Abstract
Purpose of review
Patients with chronic kidney disease (CKD) have the highest risk for atherosclerotic cardiovascular disease (CVD). Current interventions have been insufficiently effective in lessening excess incidence and mortality from CVD in CKD patients versus other high-risk groups. This review focuses on traditional and CKD-related risks as well as key mechanisms of macrophage foam cell formation that underlie the excess CVD in the setting of CKD.
Recent findings
Hyperlipidemia, particularly increased low density lipoprotein (LDL) cholesterol, is the key factor in atherogenesis in the general population, but has not been found to be the overriding risk for greater CVD in CKD, especially as renal damage progresses. Although higher incidence of CVD in CKD is not due to higher serum lipids per se, CKD is associated with abnormal lipid metabolism that is proatherogenic. CKD-related risks, including inflammation and disturbances in mineral metabolism, have been implicated. In addition, perturbations of the macrophage, a cell that is central in atherogenesis, may be important.
Summary
The mechanisms underlying the heightened risk for CVD in CKD have been the focus of intense study and may relate to the combined effects of traditional and CKD-specific risks involving inflammation and lipid metabolism, especially perturbation of macrophage cholesterol homeostasis.
Keywords: ABCA1, atherosclerosis, cholesterol, CKD, macrophage
Introduction
Accelerated atherosclerosis and increased cardiovascular events have been extensively documented in patients with end stage chronic kidney disease (CKD) [1–3]. However, accumulating evidence underscores the increased risk for cardiovascular events that prevails at every stage of CKD [4–6]. A graded association between glomerular filtration rate (GFR) and cardiovascular deaths begins with subtle decrease in GFR (<60–80ml/min/1.73m2) which imparts an independent risk of death, acute cardiovascular events, hospitalization, and more cardiovascular complications following a myocardial infarct [4,5]. Even microalbuminuria, in the absence of apparent decrease in renal function or diabetes, predicts more cardiovascular disease (CVD) and death [6]. The impact of CVD in CKD is illustrated by reports on the natural history of patients with early CKD, which find that the risk of premature CVD death is much higher than the risk of progressing to dialysis/transplantation [7,8]. Such observations are significant not only because of the high incidence and prevalence of end stage CKD, but because the number of patients with early CKD far exceeds those with end stage CKD and this trend is continuing to rise [9]. The concern is further heightened by the recent findings of low prevalence of awareness of kidney disease among adults in the United States with or without coronary artery disease [10•]. These findings have prompted new recommendations that all patients with cardiovascular disease be systematically screened for CKD [11] and that the presence of even subtle renal dysfunction should prompt intense efforts to decrease cardiovascular risks.
Risk factors
There is little doubt that renal dysfunction is associated with excessive CVD, however, it remains uncertain how renal dysfunction/failure imparts the heightened risk. Hyperlipidemia, diabetes mellitus, hypertension, obesity, smoking, advanced age and male gender are all well known risk factors for developing cardiovascular disease in the general population. Although CKD per se appears to predispose to CVD, it is also possible that CKD simply identifies individuals with more severe, long-standing, or poorly controlled risks, that is, diabetes or hypertension. This possible limitation nonwithstanding, recognition of risk factors is important, as some of these are modifiable and implementation of risk-factor-reducing programs and therapeutic interventions has successfully lessened overall morbidity and mortality in the general population. Thus, from 1980 through 2000, death rate from coronary artery disease fell by more than 40%, with half of the decline attributable to reduction in major risk factors and the other half to therapeutic interventions [12•]. By contrast, no such trend has occurred in CKD and end stage renal disease (ESRD) patients, whose mortality has been estimated to be between 5 and 500 times higher than age-matched normal populations (Fig. 1) [13,14].
Figure 1. Deaths from cardiovascular disease in the general population and chronic kidney disease.
Between 1980 and 2000, CVD mortality in the general population in the United States fell by more than 40%, whereas CVD mortality in CKD is estimated between 10 to more than 100% above the general population. CKD, chronic kidney disease; CVD, cardiovascular disease. Adapted from [12•,13,14].
The traditional, that is, Framingham, risk factors of hypertension, diabetes mellitus, older age that predict cardiovascular disease in the general population, are increased in CKD and have been shown to predict cardiovascular mortality in the CKD patients [15–18]. However, the impact of an individual risk is not uniform across the spectrum of CKD and depends on the patient population, the degree of renal dysfunction and the etiology of kidney disease [17,19–22]. In addition, some studies find that specific risks in the setting of CKD deviate from the effects observed in the general population. For example, in patients with ESRD, elevated serum cholesterol, higher blood pressure and increased BMI are not consistently associated with acute cardiac events or all-cause mortality [20,21]. Indeed, ‘reverse epidemiology’, that is, higher cholesterol, blood pressure and BMI have been reported to predict a better outcome in these individuals (see below) [22]. Further, statistical adjustments for traditional risks have not been able to fully explain the predisposition for CVD in CKD, raising the possibility of nontraditional risks that may be specific to CKD.
The cardiovascular risk factors that have been postulated to be especially relevant to CKD include malnutrition/low serum albumin, anemia, hyperhomocysteinemia, elevated fibrinogen, dysregulation of calcium/phosphorus, oxidative stress and inflammatory factors [19,23•,24–26]. Oxidative stress and inflammation have recently gained considerable support as factors relevant in CVD in the setting of CKD. For example, the highest tertiles of high sensitivity C-reactive proteins and IL-6 were each associated with doubling in the risk for sudden cardiac death compared with the lowest tertiles [21,23•,27,28]. Notably, the impact of these circulating markers of inflammation on cardiovascular deaths was independent of traditional risk factors. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide-synthase, an independent predictor of endothelial dysfunction and poor cardiovascular outcome, is increased in patients with advanced CKD [29–33]. The combination of inflammation, malnutrition (low serum albumin) and atherosclerosis was recently incorporated into the term protein–energy wasting (PEW), which has been put forth as a leading cause for the excess mortality in patients with end-stage CKD [19,24,28]. In this regard, a recent clinical trial of apparently healthy men and women with intact renal function and no hyperlipidemia but with elevated high-sensitivity C-reactive protein levels was terminated early because statin therapy significantly reduced the incidence of major cardiovascular events [34••]. The study underscored the prediction that the benefits of enrollment that were based on elevated high-sensitivity C-reactive protein were double those predicted by studies with enrollment based on elevated LDL cholesterol. Such observations emphasize the growing support for a critical role of inflammation in atherogenesis and may be especially pertinent in the chronically pro-inflammatory condition that characterizes CKD. Indeed, a recent study found that high sensitivity C-reactive protein can predict all-cause mortality in nondialysis CKD patients, that is, independent of age, estimated GFR, left ventricular mass index and vascular disease [35•].
CKD also leads to mineral–bone disorders. Laboratory abnormalities, including phosphate retention, elevated parathyroid hormone, and low 1,25-dihydroxy vitamin D levels have been extensively documented [36]. Clinical studies show that excess phosphate influences mortality and myocardial infarction in CKD patients [37,38•]. Sevelamer, a phosphate binder, delays progression of not only vascular calcification but also atherosclerotic lesion with decreased serum phosphate levels and oxidative damage in vascular intima of apolipoprotein E (apoE) -deficient mice, although in humans, benefits of such interventions may also reflect reduction in serum lipid levels [39]. Vitamin D also contributes to the regulation of renin–angiotensin system, inflammation and inhibits both cardiac hypertrophy and myocyte proliferation [40]. Although there are a number of recent studies that associate one or more of these mineral metabolism disturbances with excessive CVD in CKD, none have been definitive.
Dyslipidemia in chronic kidney disease
Among the risk factors for atherosclerotic CVD, hyperlipidemia is key; it is also the primary target for therapeutic intervention. CKD causes dyslipidemia. The magnitude and characteristics of the dyslipidemia depends on the degree of renal impairment, the etiology of the primary renal disease, and presence of nephrotic syndrome [41,42•,43]. CKD without nephrotic syndrome is typified by elevated triglycerides, low high-density lipoprotein (HDL) and total cholesterol concentration that is, at, or near, normal levels (Table 1). Although this dyslipidemic pattern does not necessarily fit into recommendations for therapeutic interventions in the general population, it belies profound disturbances in lipid metabolism. These disturbances result from overproduction as well as impairment in the clearance of apolipoproteinB (apoB)-containing lipoproteins that reflects abnormalities in lipid substrates, enzymes, lipid transfer proteins and lipoprotein receptor activity (Table 1).
Table 1.
Common feature of serum lipids, lipoproteins, apolipo-proteins, enzymes, and transfer proteins in predialysis chronic kidney disease patients
| CKD stage 1–4 | CKD stage 5 | Nephrotic syndrome | |
|---|---|---|---|
| Total cholesterol | ↔ | ↔ | ↑ |
| Triglyceride | ↔ or ↑ | ↑ | ↑ |
| LDL-cholesterol | ↔ or ↑ | ↔ or ↓ | ↑ |
| Small dense LDL | ↑ | ↑ | ↑ |
| HDL-cholesterol | ↓ or ↔ | ↓ | ↓ or ↔ |
| Lipoprotein a | ↑ | ↑ | ↑ |
| ApoA-I, A-II | ↓ | ↓ | ↑ or ↓ |
| ApoC-II | ↓ | ↓ | ↑ |
| ApoC-III | ↑ | ↑ | ↑ |
| ApoE | ↓ | ↓ | ↑ |
| LPL activity | ↓ | ↓ | ↓ |
| Hepatic lipase activity | ↓ | ↓ | ↓ |
| ACAT | ↑ | ↑ | ↑ |
| LCAT activity | ↓ | ↓ | ↓ |
| CETP | ↑ | ↑ | ↑ |
ACAT, acyl-CoA cholestereol acyltransferase; Apo, apolipoprotien; CETP, cholesterol ester transfer protein; CKD, chronic kidney disease; HDL, high density lipoprotein; LCAT, lecithin cholesterol acyltransferase; LDL, low density lipoprotein; LPL, lipoprotein lipase.
Hypertriglyceridemia is an early feature of CKD. It persists at every stage of renal dysfunction, and is found in the majority of patients with ESRD, particularly diabetics and those undergoing peritoneal dialysis. Hyper-triglyceridemia reflects increased synthesis by the liver and especially decreased clearance because of decreased activity of lipolytic enzymes, including lipoprotein lipase and hepatic lipase, and reduction in their inhibitors, such as preβ HDL, reduced apolipoprotein CII and apoE [41]. Elevation of triglyceride-rich lipoproteins also reflects upregulation of hepatic acyl-CoA cholesterol acyltransferase (ACAT) [43]. Decreased catabolism also leads to accumulation of small dense low-density lipoprotein (LDL) particles. The impaired catabolism, at least in part, reflects posttranslational modification of apolipoproteins by CKD-related oxidation, glycation, carbamylation, [44–46]. In addition, there is reduced receptor-mediated uptake of triglycerides by hepatic LDL-receptor related protein (LRP) and VLDL-receptors [41,43].
As noted, increased CVD in CKD is not because of higher levels of cholesterol. Clinicians have also been reluctant to initiate lipid-modulating therapy because of the possibility of toxicity. Until very recently, patients with renal impairment were specifically excluded from studies of treatment strategies, further limiting assessments of treatment efficacy in this population. Nonetheless, recent subgroup analysis of some secondary prevention trials, including, Cholesterol and Recurrent Events (CARE), Heart Protection Study (HPS), and Veterans’ Affairs High-Density Lipoprotein Intervention Trial (VA-HIT) found that lipid lowering agents are effective in preventing cardiovascular events in patients with mild-to-moderate CKD; however, other studies did not confirm these findings, that is, Prevention of Renal and Vascular End Stage Disease Intervention Trial (PREVEND IT) [47–50]. Interestingly, intensive lipid lowering therapy (atorvastatin 80 mg/day versus 10 mg/day) was found to be more beneficial in patients with mild-to-moderate CKD than in individuals with normal or near-normal renal function although some of the reduction in cardiovascular risk may reflect improvement in renal function observed in patients on the higher statin dose [51••].
By contrast to mild–moderate CKD, patients with advanced or end-stage kidney disease who are at the greatest risk for cardiovascular events show an inconsistent relationship to elevated lipids and have even been found to have ‘reverse epidemiology’ with increased mortality with lower cholesterol levels [20–22,52]. This paradoxical effect has been linked to protein energy wasting and/or inflammation that characterize CKD [22]. Notably, in a study of more than 15 000 ESRD patients, adjustments for malnutrition and inflammation did not improve the association between mortality and low cholesterol levels [53]. Moreover, the effects of therapeutic interventions that lower LDL are equivocal as renal damage progresses. Thus, some but not all observational studies found that lowering LDL reduced relative risk in total mortality in ESRD patients [54–57]. The only prospective randomized controlled clinical trial evaluated 1200 hemodialyzed diabetics over a period of 4 years and found that atorvastatin which decreased the serum cholesterol had a nonsignificant 8% relative risk reduction on the combined primary end point of cardiac death, nonfatal myocardial infarction or stroke [57]. It has been suggested that the negative results reflect very advanced atherosclerotic disease and/or importance of other cardiovascular disorders nonmodifiable by lipid-lowering therapies.
Thus, although there is indisputable evidence that hyper-lipidemia underlies atherosclerosis and that reduction in LDL decreases coronary events and mortality in the general population and in early stages of CKD, this abnormality may be a weaker predictor as renal damage progresses. Even in the general population, LDL-lowering therapy is not uniformly beneficial. Such findings have been interpreted to suggest that a plateau of risk reduction may have been achieved with current interventions [58]. The implication of these findings has been variously interpreted [59], including the need for more aggressive reduction in LDL cholesterol, or the possible importance of inflammation in atherogenesis, or the possibility that lipid abnormalities other than serum LDL cholesterol levels modulate cardiovascular risk, such as HDL, or disturbed lipid homeostasis at the local cellular level (see below).
Decreased HDL cholesterol has long been recognized to be a powerful negative-risk factor for CVD, and has emerged as a new target for intervention in progression and even regression of atherosclerosis [60]. Low HDL is a consistent finding at all stages of CKD [41]. Impaired maturation of HDL in CKD is primary due to downregulation of lecithin–cholesterol acyltransferase (LCAT), which esterifies cholesterol taken up by HDL that enables it to acquire subsequent cholesterol particles, increased cholesteryl ester transfer protein (CETP) and ACAT, as well as decreased hepatic lipase, Table 1. Although there is currently no therapy that specifically targets HDL, some benefits of currently used LDL-lowering interventions have been ascribed to the accompanying increase in HDL levels [61]. Moreover, in a small trial evaluating the effects of a synthetic HDL-associated apolipoprotein A-I (apoA-I Milano), there was significant regression of atherosclerotic lesions [62], providing support that targeting HDL may have important beneficial consequences for atherosclerosis.
Efforts to raise HDL have been problematic. A clinical trial with an inhibitor of CETP, torcetrapib, was terminated because of increased mortality and cardiovascular events [63••] possibly related to an off-target effect indicated by increased aldosterone. Further, the ultra-sonographic assessments did not find benefit of tocetrapib on progression of coronary atherosclerosis or carotid intimal thickness [64]. Interestingly, although low HDL levels tracked with high CETP activity in patients with CKD stage V, neither parameter was associated with cardiovascular events over a 48-month period [65]. Such findings cast doubt on the potential efficacy of CETP inhibitors in CKD. In this regard, accumulating evidence suggests that the quality of HDL may be more important than the level of circulating HDL [66]. Support for this concept includes studies showing that the antiatherogenic effects of plasma HDL depend on the ability of the lipoprotein to accept cholesterol for reverse cholesterol transport and provide antioxidative and anti-inflammatory functions [67]. CKD patients have abnormalities in the maturation, composition, stability, and antioxidant capacity of HDL [46,68,69]. These considerations are especially relevant, as a number of antioxidative functions of HDL depend on enzymes and transfer proteins known to be deranged by CKD including LCAT, paraoxonase, and phospholipid transfer protein which have the possibility of not only loosing the antiatherogenic properties but actually promoting inflammation, and thus atherosclerosis.
Among the mechanisms by which HDL exerts its antiatherogenic effects, a pivotal factor is thought to be HDL’s involvement in reverse cholesterol transport. This is a a multistep, multiorgan process, the net effect of which is to remove excess cholesterol from macrophages in peripheral tissue, transport it in plasma for delivery and processing within the liver, and excretion in bile and intestine [70,71••,72••]. CKD may confound the reverse cholesterol transport due to hepatic impairment in synthesis and function of several enzymes and lipoproteins involved in the process, such as ApoA-I, ApoE, hepatic lipase, LCAT activity (Table 1). New information suggests that the macrophage lipid homeostasis in CKD, the first step in reverse cholesterol transport, is also deranged.
Macrophage lipid homeostasis
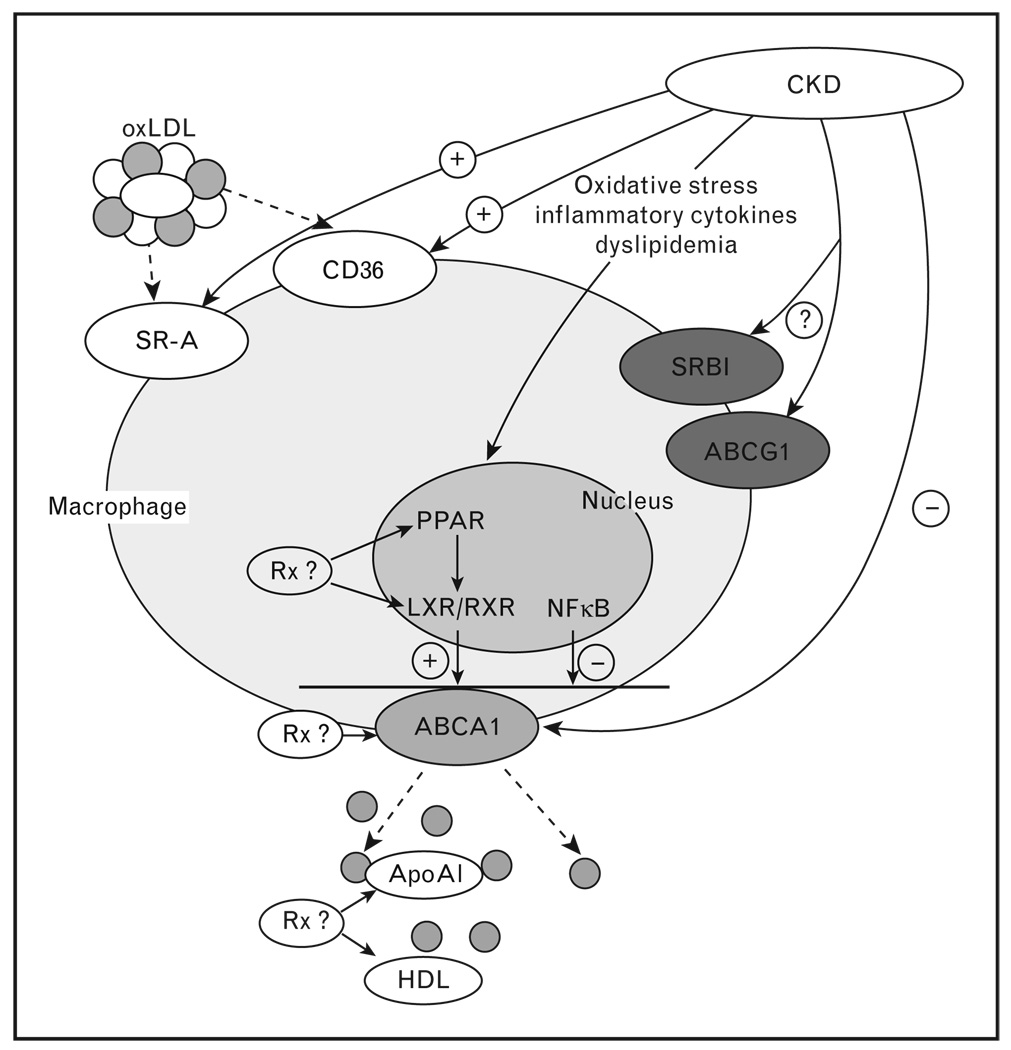
The cholesterol loaded macrophage foam cell is the hallmark of the atherogenic lesion. Foam cell formation results not only from an unrelenting uptake of lipoproteins but failure of cholesterol export mechanisms to keep pace with internalization of cholesterol from lipoproteins and cellular debris, which is mediated by scavenger receptors (Fig. 2). Increased cholesterol uptake in the setting of renal dysfunction is predicted from observations that monocytes of patients with renal damage upregulate scavenger receptor expression [73]. However, once upregulated, macrophages do not downregulate scavenger receptors or inflow of cholesterol by this pathway, and foam cell formation becomes critically dependent on lipid efflux, which involves mobilization of excess cholesterol from intracellular pools to the plasma membrane and transfer to suitable external cholesterol acceptors [70,74•]. The key pathways for cholesterol movement out of macrophages involve an energy-dependent efflux linked to the ATP-binding cassette transporter A1 (ABCA1), ABCG1, and HDL scavenger receptor class I type 1 (SR-B1) Fig. 2 [70,71••,72••,74•].
Figure 2. Macrophage lipid homeostasis in chronic renal disease.
CKD upregulation of scavenger receptors CD36 and SR-A enhances cholesterol uptake whereas downregulation of ABCA1 transporter represses cholesterol efflux that promotes foam cell formation. CKD induces oxidative stress, inflammatory cytokines, and dyslipidemia that may regulate cellular processes involved in macrophage lipid metabolism. Rx, possible therapeutic interventions; oxLDL, oxidized light density lipoprotein; SRA, scavenger receptor A; CD36, scavenger receptor 36; ABCA1, ATP binding cassette transporter A1; ABCG1, ATP binding cassette transporter G1; SRB1, scavenger receptor B1; ApoAI, apolipoprotein AI; HDL, high density lipoprotein; LXR, liver X receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; NFκB, nuclear factor kappa B.
As foam cell formation depends on perturbations in macrophage cholesterol homeostasis, we recently investigated whether renal dysfunction affects the influx of macrophages within the atherosclerotic lesion as well as the cell’s lipid metabolism. Reduction in renal mass increases atherosclerosis in apoE deficient hyperlipidemic mice, which is proportional to the extent of renal ablation. We showed that uninephrectomy (UNx), which has little effect on renal function, dramatically increases the extent of atherosclerosis and that the lesions have greater macrophage content than in mice with intact kidneys [75]. In vitro, macrophages of UNx mice had increased migratory response to a monocyte chemoattractant protein (MCP)-1 stimulus. These findings complement observations that reduction in renal mass can induce increased endothelial expression of adhesion molecules, including intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and generalized oxidative stress, findings that predict enhanced monocyte adhesion and migration into the vascular intima [76,77]. Antagonism of angiotensin II (AII) is well established to benefit CVD in the general population [78]. We, therefore, examined the effect of such intervention in our mice. Although both the AII antagonist losartan and a nonspecific vasodilator hydralazine similarly decreased systemic blood pressure, only losartan decreased atherogenesis, lesional macrophage content and macrophage migration.
Our preliminary evaluation of cellular lipid homeostasis revealed strikingly increased lipid content of peritoneal macrophages of UNx mice [79,80]. The cellular lipid expansion was not simply a reflection of the in vivo plasma lipid environment suggesting that foam cell formation and atherosclerosis do not necessarily parallel plasma lipid levels, particularly in the presence of renal dysfunction. The studies further showed that in vivo treatment with losartan reduced cholesterol content in macrophages of UNx. Since cholesterol efflux in this setting is a pivotal step in determining whether intracellular lipid homeostasis is maintained or whether the macrophage will turn into a foam cell, we assessed efflux. Cholesterol efflux was significantly in macrophages from UNx mice. This effect was linked to repression of the macrophage ABCA1 transporter. Notably, in vivo treatment with losartan restored macrophage ABCA1 expression, results that complement previous in-vivo and in-vitro findings that exogenous AII downregulates ABCA1 [80,81,82•].UNx macrophages had significantly elevated nuclear factor kappa B (NF-κB) activity and specific antagonism of the NF-κB activation pathway in macrophages lessened repression of the ABCA1 transporter. Losartan significantly decreased upregulation of NF-κB, suggesting this as a potential key regulatory step. It is of interest that plasma from CKD patients’ downregulated ABCA1 in cultured endothelial cells [83•] and that cultured human macrophages exposed to the AII receptor blocker telmisartan increased cholesterol efflux [84•]. These observations suggest a pivotal importance of ABCA1 and macrophage cholesterol homeostasis in the setting of renal dysfunction, providing a basis for possible target for excess CVD in this setting (Fig. 2). Although there is strong support for AII antagonism lessening progressive renal parenchymal damage, the impact of this intervention on CVD in CKD remains to be clarified. Post-hoc analysis of patients with early renal damage treated with angiotensin-converting enzyme inhibitors (ACEI) document fewer cardiovascular events and increased survival [85]. A 2 year follow up of 400 patients with left ventricular hypertrophy on hemodialysis found a trend for lower rate of cardiovascular events in patients treated with the ACEI, fosinopril, but the difference was not significant [86]. Although constrained by some limitations, two reports suggest that antagonism of AII actions reduces cardiovascular morbidity and mortality in hemodialysis patients [87,88].
Conclusion
Recognition of the mechanisms and risk factors of atherosclerotic disease has made a tremendous impact on morbidity and mortality in the general population. Although many of these risk factors prevail in patients with CKD, additional disturbances in lipid metabolism, inflammation and macrophage cholesterol handling contribute to the excess vascular disease, which is apparent at every level of renal impairment. Clarification of lipid abnormalities may enable more aggressive and specific therapeutic interventions. However, even available antilipidemic therapies may provide anti-inflammatory benefits in this setting. Antagonism of AII actions which predicts beneficial effects on inflammation, may also promote macrophage efflux and subsequent elimination of lipids that offer the possibility of a novel target for lessening atherosclerosis in CKD.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 3.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and sub-sequent kidney disease. Arch Intern Med. 2007;167:1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 6.Klausen KP, Scharling H, Jensen JS. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med. 2006;260:231–237. doi: 10.1111/j.1365-2796.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 7.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 8.Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333:1047. doi: 10.1136/bmj.39001.657755.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 10.McClellan WM, Newsome BB, McClure LA, et al. Chronic kidney disease is often unrecognized among patients with coronary heart disease: The REGARDS Cohort Study. Am J Nephrol. 2009;29:10–17. doi: 10.1159/000148645.This report observes not only a high prevalence of CKD and a high prevalence of CVD in CKD, but emphasizes the low prevalence of awareness of kidney disease among the United States population with or without coronary heart disease which limits the possibility of therapeutic intervention.
- 11.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935.A statistical model describes a dramatic decrease in deaths from coronary heart disease in the United States from 1980 through 2000 with approximately half the decline attributable to reductions in major risk factors and approximately half to evidence-based medical therapies.
- 13.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 14.US renal data system. Excerpts from the USRDS. Annual data report. Am J Kidney Dis. 2006;49:S1–S296. [Google Scholar]
- 15.Muntner P, Mann D, Winston J, et al. Serum cystatin C and increased coronary heart disease prevalence in US adults without chronic kidney disease. Am J Cardiol. 2008;102:54–57. doi: 10.1016/j.amjcard.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 16.Parikh NI, Hwang SJ, Larson MG, et al. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 17.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 18.Chonchol M, Whittle J, Desbien A, et al. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354–360. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 19.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between non-traditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial. 2007;20:566–569. doi: 10.1111/j.1525-139X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Shah DS, Polkinhorne KR, Pellicano R, Kerr PG. Are traditional risk factors valid for assessing cardiovascular risk in end-stage renal failure patients? Nephrology (Carlton) 2008;13:667–671. doi: 10.1111/j.1440-1797.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74:1335–1342. doi: 10.1038/ki.2008.449.A prospective study of dialysis patients found that elevated levels of high sensitivity C-reactive protein and IL-6 (markers of inflammation and malnutrition) were associated with a high risk of death, and were independent of traditional risk factors.
- 24.de Mutsert R, Grootendorst DC, Axelsson J, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 25.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:672–681. doi: 10.1038/ncpneph0954. [DOI] [PubMed] [Google Scholar]
- 27.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Soriano S, Gonzalez L, Martin-Malo A, et al. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3–5 patients. Clin Nephrol. 2007;67:352–357. doi: 10.5414/cnp67352. [DOI] [PubMed] [Google Scholar]
- 29.Kielstein JT, Boger RH, Bode-Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170–176. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- 30.Kielstein JT, Zoccali C. Asymmetric dimethylarginine: a cardiovascular risk factor and a uremic toxin coming of age? Am J Kidney Dis. 2005;46:186–202. doi: 10.1053/j.ajkd.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Meinitzer A, Seelhorst U, Wellnitz B, et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 32.Annuk M, Soveri I, Zilmer M, et al. Endothelial function, CRP and oxidative stress in chronic kidney disease. J Nephrol. 2005;18:721–726. [PubMed] [Google Scholar]
- 33.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646.Support for a key role of inflammation in atherosclerotic disease is provided by this study showing that resuvostatin-induced reduction in elevated levels of high-sensitivity C-reactive protein prevents cardiovascular events even in the absence of hyperlipidemia.
- 35.Vickery S, Webb MC, Price CP, et al. Prognostic value of cardiac biomarkers for death in a nondialysis chronic kidney disease population. Nephrol Dial Transplant. 2008;23:3546–3553. doi: 10.1093/ndt/gfn341.Along with reference 34 which reported results from apparently normal men and women, this study in individuals with preend stage CKD suggests that high sensitivity C-reactive protein is a valuable prognostic marker of mortality.
- 36.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 37.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 38.Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn613.Critical review of studies on mineral metabolic disturbances and cardiovascular events concluding a significant CVD risk with abnormal mineral metabolism in dialysis patients but not in preend stage CKD.
- 39.Phan O, Ivanovski O, Nguyen-Khoa T, et al. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation. 2005;112:2875–2882. doi: 10.1161/CIRCULATIONAHA105.541854. [DOI] [PubMed] [Google Scholar]
- 40.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005;68:1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 2008;28:958–973. doi: 10.1159/000144024. [DOI] [PubMed] [Google Scholar]
- 42.Chan DT, Irish AB, Dogra GK, Watts GF. Dyslipidaemia and cardiorenal disease: mechanisms, therapeutic opportunities and clinical trials. Atherosclerosis. 2008;196:823–834. doi: 10.1016/j.atherosclerosis.2007.01.023.A thorough and accessible review of dyslipidemia across the spectrum of chronic renal disease.
- 43.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–F272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 44.Asci G, Basci A, Shah SV, et al. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 2008;13:480–486. doi: 10.1111/j.1440-1797.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 45.Kalogerakis G, Baker AM, Christov S, et al. Oxidative stress and high-density lipoprotein function in Type I diabetes and end-stage renal disease. Clin Sci (Lond) 2005;108:497–506. doi: 10.1042/CS20040312. [DOI] [PubMed] [Google Scholar]
- 46.Jurek A, Turyna B, Kubit P, Klein A. LDL susceptibility to oxidation and HDL antioxidant capacity in patients with renal failure. Clin Biochem. 2006;39:19–27. doi: 10.1016/j.clinbiochem.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 48.Tonelli M, Collins D, Robins S, et al. Gemfibrozil for secondary prevention of cardiovascular events in mild to moderate chronic renal insufficiency. Kidney Int. 2004;66:1123–1130. doi: 10.1111/j.1523-1755.2004.00862.x. [DOI] [PubMed] [Google Scholar]
- 49.Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–178. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 50.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd J, Kastelein JP, Bittner VA, et al. Intensive lipid lowering with atorvastatin in patients with coronary artery disease, diabetes, and chronic kidney disease. Mayo Clin Proc. 2008;83:870–879.Aggressive lipid lowering therapy with atorvastatin was found to be more effective in reducing cardiovascular events in CKD than in individuals with normal or near normal renal function.
- 52.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 53.Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 54.Seliger SL, Weiss NS, Gillen DL, et al. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61:297–304. doi: 10.1046/j.1523-1755.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- 55.Andreucci VE, Fissell RB, Bragg-Gresham JL, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS) data on medications in hemodialysis patients. Am J Kidney Dis. 2004;44:61–67. doi: 10.1053/j.ajkd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Lahoz C, Mostaza JM, Mantilla MT, et al. Achievement of therapeutic goals and utilization of evidence-based cardiovascular therapies in coronary heart disease patients with chronic kidney disease. Am J Cardiol. 2008;101:1098–1102. doi: 10.1016/j.amjcard.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 58.Lee CH, Plutzky J, Liver X. receptor activation and high-density lipoprotein biology: a reversal of fortune? Circulation. 2006;113:5–8. doi: 10.1161/CIRCULATIONAHA.105.590273. [DOI] [PubMed] [Google Scholar]
- 59.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 60.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High: Density Lipoprotein Intervention Trial. Am J Cardiol. 2000;86:19L–22L. doi: 10.1016/s0002-9149(00)01464-8. [DOI] [PubMed] [Google Scholar]
- 61.Nachimuthu S, Raggi P. Novel agents to manage dyslipidemias and impact atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 2006;6:209–217. doi: 10.2174/187152906778249554. [DOI] [PubMed] [Google Scholar]
- 62.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 63.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635.Despite favorable effects on high-density lipoprotein cholesterol, the cholesteryl ester transfer protein inhibitor, torcetrapib, failed to slow atherosclerosis progression and increased mortality.
- 64.Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370:153–160. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 65.Seiler S, Schlitt A, Jiang XC, et al. Cholesteryl ester transfer protein activity and cardiovascular events in patients with chronic kidney disease stage V. Nephrol Dial Transplant. 2008;23:3599–3604. doi: 10.1093/ndt/gfn296. [DOI] [PubMed] [Google Scholar]
- 66.Navab M, Reddy S, Van Lenten BJ, et al. Role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2008 doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 68.Miida T, Miyazaki O, Hanyu O, et al. LCAT-dependent conversion of prebeta1-HDL into alpha-migrating HDL is severely delayed in hemodialysis patients. J Am Soc Nephrol. 2003;14:732–738. doi: 10.1097/01.asn.0000046962.43220.8a. [DOI] [PubMed] [Google Scholar]
- 69.Okubo K, Ikewaki K, Sakai S, et al. Abnormal HDL apolipoprotein A-I and A-II kinetics in hemodialysis patients: a stable isotope study. J Am Soc Nephrol. 2004;15:1008–1015. doi: 10.1097/01.asn.0000117286.85443.7d. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 71.Wang MD, Franklin V, Marcel YL. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler Thromb Vasc Biol. 2007;27:1837–1842. doi: 10.1161/ATVBAHA.107.146068.Along with reference 72, the first in-vivo demonstration of the importance of the ABCA1 transporter for cholesterol transport.
- 72.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057.Demonstration that in addition to ABCA1, the ABCG1 transporter contributes to reverse cholesterol transport in vivo.
- 73.Chmielewski M, Bryl E, Marzec L, et al. Expression of scavenger receptor CD36 in chronic renal failure patients. Artif Organs. 2005;29:608–614. doi: 10.1111/j.1525-1594.2005.29097.x. [DOI] [PubMed] [Google Scholar]
- 74.Yvan-Charvet L, Ranalletta M, Wang N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372.Transplantation of ABCA1−/−:ABCG1−/− bone marrow into LDLr−/− mice underscored a relationship between lipid homeostasis and inflammation in macrophages where acceleration in atherosclerosis is related to increased inflammation and apoptosis in lipid engorged cells.
- 75.Suganuma E, Zuo Y, Ayabe N, et al. Antiatherogenic effects of angiotensin receptor antagonism in mild renal dysfunction. J Am Soc Nephrol. 2006;17:433–441. doi: 10.1681/ASN.2005080883. [DOI] [PubMed] [Google Scholar]
- 76.Bro S, Bentzon JF, Falk E, et al. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 77.Massy ZA, Ivanovski O, Nguyen-Khoa T, et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 78.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 79.Zuo YY, Linton P, Fazio MF, Ichikawa S, Kon I. V. Chronic renal damage (CKD) represses ATP-binding cassette transporter A1 (ABCA1) to decrease macrophage efflux and promote foam cell formation: role of angiotensin II (AII) JASN. 2007;18:636A. [Google Scholar]
- 80.Kaplan M, Aviram M, Knopf C, Keidar S. Angiotensin II reduces macrophage cholesterol efflux: a role for the AT-1 receptor but not for the ABC1 transporter. Biochem Biophys Res Commun. 2002;290:1529–1534. doi: 10.1006/bbrc.2002.6376. [DOI] [PubMed] [Google Scholar]
- 81.Takata Y, Chu V, Collins AR, et al. Transcriptional repression of ATP-binding cassette transporter A1 gene in macrophages: a novel atherosclerotic effect of angiotensin II. Circ Res. 2005;97:e88–e96. doi: 10.1161/01.RES.0000190400.46267.7e. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Chen Z, Liao Y, et al. Angiotensin II increases the cholesterol content of foam cells via down-regulating the expression of ATP-binding cassette transporter A1. Biochem Biophys Res Commun. 2007;353:650–654. doi: 10.1016/j.bbrc.2006.12.067.Demonstration that angiotensin II-induced foam cell formation involves down-regulation of the ABCA1 transporter.
- 83.Cardinal H, Raymond MA, Hebert MJ, Madore F, et al. Uraemic plasma decreases the expression of ABCA1, ABCG1 and cell-cycle genes in human coronary arterial endothelial cells. Nephrol Dial Transplant. 2007;22:409–416. doi: 10.1093/ndt/gfl619.Intriguing observations that uremic sera can regulate genes involved in cholesterol efflux.
- 84.Nakaya K, Ayaori M, Hisada T, et al. Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARgamma. J Atheroscler Thromb. 2007;14:133–141. doi: 10.5551/jat.14.133.Along with reference 82, demonstration that angiotensin II-induced down-regulation in ABCA1 contributes to foam cell formation and the possibility that pharmacologic antagonism of angiotensin II actions can reverse this process.
- 85.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 86.Kessler M, Zannad F, Lehert P, et al. Predictors of cardiovascular events in patients with end-stage renal disease: an analysis from the Fosinopril in dialysis study. Nephrol Dial Transplant. 2007;22:3573–3579. doi: 10.1093/ndt/gfm417. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis–a randomized study. Nephrol Dial Transplant. 2006;21:2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki H, Kanno Y, Sugahara S, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]