DOI] [PubMed] [Google Scholar]
- 36.Kalcioglu MT, Kizilay A, Gulec M, et al. The protective effect of erdosteine against ototoxicity induced by cisplatin in rats. European Archives of Oto-Rhino-Laryngology. 2005;262:856–863. doi: 10.1007/s00405-004-0909-7. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Camacho R, Citores MJ, Trinidad A, et al. HSP-70 as a nonspecific early marker in cisplatin ototoxicity. Acta Oto-Laryngologica. 2007;127:564–567. doi: 10.1080/00016480600951483. [DOI] [PubMed] [Google Scholar]