Abstract
Background. Immunofluorescence (IF) staining of type IV collagen α chains on fresh frozen renal tissue is a convenient and accurate diagnosis technique for Alport syndrome (AS), which is restricted in the application with a non-frozen section. An alternative method for a paraffin-embedded section is needed in order to extend the application in various specimens. In this study, immunohistochemical staining of type IV collagen α chains on paraffin-embedded renal sections was investigated.
Methods. Three antigen retrieval methods including autoclave heating, pepsin digestion and protease digestion were tried on paraffin-embedded renal sections from two X-linked male AS patients, two X-linked female AS patients and two autosomal recessive AS patients. Two patients with isolated haematuria were also involved. Normal portions of nephrectomized kidneys from two patients with renal tumours were used as controls. The immunohistochemical staining pattern of type IV collagen was compared with that of IF staining.
Results. The results showed that both the autoclave heating method and protease digestion can be used for antigen retrieval for type IV collagen α chains. Compared to autoclave heating, protease digestion had the advantage of being less time consuming, with less renal sections being lost from the slides; it was the most optimal antigen retrieval method in this study. After protease digestion for 40 min, type IV collagen α3 and α5 chains showed a clear and proper distribution pattern in AS patients, haematuria patients and controls, which is the same as that of IF staining.
Conclusions. Compared to the autoclave heating method, protease antigen retrieval is more convenient and effective and can be used to restore type IV collagen α chains on paraffin-embedded renal sections for the diagnosis of AS.
Keywords: Alport syndrome, antigen retrieval, diagnosis, paraffin-embedded renal sections, protease digestion
Introduction
Alport syndrome (AS) is a hereditary renal disease manifested by haematuria, sensorineural hearing loss and progressive renal failure; it is caused by mutations of COL4A3, COL4A4 and COL4A5 genes that encode type IV collagen α3, α4 and α5 chains, respectively. Type IV collagen is the main component of the glomerular basement membrane (GBM); it is composed of six α chains, including α1–α6 chains. The gene mutations result in the characteristic abnormal immunofluorescence (IF) staining pattern of type IV collagen α chains on renal tissues [1,2]. The X-linked form of AS is caused by COL4A5 mutations, which results in negative staining of α3 (IV), α4 (IV) and α5 (IV) along the GBM and Bowman's capsule (BC) in male patients and mosaic linear staining of α3 (IV), α4 (IV) and α5 (IV) on GBM and BC in female patients. In autosomal recessive (AR) AS, which is caused by COL4A3 or COL4A4 mutation, the staining of α3 (IV), α4 (IV) and α5 (IV) is negative along the GBM, but the staining of α5 (IV) remains positive along the BC [3]. The type IV collagen α1 chain is widely distributed in the basement membrane irrespective of AS. IF staining of type IV collagen α chains on a renal frozen section has been established for the diagnosis of AS and is applied in practice in our laboratory [1] as well as some other laboratories routinely. However, the method is restricted in the application with non-frozen sections [4]. In addition, some archival paraffin-embedded renal tissues need to be reassessed for the patients who are suspected of having AS. An alternate technique for paraffin-embedded sections, which is more convenient to store and transport, needs to be investigated. To our knowledge, the immunostaining method for collagen IV α chains on paraffin-embedded renal sections is rare; only an autoclave heating antigen retrieval method has been developed in Japan [5]. For immunostaining on a paraffin-embedded section, antigen retrieval is the most crucial step. In this study, we investigated three different antigen retrieval methods including autoclave heating, pepsin digestion and protease digestion in order to find an optimal immunohistochemistry diagnosis technique for AS on paraffin-embedded sections.
Subjects and methods
Specimens
Renal biopsy specimens were obtained from two X-linked AS male patients, two X-linked AS female patients and two autosomal recessive AS patients. The age ranged from 4 to 13 years. The diagnosis of AS was made under the criteria proposed by Flinter [6]. Five patients were diagnosed with AS as well as their inheritance patterns with the IF staining of α(IV) chains on basement membranes and ultrastructural changes of the GBM revealed by electron microscopy. One patient was diagnosed with the IF staining of α(IV) chains on basement membranes and molecular study, and COL4A5 4183 del C was revealed. All patients with AS in this study presented with haematuria and proteinuria. Two patients presented with hypertension and two with mild sensorial hearing loss. Two patients with isolated haematuria (one male and one female) who have been excluded from the diagnosis of AS by gene analysis were also involved; they presented with microscopic haematuria: one glomerular haematuria and one non-glomerular haematuria assessed by urinary red blood cell morphology analysis. Normal portions of nephrectomized kidneys due to renal tumours from two adults were used as controls. Both of them did not have glomerulonephritis, AS and AS family history. The fresh frozen specimens from patients and controls were cut into sections with a thickness of 6 μm. Formalin-fixed specimens were embedded by paraffin and cut into 4-μm sections. This study was approved by the Ethic Committee of Peking University First Hospital.
Antibodies
The murine monoclonal antibodies (Wieslab, Lund, Sweden) against NC1 domain of α1(IV), α3(IV) and α5 (IV) were used.
Antigen retrieval
Three different antigen retrieval methods including autoclave heating, pepsin digestion and protease digestion were investigated on control sections. According to the reports from Natio [5], after removal of paraffin, the sections were put into a 3.65 g/L HCL solution and autoclave (SANYO MLS-3020, Japan) heated at 115°C for 6 min and 121°C for 6 min, respectively. In the pepsin digestion method group, after removal of paraffin, the sections were digested by pepsin (Invitrogen, USA) for 5, 10, 15 and 30 min, respectively, in chambers at 37°C. In the protease digestion group, the sections were incubated with protease (Lab Vision Corporation, USA) for 10, 20, 30 and 40 min, respectively, in chambers at 37°C. The effect of antigen retrieval was compared by the damage of renal sections after staining, staining intensity with different antibodies and the proper distribution pattern of type IV collagen α chains.
Immunoperoxidase staining
After antigen retrieval, the sections were incubated with monoclonal antibodies against type IV collagen α3 and α5 chains, respectively, for 30 min at room temperature; the phosphate buffered saline instead of primary antibodies was used as a negative control and monoclonal antibody against type IV collagen α1 chain as a positive control. After rinsing with phosphate buffered saline, the antigens were detected by a PV 9000 two-step Immuno-histological Staining kit (GBI Labs, Mukilteo, WA, USA) according to the procedure provided by the company.
Indirect IF staining
Indirect IF staining of type IV collagen α chains was performed on the frozen sections according to the procedure reported previously [1,2]. After incubation with primary antibodies and secondary antibodies, the sections were observed under IF microscopy.
Results
The staining pattern of type IV collagen α chains on fresh frozen sections
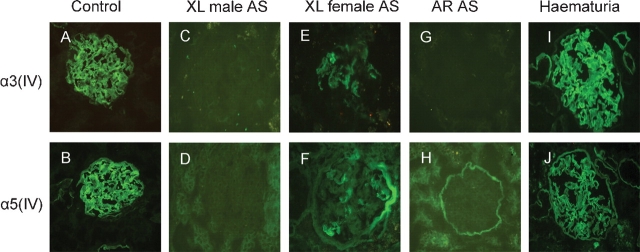
On renal sections from controls, the IF staining of the type IV collagen α3 chain showed a continuous linear pattern along the GBM and tubular basement membrane (TBM) (Figure 1A), negative on BC; the type IV collagen α5 chain showed a continuous linear pattern along the GBM, BC and partial TBM (Figure 1B). The same α3(IV) and α5(IV) staining patterns on renal sections from haematuria patients (Figure 1I and J) as that of controls were revealed. The IF staining of α3(IV) and α5(IV) was negative on the GBM, TBM and BC on sections from X-linked AS male patients (Figure 1C and D). On renal sections from X-linked AS females, the IF staining of α3(IV) showed a mosaic linear pattern along the GBM, partial TBM and negative on BC; α5(IV) showed a mosaic linear pattern along the GBM, partial TBM and BC (Figure 1E and F). On renal sections from autosomal recessive AS patients (Figure 1G and H), IF stainings of α3(IV) and α5(IV) were negative on the GBM, whereas α5(IV) was still positive on the BC and partial TBM. The type IV collagen α1 chain was widely distributed on renal basement membranes in all patients.
Fig. 1.
Immunofluorescence staining of collagen IV α3, α5 chains on fresh frozen renal sections of controls, Alport syndrome patients and haematuria patients (IF, ×400). A, B: The staining of type IV collagen α3 and α5 chains on renal sections from controls. C, D: Type IV collagen α3 and α5 chains on sections from X-linked male Alport syndrome. E, F: Type IV collagen α3 and α5 chains on renal sections of X-linked female Alport syndrome. G, H: Type IV collagen α3 and α5 chains on renal sections of autosomal recessive Alport syndrome. I, J: The staining patterns of type IV collagen α chains on renal sections of haematuria patients.
The distribution of type IV collagen α chains with different antigen retrieval methods (Table 1)
Table 1.
The effect of different antigen retrieval methods on the staining of type IV collagen α chains in control renal specimens
| Autoclave heating | Pepsin digestion | Protease digestion | IF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 115°C | 121°C | 5′ | 10′ | 15′ | 30′ | 10′ | 15′ | 30′ | 40′ | |||
| Integrity of the tissue | Partial lost | Partial lost | Well | Well | Well | Partial lost | Well | Well | Well | Well | Well | |
| α3(IV) | GBM | − | + | − | − | − | ± | ± | ± | + | + | |
| BC | / | / | / | / | / | / | / | / | / | / | / | |
| TBM | ± | + | − | − | − | ± | ± | ± | + | + | ||
| α5(IV) | GBM | − | + | ± | ± | + | ± | ± | ± | + | + | |
| BC | + | − | ± | ± | + | ± | ± | ± | + | + | ||
| TBM | + | + | ± | ± | + | ± | ± | ± | + | + | ||
GBM: glomerular basement membrane; BC: Bowman's capsule; TBM: tubular basement membrane; IF: immunofluorescence.
−: negative; ±: weak positive; +: positive; /: no expression in normal kidneys.
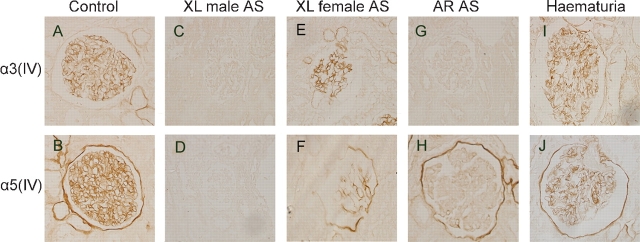
The effects of different antigen retrieval methods were compared by the staining intensity and pattern of type IV collagen α chains, as well as the degree of renal tissue damage after antigen retrieval. By autoclave heating, although both α3(IV) and α5(IV) could be restored like previous reports, two different temperatures were needed to restore two different antigens. Unexpectedly, partial tissue sections (three out of eight sections) were damaged and lost from the slides during the process. After pepsin digestion, only α5(IV) could be restored whereas α3(IV) could not. After protease digestion for 40 min, both α3(IV) and α5(IV) were clearly stained and properly distributed on paraffin-embedded renal sections (Figure 2A and B), which was the same as the result of IF staining on frozen sections, and the tissue was preserved well.
Fig. 2.
Immunostaining of type IV collagen α3 and α5 chains on paraffin-embedded renal sections of controls, Alport syndrome patients and haematuria patients (LM, ×400). A, B: The staining of type IV collagen α3 and α5 chains on renal sections from controls. C, D: Type IV collagen α3 and α5 chains on sections from X-linked male Alport syndrome. E, F: Type IV collagen α3 and α5 chains on renal sections of X-linked female Alport syndrome. G, H: Type IV collagen α3 and α5 chains on renal sections of autosomal recessive Alport syndrome. I, J: The staining patterns of type IV collagen α chains on renal sections of haematuria patients.
The immunostaining of type IV collagen α chains on paraffin-embedded sections from patients
After antigen retrieval by protease digestion for 40 min, immunoperoxidase staining was performed on patients with AS and haematuria. On sections from the X-linked form of AS male patients (Figure 2C and D), both α3 (IV) and α5 (IV) were negative on the GBM and BC. On renal sections from the X-linked form of AS female patients (Figure 2E and F), the staining of α3 (IV) showed a mosaic linear pattern along the GBM and negative on the BC, and α5 (IV) showed a mosaic linear pattern along the GBM and BC. On sections from autosomal recessive AS patients (Figure 2G and H), the staining of α3 (IV) and α5 (IV) were negative on the GBM, but the staining of α5 (IV) was positive along the BC. The staining pattern of type IV collagen α chains on specimens from haematuria was the same as controls (Figure 2I and J). All the immunostaining patterns of type IV collagen α chains on paraffin-embedded renal sections were comparable to that with IF staining.
Discussion
IF staining of type IV collagen α chains on renal sections has been used for diagnosis of AS for >10 years. Although valuable and believable [2], the method is restricted in the application with non-frozen sections. Unlike a paraffin-embedded section, frozen section preparation and microscopy for the IF method need special facilities that are not available in a lot of local hospitals as well as laboratories. Furthermore, the archival renal tissues of suspected patients need to be reassessed for definite diagnosis. Therefore, it is necessary to set up an alternative method of staining type IV collagen α chains on paraffin-embedded sections.
The antigen retrieval step was thought to be as the key process influencing the immunohistochemical staining on paraffin-embedded sections [7]. The common antigen retrieval methods available now include a heating method [8] and enzyme digestion. Among heating methods, the autoclave heating method has been successfully used for diagnosis of AS, but the procedure is time consuming because it needs two different steps to restore different antigens on the GBM and BC separately [5]. Pepsin digestion is a common way of enzyme digestion used for antigen retrieval, which has been successfully used for collagen IV staining in mouse brain [9]. For formalin-fixed specimens, protease digestion was thought to be a better antigen retrieval method; it has been successfully used for other antigen retrieval in renal tissues but not used for type IV collagen [10,11]. The effects of different antigen retrieval methods for collagen IV alpha chains were compared by the tissue integrity, the staining intensity and pattern of the antigen restored. For control specimens, protease digestion of 40 min was the most optimal method for glomerular type IV collagen α chains. Although the results showed that the autoclave heating method can restore type IV collagen α3 and α5 chains and help diagnosis of AS as previously reported, during the antigen retrieval process, some tissues on the specimen (three out of eight sections) were destroyed and lost from the slides. The tissue loss might be due to the heating process [12]. Only alpha 5(IV) can be restored by pepsin digestion antigen retrieval method, the mechanism underlying it is not clear. After protease antigen retrieval at 37°C for 40 min, type IV collagen α3 and α5 chains were restored perfectly, and the staining was clear and properly distributed without tissue loss, which demonstrated the superiority of this method compared to other methods in this study.
To further confirm the effect of the protease antigen retrieval method, we applied it to the paraffin-embedded renal sections from AS patients and haematuria patients; the same results as those with the IF method were achieved. We also repeated the immunostaining on patients with different genotypes of AS, haematuria and control and confirmed the stability of the method for the diagnosis of AS. The antigen retrieval method of protease digestion has been used for other antigen retrievals on renal tissues and was thought to be a better antigen retrieval method for formalin-fixed paraffin-embedded renal sections, but it has not been used in the staining of type IV collagen. For the first time, our results showed that protease digestion could be used for the antigen retrieval of type IV collagen α chains and applied to the diagnosis of AS. Compared with autoclave heating, the protease digestion antigen retrieval method is easy to operate, less time consuming and there is less tissue wastage. This technique can also be used in retrospective analysis on paraffin-embedded specimens and in specimen convenient transportation between different laboratories. Although the sensitivity and specificity of the technique need further confirmation, we conclude that the protease antigen retrieval method can assist in a more convenient diagnosis and even retrospective diagnoses. It provides a new alternative technique for diagnosing AS. Although the method worked well for the antibodies we used in this study, additional tests are needed for other anti-type IV collagen chains antibodies.
Acknowledgments
This study was granted by National Scientific Foundation of Chinese (39770780, 39970775, 30371495, 30400482), and International Collaborative Genetic Research Training Grant, NIH/FIC, D43 TW06176-International Collaborative Genetics Research Training Program. We appreciate the support from all the doctors and nurses who participated in the diagnosis practice of AS in Pediatric Nephrology Department of Peking University First Hospital.
Conflict of interest statement. None declared.
References
- 1.Ding J, Yao Y, Huang J, et al. Detection of type IV collagen α chains in basement membranes for determining the hereditary modes of Alport syndrome. Zhonghua Er Ke Za Zhi. 1999;37:90–93. [Google Scholar]
- 2.Nakanishi K, Yoshikawa N, Iijima K, et al. Immunohistochemical study of alpha 1–5 chains of type IV collagen in hereditary nephritis. Kidney Int. 1994;46:1413–1421. doi: 10.1038/ki.1994.413. [DOI] [PubMed] [Google Scholar]
- 3.Gubler MC, Knebelmann B, Beziau A, et al. Autosomal recessive Alport syndrome: immunohistochemical study of type IV collagen chain distribution. Kidney Int. 1995;47:1142–1147. doi: 10.1038/ki.1995.163. [DOI] [PubMed] [Google Scholar]
- 4.Mölne J, Breimer ME, Svalander CT. Immunoperoxidase versus immunofluorescence in the assessment of human renal biopsies. Am J Kidney Dis. 2005;45:674–683. doi: 10.1053/j.ajkd.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Naito I, Ninomiya Y, Nomura S. Immunohistochemical diagnosis of Alport's syndrome in paraffin-embedded renal sections: antigen retrieval with autoclave heating. Med Electron Microsc. 2003;36:1–7. doi: 10.1007/s007950300000. [DOI] [PubMed] [Google Scholar]
- 6.Flinter F. Alport's syndrome. J Med Genet. 1997;34:326–330. doi: 10.1136/jmg.34.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong AS. Pitfalls in diagnostic immunohistology. Adv Anat Pathol. 2004;11:86–93. doi: 10.1097/00125480-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita S. Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem. 2007;41:141–200. doi: 10.1016/j.proghi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Franciosi S, De Gasperi R, Dickstein DL, et al. Pepsin pretreatment allows collagen IV immunostaining of blood vessels in adult mouse brain. J Neurosci Methods. 2007;163:76–82. doi: 10.1016/j.jneumeth.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr SH, Galgano SJ, Markowitz GS, et al. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70:2148–2151. doi: 10.1038/sj.ki.5001990. [DOI] [PubMed] [Google Scholar]
- 11.Miura M, Tomino Y, Suga T, et al. Evaluation of the staining findings of immunofluorescence in unfixed or fixed renal biopsy specimens from patients with IgA nephropathy and membranous nephropathy. Acta Pathol Jpn. 1985;35:315–321. doi: 10.1111/j.1440-1827.1985.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 12.Frost AR, Sparks D, Grizzle WE. Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl Immunohistochem Mol Morphol. 2000;8:236–243. doi: 10.1097/00129039-200009000-00011. [DOI] [PubMed] [Google Scholar]