Background
The incidence of diabetes mellitus, obesity and the metabolic syndrome is rapidly rising to epidemic levels worldwide. Hyperglycemia, the metabolic hallmark of the pathology, is a significant causative factor for the complications of diabetes mellitus which result in significant morbidity and mortality for millions. Hyperglycemia is clearly associated with microvascular complications in many organs including the kidney, and diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) in developed countries [1,2]. In addition, diabetes may lead to other vascular complications, including systemic hypertension [1,2]. Recent advances on the cellular and kidney-specific effects of hyperglycemia place activation of the local, intra-renal renin–angiotensin system (RAS) as a strong candidate for the core abnormality that leads to renal tissue injury [1–3].
The nature of RAS activation in diabetes is, however, controversial [3]. It has been difficult to isolate the acute and direct actions of hyperglycemia per se from the many other systemic factors and intra-renal, macula densa-mediated feedback mechanisms that can indirectly activate the intra-renal RAS. Therefore, the primary cause and exact mechanism of RAS activation in early diabetes have been unknown. The prevailing paradigm, the ‘tubular hypothesis of glomerular filtration’ [4], argues that the two hallmarks of early changes, glomerular hyperfiltration and renin activation, originate from the primary effects of glucose on proximal tubule salt reabsorption that secondarily activate macula densa-mediated feedback mechanisms. However, the development of diabetes-induced glomerular hyperfiltration was intact, or even augmented, in mice that lacked tubuloglomerular feedback (TGF), demonstrating that the TGF mechanism could not be the major cause of the development of hyperfiltration [5,6].
The recent discovery of the G-protein-coupled receptor GPR91 [7] which is activated by the citric acid cycle intermediate succinate prompted the discovery of a new, direct link between high glucose levels and renin release from the juxtaglomerular apparatus (JGA) in the kidney [8].
Succinate and GPR91 directly link high glucose levels to JGA renin release
GPR91, a metabolic receptor which is highly expressed in the kidney, can lead to renin-dependent activation of RAS and increased systemic blood pressure [7]. Its ligand, the citric acid cycle intermediate succinate, has long been known to cause renin release from the JGA [9]. However, the importance of the local interstitial accumulation of succinate as a sign of ischemic organ damage in sites like the brain [10] or its role as an indicator of the imbalance between tissue energy supply and demand was only recently recognized [8,11,12]. At least in the liver and the kidneys [8,11,12], succinate triggers paracrine signaling through GPR91 leading to (patho)physiological alterations in organ function.
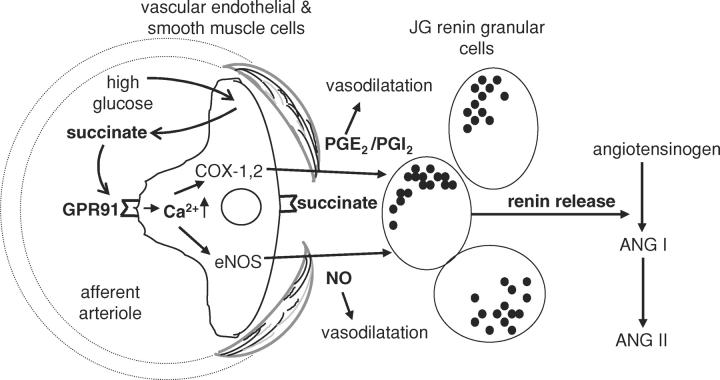
In fact, new data demonstrate that localized succinate accumulation occurs in the intact diabetic kidney as well as in the dissected, in vitro microperfused JGA preparation acutely subjected to high glucose levels [8]. High glucose and succinate-induced GPR91 activation trigger paracrine signaling (summarized in Figure 1) from the (juxta)glomerular endothelium to the adjacent renin-producing JG cells to increase renin synthesis and release [8], the rate-limiting step of RAS activation. Elements of the signal transduction cascade involve succinate and GPR91-dependent elevations in vascular endothelial [Ca2+]i as well as the synthesis and release of NO and PGE2, classic mediators of renin release [13]. Endothelial NO and prostaglandin production also directly causes vasodilatation of the afferent arteriole, which may be important in the development of glomerular hyperfiltration. In summary, this GPR91-mediated paracrine signaling pathway provides an alternative to the ‘tubular hypothesis’ and offers a direct mechanism for the development of both hallmarks of diabetes: glomerular hyperfiltration and JGA renin activation.
Fig. 1.
Schematic illustration of the newly described (juxta)glomerular paracrine signaling cascade involving succinate activation of GPR91, leading to renin synthesis and release. High glucose levels result in the accumulation of the metabolic intermediate succinate, which acts directly on the vascular endothelium and triggers cell-to-cell signaling to culminate in renin release from the juxtaglomerular apparatus. The chain of events involves elevating glucose levels, succinate accumulation in the plasma and local interstitium, GPR91 activation, increases in endothelial cytosolic calcium, nitric oxide (NO) and prostaglandin (at least PGE2) production and release from endothelium, PG actions on renin-producing JG cells, renin release, angiotensin (Ang I and Ang II) synthesis and RAS activation. Endothelial NO and PG production also causes vasodilatation that may be important in the development of glomerular hyperfiltration.
In vivo imaging of (pro)renin
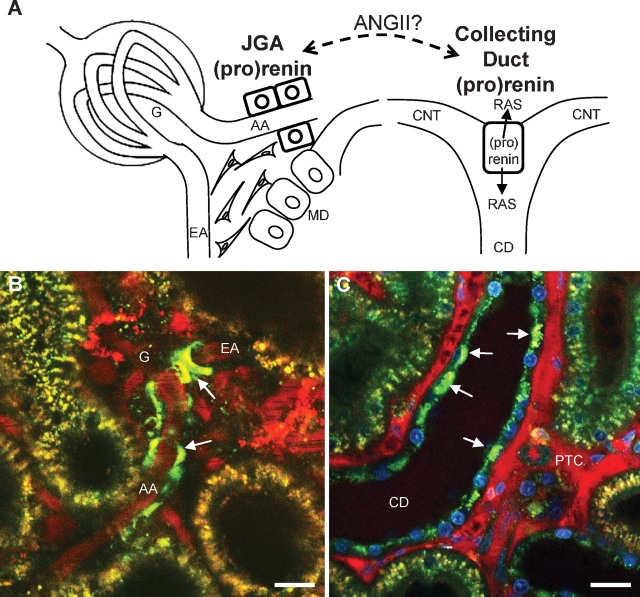
Characterization of this novel GPR91-mediated renin release pathway was made possible, at least in part, by the recent development of a quantitative in vivo imaging model based on multi-photon excitation fluorescence confocal microscopy [14,15]. Using this new imaging approach, it is now possible to directly visualize JGA renin granular content, release, and tissue activity in the intact living kidney with high temporal and spatial resolution [16–18]. A recent study provided quantitative, functional and in vivo visual analysis of (pro)renin (a term denoting both renin and its precursor prorenin) in the diabetic rat kidney [19]. In addition to the JGA, which is the classic site of (pro)renin synthesis, significant amounts of (pro)renin were present in principal cells of the collecting duct (CD), especially in diabetes. The two most important intra-renal locations of (pro)renin synthesis and release and the in vivo imaging of these two sites by multi-photon microscopy are shown in Figure 2.
Fig. 2.
The two major sites of intra-renal (pro)renin synthesis: the juxtaglomerular apparatus (JGA) and the collecting duct (CD). Schematic drawing (A) and in vivo multi-photon fluorescence images (B, C) of these two sites. Direct in vivo visualization of quinacrine-labeled renin granules (green) in the JGA (B) and CD (C) in the intact kidney using multi-photon confocal microscopy. Control rat (B) and diabetic mouse kidney (C) are shown. A dextran-rhodamine B conjugate (70 kDa) labeled the intravascular space (plasma) red. (B) Quinacrine identified JGA renin granules in the terminal afferent arteriole (AA). The efferent arteriole (EA) is seen leaving the glomerulus (G). (C) Note the abundance of quinacrine staining in the bulging apical aspects of CD principal cells in diabetes (arrows). Nuclei are labeled blue with Hoechst 33342. Bars are 20 μm. MD: macula densa; CNT: connecting tubule; PTC: peritubular capillaries.
The distal nephron is the major source of (pro)renin in diabetes
While the JGA is recognized as the primary source of renin and GPR91-mediated renin release in early diabetes could serve as the gatekeeper responsible for early RAS activation, its negative feedback function dictates that elevating Ang II levels eventually suppress JGA renin [13]. At the same time when Ang II suppresses JGA renin, it activates CD (pro)renin production in a number of disease models including high Ang II states, renovascular hypertension and diabetes [19–21]. Significant levels of de novo renin synthesis in the connecting segment (CNT) and CD were confirmed earlier [22]. The vast CD (pro)renin upregulation in diabetes is most likely mediated by Ang II, which appears to promote the buildup and release of CD prorenin [19]. CD prorenin may be released to cause systemic or local pathological actions at the recently identified and characterized (pro)renin receptor [23–26], or may be cleaved to serve as a source of active renin in the face of JG renin suppression. This would be consistent with the existence of a local distal tubular RAS [22] and its possible regulatory effects on salt reabsorption [27,28].
Another thus far unrecognized and potentially very important feature of the diabetic kidney is significant proliferation of the CNT [19]. While Ang II up-regulates (pro)renin synthesis on the individual cellular level in this part of the nephron, proliferation of the entire CNT has larger scale effects on RAS activation by increasing the entire population of the (pro)renin-producing principal cells.
Conclusion
GPR91-induced JGA renin release and the identification of the CD as the major site of intrarenal (pro)renin synthesis in diabetes are two innovative, potentially paradigm-shifting discoveries. Physiologically, GPR91-mediated paracrine signaling pathway in the JGA may serve to modulate glomerular filtration rate and RAS activity in relation to changes in whole body metabolism (especially in the postprandial phase). Pathologically, it could link metabolic diseases (diabetes, metabolic syndrome) with RAS overactivation, systemic hypertension and organ injury. In diabetes, the proximity of CD prorenin synthesis to the (pro)renin receptor [24] establishes a volatile setting for persistent, local RAS activation in the kidney, and end-organ damage. GPR91 and CD (pro)renin are critical new sites to target in the treatment of diabetic complications.
Acknowledgments
This work was supported by NIH grants DK64324 and DK74754 and an American Heart Association Established Investigator award (0640056N) to J.P.P.
Conflict of interest statement. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Ritz E, Dikow R. Hypertension and antihypertensive treatment of diabetic nephropathy. Nat Clin Pract Nephrol. 2006;2:562–567. doi: 10.1038/ncpneph0298. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, et al. for the RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 3.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Thomson SC, Deng A, Bao D, et al. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulhaber-Walter R, Chen L, Oppermann M, et al. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19:722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sällström J, Carlsson PO, Fredholm BB, et al. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 2007;190:253–259. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 7.He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 8.Toma I, Kang JJ, Sipos A, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118:2526–2534. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumbach L, Leyssac PP, Skinner SL. Studies on renin release from isolated superfused glomeruli: effects of temperature, urea, ouabain and ethacrynic acid. J Physiol. 1976;258:243–256. doi: 10.1113/jphysiol.1976.sp011417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966;241:3997–4003. [PubMed] [Google Scholar]
- 11.Correa PR, Kruglov EA, Thompson M, et al. Succinate is a paracrine signal for liver damage. J Hepatol. 2007;47:262–269. doi: 10.1016/j.jhep.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert SC. Physiology: orphan detectors of metabolism. Nature 2004. 2004;429:143–145. doi: 10.1038/429143a. [DOI] [PubMed] [Google Scholar]
- 13.Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Alpern RJ, Hebert SC, editors. The Kidney Physiology and Pathophysiology. Burlington/San Diego/London: Elsevier Academic Press; 2008. pp. 589–626. [Google Scholar]
- 14.Dunn KW, Sandoval RM, Kelly KJ, et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kang JJ, Toma I, Sipos A, et al. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–F502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 16.Peti-Peterdi J, Fintha A, Fuson AL, et al. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 2004;287:F329–F335. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 17.Toma I, Kang JJ, Peti-Peterdi J. Imaging renin content and release in the living kidney. Nephron Physiol. 2006;103:71–74. doi: 10.1159/000090622. [DOI] [PubMed] [Google Scholar]
- 18.Kang JJ, Toma I, Sipos A, et al. Imaging the renin-angiotensin system: an important target of anti-hypertensive therapy. Adv Drug Deliv Rev. 2006;58:824–833. doi: 10.1016/j.addr.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Kang JJ, Toma I, Sipos A, et al. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Carrasquero MC, Botros FT, Pagan J, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen G, Delarue F, Burckle C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen G. Increased cyclooxygenase-2, hyperfiltration, glomerulosclerosis, and diabetic nephropathy: put the blame on the (pro)renin receptor? Kidney Int. 2006;70:618–620. doi: 10.1038/sj.ki.5001723. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Ichihara A, Kaneshiro Y, et al. Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J Am Soc Nephrol. 2007;18:2054–2061. doi: 10.1681/ASN.2006080820. [DOI] [PubMed] [Google Scholar]
- 26.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 27.Komlosi P, Fuson AL, Fintha A, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 28.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]