Abstract
Background
Impedance cardiography (ICG) is a noninvasive modality that utilizes changes in impedance across the thorax to assess hemodynamic parameters, including cardiac output (CO). The utility of ICG in patients hospitalized with heart failure (HF) is uncertain.
Methods
The BioImpedance CardioGraphy in Advanced Heart Failure (BIG) study was a prospective substudy of ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness). A total of 170 subjects underwent blinded ICG measurements using BioZ (CardioDynamics); of these, 82 underwent right heart catheterization. We compared ICG with invasively measured hemodynamics by simple correlation, and compared overall ICG hemodynamic profiles (“wet” [thoracic fluid content [TFC] ≥47 in men, ≥37 in women] and “cold” [cardiac index [CI] ≤2.2 L/min/m2]) versus those determined by invasive measurements (“wet” [pulmonary capillary wedge pressure [PCWP] ≥22 mm Hg] and “cold” [CI ≤2.2 L/min/m2]). We also determined whether ICG measurements were associated with subsequent death or hospitalization within 6 months.
Results
There was modest correlation between ICG and invasively measured CO (r=0.4 to 0.6 on serial measurement). TFC measured by ICG was not a reliable measure of PCWP. There was poor agreement between ICG and invasively measured hemodynamic profiles (kappa ≤0.1). No ICG variable alone or in combination was associated with outcome.
Conclusions
In hospitalized patients with advanced HF, ICG provides some information about CO but not left-sided filling pressures. ICG did not have prognostic utility in this patient population.
Keywords: impedance cardiography, hemodynamics, heart failure, pulmonary artery catheterization
Impedance cardiography (ICG) is a noninvasive modality that has provided reasonably accurate measurements of cardiac output (CO) in patients with heart failure (HF)1,2 in carefully monitored settings. However, it remains entirely unclear how ICG should be incorporated into HF management. A risk score comprised of a composite of ICG parameters has recently been shown to be associated with an increased risk of rehospitalization among recently discharged ambulatory patients with systolic HF, though that observation awaits confirmation in a validation cohort.3
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial,4 a randomized comparison assessing the utility of therapy guided by right heart catheterization, afforded an opportunity to further assess the utility of ICG in a well characterized cohort of patients with advanced HF. As such, the BioImpedance CardioGraphy in Advanced Heart Failure (BIG) substudy was conducted within the ESCAPE trial, and was designed to determine the utility of bioimpedance cardiography as an adjunct tool for HF monitoring in hospitalized patients with advanced HF.
METHODS
ESCAPE study
The ESCAPE study, sponsored by the National Heart, Lung, and Blood Institute, was a randomized trial conducted at 26 experienced HF centers in the United States and Canada testing the safety and efficacy of pulmonary artery catheters (PAC) in patients with advanced HF. Patients were randomly assigned in a 1:1 fashion to receive either PAC-guided therapy (PAC arm) or clinical assessment (CLIN arm). Inclusion criteria were designed to select patients with severe symptomatic HF despite recommended therapies, and were met by (1) hospitalization for HF within the past year; (2) urgent visit to the emergency department; or (3) treatment during the preceding month with more than 160 mg of furosemide daily (or equivalent). Randomization required at least 3 months of symptoms despite angiotensin-converting enzyme inhibitors and diuretics, left ventricular (LV) ejection fraction ≤30%, systolic blood pressure ≤125 mm Hg, and at least 1 sign and 1 symptom of congestion. Exclusion criteria included creatinine level >3.5 mg/dL (309.4 µmol/L), prior use of dobutamine or dopamine >3 µg/kg/min, or any prior use of milrinone during the current hospitalization. Further details of the ESCAPE trial have been published.4,5
Impedance cardiography and derived measurements
ICG applies Ohm’s relationship to the thorax to allow changes in voltage and impedance to be translated into hemodynamic parameters of cardiac function. Because blood is a strong conductor of current when compared with the surrounding thoracic tissues, variations in blood flow through the great vessels results in a measurable change in impedance that allows calculation of effective stroke volume. This technique is described in greater detail elsewhere.6
BioZ (CardioDynamics, San Diego, CA) is a proprietary bioimpedance cardiography device that uses unique computation algorithms to filter the raw impedance data and compute hemodynamic parameters. The ICG variables assessed in the present study include: CO, cardiac index (CI), stroke volume, velocity index (maximum rate of impedance change, related to changes in aortic blood velocity), acceleration index (maximum rate of change of blood velocity, related to changes in aortic blood acceleration), thoracic fluid content (TFC; inverse of impedance measurement, representative of total fluid volume in the chest), TFC index, pre-ejection period (measured interval from the onset of ventricular depolarization [Q-wave in an electrocardiogram] to the beginning of mechanical contraction [first upslope of the impedance waveform B point]), LV ejection time (time from aortic valve opening [B point on the impedance waveform] to aortic valve closing [X point on the impedance waveform]), and systolic time ratio (inversely proportional to LV function; pre-ejection period divided by LV ejection time). Detailed descriptions of these ICG parameters and their calculations have been published elsewhere.3,7
BIG substudy
The 3 prespecified objectives of the BIG substudy were (1) to evaluate the relationship between hemodynamic data derived from bioimpedance cardiography with hemodynamic data obtained from invasive hemodynamic monitoring; (2) to compare the clinical profiles generated from the ICG parameters with those from the PAC parameters; and (3) to evaluate how accurately hemodynamic parameters obtained from bioimpedance cardiography predict clinical outcomes. The study was prospectively designed to include 300 patients within ESCAPE, with equal numbers of patients from the PAC and CLIN arms. All patients eligible for the parent trial were eligible for this substudy, with 3 notable exceptions (1) patients with minute ventilation pacemakers (which also use impedance technology and could cause pacing and/or monitoring interference); (2) patients with a known allergy or sensitivity to the sensor gel or adhesive; and (3) patients with skin lesions at sensor placement sites. All subjects provided written informed consent (in addition to that provided for the parent trial) prior to study entry.
Right Heart Catheterization and ICG Measurements
Centers participating in the ESCAPE trial were selected based on expertise in invasive monitoring and clinical management of patients with HF. Training was provided in methods for collection and interpretation of hemodynamic data. The baseline value for each hemodynamic parameter represented an average of 3 readings: 1 at PAC insertion and 2 subsequent baseline readings. The mean of the A wave was used as the PCWP if at all possible. If the A wave was not recorded, then the overall mean of all waves was used to minimize missing data. The A wave was available in 94% of subjects, recorded at either 3 (n=130), 2 (n=21), or 1 (n=11) of the PAC measurements. CO was calculated as an average of 3 thermodilution readings.8
Study personnel in the BIG substudy were already experienced or were trained by the sponsor to perform ICG properly. The ability to acquire accurate information was evaluated under supervision, and once the sponsor determined that personnel were properly trained to use the ICG device, study enrollment was allowed to proceed. Investigators and study coordinators were blinded to the data obtained from the ICG device. To accomplish a complete blind, study personnel were not able to view real-time data acquired with the BioZ. For this reason, 3 independent ICG readings were made at each time point to ensure that an interpretable tracing was obtained. The raw ICG data were interpreted at a central location by the sponsor, who was blinded to all clinical and hemodynamic data. At no time was the sponsor able to access hemodynamic data or clinical outcome data.
Data analysis
For the first objective, the following PAC-derived variables were assessed: right atrial pressure (RAP), pulmonary artery (PA) systolic pressure, PA diastolic pressure, PA mean pressure, mean pulmonary capillary wedge pressure (PCWP mean), transpulmonary gradient, PCWP A wave, PCWP V wave, systemic vascular resistance, mixed venous oxygen saturation, CO, and CI. The specific hypotheses were as follows, with the PAC variable followed by the hypothesized correlating ICG variable: CO/CO, mixed venous oxygen saturation/CO, CI/CI, mixed venous oxygen saturation/CI, PCWP mean/TFC, RAP/TFC, mixed venous oxygen saturation/TFC, transpulmonary gradient/TFC, stroke volume/stroke volume, and systemic vascular resistance/systemic vascular resistance. These relationships were evaluated at baseline, discharge, and difference in baseline and discharge. In cases where the first baseline reading was not available, the second baseline reading was used. In cases where the discharge reading was not available, the last reading was used. In general, the PAC parameter values represent an average of 3 readings.
For the second objective, the following cutoff values were prospectively identified to define the PAC-generated clinical profiles: dry/warm (PCWP <22 mm Hg, CI ≥2.2 L/min/m2); wet/warm (PCWP ≥22 mm Hg, CI ≥2.2 L/min/m2); wet/cold (PCWP ≥22 mm Hg, CI <2.2 L/min/m2); dry/cold (PCWP <22 mm Hg, CI <2.2 L/min/m2). The following cutoff values were prospectively identified to define the ICG-generated clinical profiles: dry/warm (TFC <47 mm Hg, CI ≥2.2 L/min/m2); wet/warm (TFC ≥47 mm Hg, CI ≥2.2 L/min/m2); wet/cold (TFC ≥47 mm Hg, CI <2.2 L/min/m2); dry/cold (TFC <47 mm Hg, CI <2.2 L/min/m2). For female subjects, the TFC cutoff was 37 mm Hg. These ICG cutoff values represent empiric thresholds determined based on significant prior clinical experience of one of the investigators (CWY) and corroborated by internal data from the sponsor. The 2 different categorizations (by PAC and ICG parameters) were compared using a Kappa statistic. Sensitivity, specificity, and positive/negative predictive values were calculated for the ability of ICG parameters to predict normal/abnormal PAC parameters. The following ICG parameters and corresponding PAC parameters were assessed as follows: ICG CI, normal ≥2.2 L/min/m2, abnormal ς.2 L/min/m2 as a predictor of PAC CI, abnormal <2.2 L/min/m2; ICG TFC, abnormal ≥47 mm Hg in men and ≥37 mm Hg in women as a predictor of PAC PCWP, abnormal ≥22 mm Hg; ICG SVR, abnormal >1600 dynes/sec*cm−5 as a predictor of PAC SVR, abnormal >1600 dynes/sec*cm−5.
For the third objective, the primary outcome variable was identical to the main trial—days alive outside of the hospital during the 6 months following randomization. This was analyzed using the Cox proportional hazards model. A secondary outcome variable, time to death, was also analyzed. Endpoints were calculated with patients receiving transplant or assist devices coded as dead, then recalculated coded as alive. The following ICG variables were prospectively selected and analyzed: CO, LV cardiac work index, LV ejection time, pre-ejection period, systolic time ratio, stroke volume, systemic vascular resistance, TFC, velocity index, acceleration index, and TFC index. The utility of an ICG risk score derived from a published study of ambulatory HF patients3 was also assessed. Models were calculated for the parameters at baseline, at discharge, and for changes in parameter values between baseline and discharge. For the latter, only endpoints measured after the last measurement were included. The models were compared using Akaike’s information criteria. Bootstrap methods were also used to determine the sensitivity of various parameters to the particular data set.
All data collection and statistical analyses were performed at the Duke Clinical Research Institute (Durham, NC). SAS software version 8.2 (SAS Institute Inc, Cary, NC) was used to perform all statistical calculations. The substudy was sponsored by CardioDynamics, manufacturer of the BioZ impedance cardiography system.
RESULTS
Baseline characteristics and clinical outcomes
A total of 170 subjects (roughly 40% of the total number of ESCAPE trial participants) at 17 centers in the United States were enrolled with nearly equal numbers from the PAC and CLIN arms of the parent trial (Table 1). Subjects were middle-aged, predominantly male, with New York Heart Association class IV symptoms, and severely reduced LV ejection fraction. Nearly equal numbers of whites and non-whites were enrolled. These characteristics were nearly identical to those of the parent trial.4 As in ESCAPE, subjects had a high rate of adverse outcomes at 6 months as shown.
Table 1.
Baseline Characteristics and Outcomes
| PAC Treatment | CLIN Treatment | Total | |
|---|---|---|---|
| (n=82) | (n=88) | (n=170) | |
| Characteristics | |||
| Age (yrs) | 54±13 | 56±14 | 55±14 |
| Male sex (%) | 67.1 | 75 | 71.2 |
| White race (%) | 51.2 | 52.3 | 51.8 |
| NYHA class IV (%) | 82.9 | 83.9 | 83.4 |
| Ischemic etiology of HF (%) | 43.9 | 41.4 | 42.6 |
| Systolic BP (mm Hg) | 107±17 | 107±16 | 107±16 |
| Diastolic BP (mm Hg) | 69±13 | 67±12 | 68±12 |
| Pulse (bpm) | 84±15 | 81±16 | 83±16 |
| Serum sodium (mmol/L) | 137±4 | 137±4 | 137±4 |
| Serum creatinine (mg/dL) | 1.4±0.5 | 1.4±0.5 | 1.4±0.5 |
| LVEF (%) | 20±7 | 21±6 | 20±7 |
| Time from admit to randomization (d) | 1.1±1.4 | 1.4±1.8 | 1.3±1.6 |
| Outcomes | |||
| Time from randomization to discharge (d) | 6.4±4.4 | 6.5±5.2 | 6.5±4.8 |
| Length of initial hospitalization (d) | 7.6±4.5 | 8.0±5.4 | 7.8±5.0 |
| Number dead at 180 days, No. (%) | 12 (15) | 12 (14) | 24 (14) |
| Number rehospitalized for HF at 180 days, No. (%) | 40 (49) | 52 (59) | 92 (54) |
| Number rehospitalized more than once, No. (%) | 23 (28) | 24 (27) | 47 (28) |
| Days dead or hospitalized at 180 days | 27.8±43.2 | 28.3±40.2 | 28.0±41.5 |
Abbreviations: BP=blood pressure; bpm=beats per minute; CLIN=clinical assessment; HF=heart failure; LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; PAC=pulmonary artery catheter.
Correlation of ICG and PAC parameters
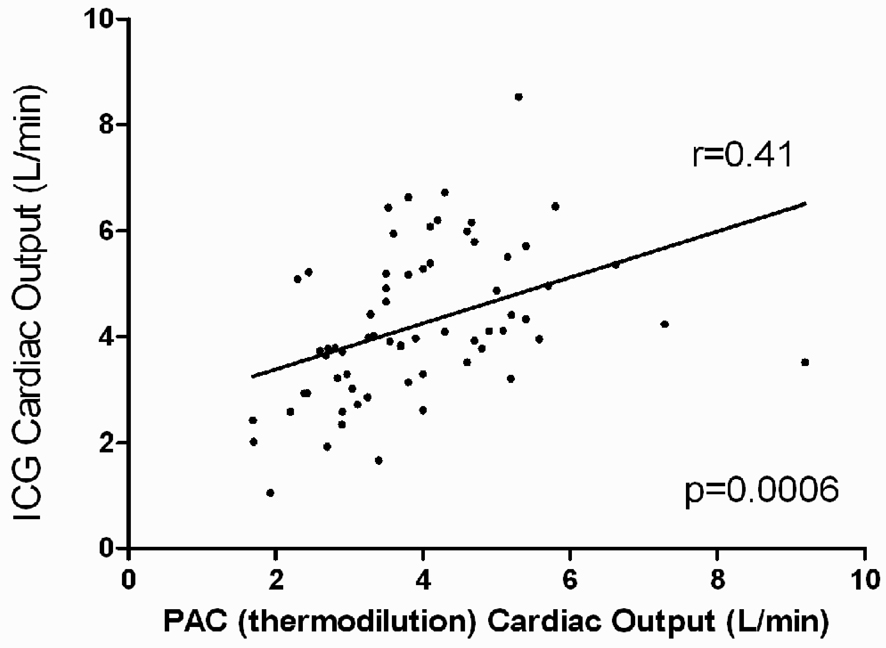
The correlation coefficients for ICG CO with invasive CO ranged from 0.4 to 0.6 (Figure 1, Table 2). The correlation between these 2 modalities for change in CO from baseline to discharge was less robust. ICG CO/CI had weak correlation with mixed venous oxygen saturation. The correlation coefficients of TFC with RAP, PCWP, and transpulmonary gradient were less robust. The presence or absence of severe mitral regurgitation had no effect on these correlations (P=0.75 for comparison). To examine whether increased experience with ICG improved these correlations, the analysis was repeated in only those centers with high study participant volume (determined as ≥11 participants; 8 total centers, n=49 subjects randomized to PAC arm of trial). The relationships were qualitatively similar with no major differences from the analysis of the entire patient cohort.
Figure 1.
Correlation of PAC-derived and ICG-derived cardiac output. Scatter plot of baseline cardiac output by the 2 methods is depicted (N=66). Abbreviations: ICG=impedance cardiography, PAC=pulmonary artery catheter.
Table 2.
Correlation of BioZ and PAC parameters.
| BioZ Parameter | PAC Parameter | Correlation Coefficient | |||
|---|---|---|---|---|---|
| Randomization | Next day | Final Hemo | Δ* | ||
| CO | CO | 0.41 | 0.55 | 0.62 | 0.33 |
| CO | MVOS | 0.06 | 0.03 | 0.25 | N/A† |
| CI | CI | 0.32 | 0.35 | 0.38 | 0.27 |
| CI | MVOS | 0.31 | 0.15 | 0.24 | N/A† |
| TFC | PCWP mean | 0.18 | 0.52 | −0.01 | 0.32 |
| TFC | RAP | 0.40 | 0.38 | −0.08 | 0.26 |
| TFC | TPG | −0.13 | −0.29 | 0.27 | 0.19 |
| SV | CO/HR | 0.46 | 0.61 | 0.66 | 0.44 |
| SVR | SVR | 0.66 | 0.48 | 0.43 | 0.46 |
Change from randomization to final hemodynamic measurement.
N/A due to missing mixed venous oxygen saturation observations.
Abbreviations: CO=cardiac output; CI=cardiac index; MVOS=mixed venous oxygen saturation; TFC=thoracic fluid content; PCWP=pulmonary capillary wedge pressure; SV=stroke volume; TPG=transpulmonary gradient; HR=heart rate; SVR=systemic vascular resistance.
The number of subjects who contributed observations for the various parameters ranges from 58 to 67.
Decision Statistics
Sensitivity, Specificity, positive predictive value, and negative predictive value for the ability of various ICG parameters to predict corresponding PAC parameters are displayed in Table 3. In general, values were low at baseline and at discharge.
Table 3.
Decision statistics for various ICG parameters. Corresponding PAC parameter is displayed in parentheses.
| Baseline | ||||
|---|---|---|---|---|
| Variable | Sensitivity | Specificity | PPV | NPV |
| (%) | (%) | (%) | (%) | |
| CI (CI) | 53.5 | 60.9 | 71.9 | 41.2 |
| TFC (PCWP) | 36.4 | 80.0 | 76.2 | 41.7 |
| SVR (SVR) | 50.0 | 56.8 | 34.5 | 71.4 |
| Discharge | ||||
| Variable | Sensitivity | Specificity | PPV | NPV |
| (%) | (%) | (%) | (%) | |
| CI (CI) | 35.0 | 73.1 | 50.0 | 59.4 |
| TFC (PCWP) | 30.8 | 80.0 | 44.4 | 69.0 |
| SVR (SVR) | 66.7 | 83.3 | 40.0 | 93.8 |
Abbreviations: CI=cardiac index; TFC=thoracic fluid content; PCWP=pulmonary capillary wedge pressure; SV=stroke volume; SVR=systemic vascular resistance.
The number of subjects who contributed observations for the various parameters ranges from 38 to 46.
Correlation of ICG-derived and PAC-derived clinical profiles
The agreement between ICG-derived and PAC-derived hemodynamic profiles was poor (Table 4), both at baseline (kappa=0.1) and discharge (kappa=0.06). In an attempt to define characteristics associated with a discordant assessment of cardiac index by impedance cardiography versus PAC, we compared baseline characteristics among those with a “warm” profile by impedance cardiography and a “cold” profile by PAC (n=18) to those with a “warm” profile by both impedance cardiography and PAC (n=14). There were no significant differences in age, gender, ethnicity, etiology of cardiomyopathy, heart rate, systolic blood pressure, serum sodium or creatinine, although left ventricular ejection fraction was slightly higher in the former (20%) versus latter group (17%, p=0.03).”
Table 4.
Number of subjects classified into BioZ and PAC-derived clinical profiles.
| Baseline | BioZ-based Clinical Profile* | ||||
|---|---|---|---|---|---|
| Warm/Dry | Warm/Wet | Cold/Dry | Cold/Wet | ||
| PAC-based Clinical Profile† | Warm/Dry | 7 | 1 | 0 | 3 |
| Warm/Wet | 3 | 3 | 3 | 3 | |
| Cold/Dry | 4 | 2 | 1 | 4 | |
| Cold/Wet | 7 | 5 | 3 | 12 | |
| Discharge | BioZ-based Clinical Profile | ||||
| Warm/Dry | Warm/Wet | Cold/Dry | Cold/Wet | ||
| PAC-based Clinical Profile | Warm/Dry | 11 | 2 | 1 | 4 |
| Warm/Wet | 1 | 2 | 1 | 1 | |
| Cold/Dry | 7 | 1 | 0 | 4 | |
| Cold/Wet | 3 | 0 | 0 | 3 | |
PAC-based clinical profiles: warm/dry: PCWP <22 mm Hg, CI ≥2.2 L/min/m2; warm/wet: PCWP ≥22 mm Hg, CI ≥2.2 L/min/m2; cold/wet: PCWP ≥22 mm Hg, CI <2.2 L/min/m2; cold/dry: PCWP <22 mm Hg, CI <2.2 L/min/m2
BioZ-based clinical profiles: warm/dry TFC <47 mm Hg, CI ≥2.2 L/min/m2; warm/wet TFC ≥47 mm Hg, CI ≥2.2 L/min/m2; cold/wet TFC ≥47 mm Hg, CI <2.2 L/min/m2; cold/dry TFC <47 mm Hg, CI <2.2 L/min/m2. For female subjects, the TFC cutoff was 37 mm Hg.
Abbreviations: PAC=pulmonary artery catheter.
Correlation between ICG and PAC variables and outcomes
None of the prospectively identified individual ICG variables predicted outcomes (baseline, discharge, or change from baseline to discharge). The results for baseline and discharge variables are displayed in Table 5. Additionally, the ICG score3 (at baseline, discharge, or change from baseline to discharge) failed to predict 6-month outcomes (chi-square for time to death/rehospitalization at baseline, discharge, and change from baseline to discharge=0.59, 0.51, and 0.19, respectively; P=NS for all). The above ICG variables and the ICG score also failed to predict death or rehospitalization at 6 months (logistic regression, P=NS for all comparisons, not shown).
Table 5.
Relationship of BioZ parameters with clinical outcomes.
| Hazard Ratio (95% CL) | |||
|---|---|---|---|
| Baseline BioZ variable | ESCAPE Primary Endpoint | Time to Death | Time to Death/Rehospitalization |
| CO | 0.95 (0.86,1.07) | 0.76 (0.56, 1.03) | 0.94 (0.82, 1.09) |
| LCWI | 1.01 (0.97, 1.06) | 0.54 (0.30, 0.98) | 1.04 (1.00, 1.09) |
| LVET | 1.00 (0.63, 1.58) | 2.71 (0.86, 8.56) | 1.04 (0.58, 1.84) |
| STR | 0.86 (0.36, 2.08) | 0.33 (0.32, 3.32) | 1.18 (0.38, 3.65) |
| SV | 0.99 (0.99, 1.00) | 0.99 (0.97, 1.01) | 1.00 (0.99, 1.01) |
| SVR | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| TFC | 1.01 (0.99, 1.02) | 1.01 (0.97, 1.04) | 1.00 (0.98, 1.02) |
| VI | 0.99 (0.98, 1.00) | 0.99 (0.97, 1.02) | 0.98 (0.97, 1.00) |
| AI | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.02) | 0.99 (0.98, 1.00) |
| TFCI | 1.01 (0.99, 1.03) | 1.02 (0.97, 1.07) | 0.99 (0.96, 1.02) |
| ICG score | 1.04 (0.97, 1.11) | 1.02 (0.85, 1.22) | 1.03 (0.95, 1.13) |
| Discharge BioZ Variable | Time to Death | Time to Death/Rehospitalization | |
| CO | 0.73 (0.51, 1.06) | 0.97 (0.85, 1.11) | |
| LCWI | 0.48 (0.21, 1.07) | 0.98 (0.92, 1.04) | |
| STR | 2.2 (0.21, 23.49) | 2.5 (0.83, 7.69) | |
| SV | 0.98 (0.96, 1.01) | 1.00 (0.99, 1.01) | |
| SVR | 1.02 (1.00, 1.03) | 1.00 (0.99, 1.02) | |
| TFC | 1.02 (0.96, 1.08) | 1.00 (0.97, 1.03) | |
| VI | 0.99 (0.96, 1.03) | 0.99 (0.97, 1.01) | |
| AI | 1.01 (0.99, 1.02) | 1.00 (0.99, 1.01) | |
| TFCI | 1.05 (0.96, 1.16) | 0.99 (0.95,1.03) | |
| ICG score | 1.07 (0.84, 1.37) | 1.04 (0.93, 1.16) | |
Abbreviations: AC=acceleration index; CO=cardiac output; ICG=impedance cardiography; LCWI=left cardiac work index; LVET=left ventricular ejection time; STR=systolic time ratio; SV=stroke volume; SVR=systemic vascular resistance; TFC=thoracic fluid content; TFCI=thoracic fluid content index; VI=velocity index.
The number of subjects who contributed observations for the various parameters is 157 at baseline and 123 at discharge.
Among the subjects in BIG who had a PAC performed, the final PCWP measured (n=69) was associated with death (HR 1.13 (1.05, 1.22, p=0.002) at 6 months post-randomization. Similarly, there was a trend for an association between the final PCWP with the composite endpoint of death and rehospitalization (HR 1.04, (0.99, 1.09, p=0.09). In contrast, the invasively measured cardiac index was not associated with prognosis (p>0.5 for both).
DISCUSSION
The present study is the largest and most comprehensive assessment to date of ICG (specifically the BioZ device) in patients hospitalized with advanced decompensated HF. To date, it is the only randomized and blinded assessment of ICG in this setting. There are 2 major findings of this study. First, there was modest correlation of ICG with CO, but poor correlation with measures of ventricular filling pressures. Second, ICG variables, either alone or in combination,3 did not correlate with 6-month outcomes.
A recently published evaluation of 212 patients with New York Heart Association class II–III HF status after recent hospitalization with mean LV ejection fraction <30% who underwent serial and blinded assessment with ICG every 2 weeks found several ICG parameters that were independently associated with a HF event (within 14 days) within the 26-week study period.3 TFC index was one of these parameters. A risk score, consisting of a composite of TFC index, LV ejection time, and velocity index (all univariate predictors of a future HF event) was derived post-hoc and found to remain significantly associated with a future HF event in multivariable analysis including New York Heart Association class, systolic blood pressure, and patient visual analog score, but prospective validation in the outpatient population is still under investigation. In BIG, neither the ICG risk score nor any individual ICG variable were associated with adverse outcomes at 6 months. There are several explanations for these discordant findings. First, ESCAPE was a hospitalized, decompensated, class IV population, while the derivation cohort was ambulatory and class II–III. There may be differences in impedance characteristics between ambulatory and decompensated patients. An alternative explanation is that the ICG score, which was derived post-hoc, described the population studied in an ambulatory outpatient heart failure trial but would not be generalizable to other heart failure populations when studied prospectively.
Prior studies with ICG have demonstrated good correlation with invasively measured CO. In patients without HF (post-CABG,9,10 intensive care unit patients receiving mechanical ventilation,11 pulmonary hypertension12), correlation between ICG-derived CO and invasively measured CO (thermodilution technique) was good, with r values ranging from 0.81 to 0.93. In a series of 50 consecutive patients with advanced HF referred for elective PAC,2 we demonstrated a good correlation between ICG and thermodilution CO (r=0.76). In another study of 29 consecutive patients with severe LV systolic dysfunction admitted to the intensive care unit with a primary diagnosis of HF and in whom a PAC was already placed, excellent correlation was found between ICG-derived CO and thermodilution CO (r=0.89).1 In the present multicenter study, it is possible that the less favorable correlation is, in part, secondary to a reduced accuracy of PAC-derived CO in this patient cohort due to their advanced HF with associated tricuspid regurgitation and/or low CO. It is also possible that as-of-yet unrecognized factors contributed to these disparate findings.
The utility of ICG, a noninvasive strategy, as an acceptable alternative to invasively derived ventricular filling pressures has been a hope that has not been realized. In a study of 63 patients with compensated HF, the combination of a high TFC and low stroke volume index was associated with a higher LV end-diastolic pressure when compared with the combination of a low TFC and high stroke volume index.13 However, that study included subjects both with reduced and preserved ejection fraction. We previously demonstrated in patients with systolic HF that TFC was poorly correlated with invasively measured RAP and PCWP (r=0.08 and r=0.05, respectively).2 That finding has now been corroborated in the BIG substudy. In the majority (75 to 80%) of patients with advanced heart failure, right-sided and left-sided filling pressures correlate with one another14; however, in the minority of cases where they do not, it would be particularly useful to have an independent measure of left-sided filling pressures. Unfortunately, ICG does not appear to fill this need, and we would advocate that TFC should not be used as a surrogate for left or right ventricular filling pressures. It remains plausible that TFC describes a separate measure of thoracic fluid that is not well represented by RAP or PCWP.
The ESCAPE trial4 showed no benefit to the routine use of the PAC to guide clinical decision making in advanced HF patients admitted with recurrent decompensation. However, there are several instances when the hemodynamic data obtained via the insertion of a PAC may prove useful.15 It can be inferred from the poor correlation between the PAC-derived and ICG-derived clinical profiles and the lack of correlation between PAC-derived and ICG-derived hemodynamic data, in general, that ICG is a poor surrogate for PAC-derived data in these patients and should not be used as an alternative. A prior study suggested that ICG could be used as an alternative to the PAC to guide medical decision making,16 but this unblinded and non-randomized study was preliminary in nature, involving only 14 patients who needed a PAC. To our knowledge, there are no other studies that have demonstrated a benefit to using ICG to guide clinical decision making in the setting of hospitalized HF. Ongoing work in stable ambulatory outpatient populations will attempt to validate adjunctive benefit.
This study has clinical implications. Based on our study, ICG does not provide prognostic information and is not an effective surrogate for PAC in advanced HF patients admitted to the hospital with recurrent decompensation, indicating that its utility in this setting is minimal.
Limitations
This substudy was initiated after the initiation of the ESCAPE trial and participation was voluntary. Not all centers that participated in ESCAPE were participants of this substudy. These were both sources of possible recruitment bias. However, major baseline characteristics of substudy participants and those of the parent trial appear similar. Only 170 of an anticipated 300 subjects were enrolled in BIG, and this may have limited our power to demonstrate an association with clinical outcomes. However, even when we restricted an analysis to those who had PAC performed (n=69), the invasively measured PCWP was associated with outcome, suggesting that limited power does not explain the lack of prognostic utility of impedance cardiography in our study. Importantly, to protect the blind, the bioimpedance data were collected without the benefit of concurrent display so real time adjustments in signal fidelity were not possible. However, study personnel were trained by the sponsor on the proper use of the device including proper lead placement. Quality issues were identified by the sponsor at several sites which required retraining and those issues were corrected. These quality issues would tend to bias the study toward the null hypothesis. However, analyses were performed with and without data from low volume or minimally experienced centers. The lack of difference in study results between high and low volume centers suggests that experience was not a major factor limiting the utility of bio-impedance in this study. PAC-derived CO was only measured by the thermodilution technique as Fick CO was not assessed. This sub-study was designed to evaluate ICG in the inpatient setting in decompensated patients in whom a PAC might be considered to guide therapy. The results may not be generalizable to ambulatory and compensated patients or those with less advanced heart failure.
CONCLUSIONS
In patients hospitalized with advanced decompensated HF, ICG provides an assessment of CO that correlates modestly with invasive measurements, but does not provide information on ventricular filling pressures. Further, ICG did not provide prognostic information in this patient population. There does not appear to be specific utility for ICG in patients hospitalized with advanced HF. Ongoing research will more fully address the role of ICG in ambulatory patients with less severe HF.
Acknowledgments
Sources of support: The ESCAPE trial was supported by contract number N01-HV-98177 from the National Heart, Lung, and Blood Institute to Duke University Medical Center. This substudy of impedance cardiography was funded by CardioDynamics Inc, San Diego, Calif.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Kamath—None to report
Drazner—None to report
Tasissa—None to report
Rogers—None to report
Stevenson—None to report
Yancy—Served as a consultant for CardioDynamics (modest relationship)
References
- 1.Albert NM, Hail MD, Li J, et al. Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care. 2004;13:469–479. [PubMed] [Google Scholar]
- 2.Drazner MH, Thompson B, Rosenberg PB, et al. Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2002;89:993–995. doi: 10.1016/s0002-9149(02)02257-9. [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Abraham WT, Mehra MR, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47:2245–2252. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 4.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 5.Shah MR, O'Connor CM, Sopko G, et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: an emphasis on bioimpedance cardiography. Curr Opin Cardiol. 2000;15:151–155. doi: 10.1097/00001573-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail. 2003;9:241–250. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 8.Drazner MH, Hellkamp MS, Leier CV, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circulation Heart Failure. 2008;1:170–177. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sageman WS, Riffenburgh RH, Spiess BD. Equivalence of bioimpedance and thermodilution in measuring cardiac index after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:8–14. doi: 10.1053/jcan.2002.29635. [DOI] [PubMed] [Google Scholar]
- 10.Van De Water JM, Miller TW, Vogel RL, et al. Impedance cardiography: the next vital sign technology? Chest. 2003;123:2028–2033. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler D, Grotti L, Krucke G. Comparison of cardiac output measurements by TEB vs. intermittent bolus thermodilution in mechanical ventilated patients. Chest. 1999;116:281S. (abstract) [Google Scholar]
- 12.Yung G, Fletcher C, Fedullo P, et al. Noninvasive cardiac index using bioimpedance in comparison to direct Fick and thermodilution methods in patients with pulmonary hypertension. Chest. 1999;116:281S. (abstract) [Google Scholar]
- 13.Velazquez-Cecena JL, Sharma S, Nagajothi N, et al. Left ventricular end diastolic pressure and serum brain natriuretic peptide levels in patients with abnormal impedance cardiography parameters. Arch Med Res. 2008;39:408–411. doi: 10.1016/j.arcmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18:1126–1132. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.Shah MR, Miller L. Use of pulmonary artery catheters in advanced heart failure. Curr Opin Cardiol. 2007;22:220–224. doi: 10.1097/HCO.0b013e32810c00e1. [DOI] [PubMed] [Google Scholar]
- 16.Silver MA, Cianci P, Brennan S, et al. Evaluation of impedance cardiography as an alternative to pulmonary artery catheterization in critically ill patients. Congest Heart Fail. 2004;10:17–21. doi: 10.1111/j.1527-5299.2004.03410.x. [DOI] [PubMed] [Google Scholar]