Abstract
Objective
To assess the relationship of empathy in medical consultations to subsequent cold outcomes.
Methods
350 subjects, ≥12 years of age received either a standard or enhanced physician visit as part of a randomized controlled trial. The patient-scored Consultation and Relational Empathy (CARE) questionnaire assessed practitioner-patient interaction, especially empathy. Cold severity and duration were assessed from twice daily symptom reports. Nasal wash was performed to measure the immune cytokine IL-8.
Results
84 individuals reported perfect (score of 50) CARE scores. They tended to be older with less education, but reported similar health status, quality of life, and levels of optimism. In those with perfect CARE scores, cold duration was shorter (mean 7.10 days vs. 8.01 days, p=0.032), and there was a trend towards reduced severity (mean AUC 240.40 vs. 284.49, p=0.118). After accounting for possible confounding variables, cold severity and duration were significantly lower in those reporting perfect CARE scores (p=0.037 and p=0.017 respectively). In these models, a perfect score also correlates with a larger increase in IL-8 levels (p=0.015).
Conclusions
Clinician empathy, as perceived by patients with the common cold, significantly predicts subsequent duration and severity of illness and is associated with immune system changes.
Keywords: CARE, cold, empathy, practitioner-patient interaction
BACKGROUND AND OBJECTIVES
Practitioner-patient interactions have been discussed at length in the literature 1–3, but there is limited data on their specific influence on medical outcomes, particularly regarding patients’ perceptions of empathy in clinical encounters. However, there is evidence that empathy is a positive component in practitioner-patient interactions. In 2001, Di Blasi et al. published a systematic review of “context effects on health outcomes” that looked at influences of the practitioner-patient interaction. A summary of 25 randomized trials stated, “One relatively consistent finding is that physicians who adopt a warm, friendly, and reassuring manner are more effective than those who keep consultations formal and do not offer reassurance.”4
The feeling of empathy between practitioner and patient includes a combination of complex interactions that are difficult to define. Mercer 5 defines empathy as the ability: to understand the patient’s situation, perspective and feelings (and their attached meanings); to communicate that understanding and check its accuracy; and to act on that understanding with the patient in a helpful (therapeutic) way. Empathy is a foundational ingredient that defines holistic, relationship-centered care that is the essence of the bio-psycho-social model in primary care.6,7 Preliminary research on the health benefits of empathy in the therapeutic encounter shows promising correlations with satisfaction, compliance and enablement.8–10 Further data and better methodology are needed to assess potential influences on medical outcomes.
Our objective was to assess the relationship of empathy in the medical consultation to subsequent cold outcomes. This paper will present interim data from the “PEP trial” (Physician, Echinacea and Placebo: A Randomized Controlled Trial in a Common Cold Model) related to the association between empathy in clinical encounters and the subsequent duration and severity of the common cold.
METHODS
This study was approved by the Health Sciences IRB at the University of Wisconsin-Madison. The larger PEP trial was designed to assess pill-related placebo effects and practitioner- patient interaction effects on the common cold. Articles describing conceptual framework11 and methodology12 for this trial are available.
Subjects and setting
The study took place in two clinics: a primary care clinic in Verona, Wisconsin, and the Employee Health Clinic at St. Mary’s Hospital in Madison, Wisconsin. Study clinicians received special training for this research and were not the subjects’ primary care providers.
Individuals were eligible to be enrolled in the study if they were at least 12 years of age and answered “yes” to “Do you think that you have a cold?” They also needed to report one of the following four symptoms: 1) nasal discharge (runny nose), 2) nasal obstruction (nasal congestion, stopped up nose, stuffiness), 3) sneezing, and 4) sore throat (raw throat, scratchy throat). They were not eligible if any of these symptoms arose more than 36 hours prior to the intake evaluation.
Instruments and measures
The Consultation and Relational Empathy (CARE) measure is a questionnaire designed to measure several aspects of the clinical encounter related to empathy.13,14 CARE assesses empathy from the patient’s perspective and has been validated in the primary care setting. Patients completed the CARE measure once, directly following their clinician visits at the time of enrollment. CARE assesses 10 areas of consultations to see if clinicians: 1) made them feel at ease, 2) allowed them to “tell their story,” 3) really listened, 4) were interested in them as a whole person, 5) fully understood their concerns, 6) showed care and compassion, 7) were positive, 8) explained things clearly, 9) helped them take control, and 10) helped create a plan of action. For each item, clinicians were rated on a scale from 1–5, from poor to excellent. Ratings were summed to produce a possible CARE score range from 0–50, 50 being a perfect score.13 Clinicians were also asked to rate the practitioner-patient interaction with two questions assessing how much they liked the patient and how connected they felt to the patient. Both questions were answered on a 0–100 visual analog scale (VAS).
Outcome measures collected assess the self-rated severity of the common cold, illness duration from enrollment in the study, and the individuals’ immune responses. The Wisconsin Upper Respiratory Symptom Survey (WURSS-44)15,16 was self-reported by the patient at enrollment. A shorter version, the WURSS-21, was reported twice daily to assess severity over time. Overall cold severity was determined as the area under the time-severity curve (AUC) delineated by the WURSS-21 scores. Duration was assessed from enrollment as the number of days the individual continued to report “yes” to, “Do you think you still have a cold?” before reporting two consecutive “no” responses to this question. If the individual still reported a cold on the last day of the study (day 14), the duration of the cold totaled 14 days. Concentration of IL-8 was assessed from nasal wash at enrollment and at a follow-up visit approximately 48 hours later. IL-8, an inflammatory cytokine found in nasal secretions, rises rapidly with viral upper respiratory infection, then falls over days to weeks. It is one of the best single markers of immune response in community-acquired upper respiratory infection, as it correlates well with symptoms, and can be reliably measured.
Study participants filled out questionnaires assessing their level of optimism (LOT)17 and perceived stress (PSS-4)18 at enrollment and again two days later. Health-related quality of life was assessed every day with the physical and mental subscales of the SF-819 and with the feeling thermometer, a 100mm visual analogue scale derived from the EuroQol.20 The first item of the WURSS (“How sick do you feel today?”) was also considered to indicate overall health and was analyzed separately. These measures and several demographic variables were used to assess possible differences between individuals reporting high or lower CARE scores. (Table 1)
Table 1.
Evaluation Tools
| Study Tool | What the Tool Measured | Format | Result (sum score/range) |
|---|---|---|---|
| CARE Questionnaire | Perceived Empathy | 10 questions Likert scale |
10–50 |
| Clinician Questionnaire | How much clinician “liked” and felt “connected to” the patient. | 2 questions Visual Analog Scale |
0–100 |
| WURSS-21 (AUC) | Severity and Duration of the Cold | WURSS-21: 21 questions assessed twice daily Likert scale |
0–3920 |
| IL-8 | Cytokine associated with immune response | Biological marker for inflammation | 15.6 pg/mL to no upper limit |
|
| |||
| Tools to Assess Confounding Variables | |||
|
| |||
| LOT | Optimism | 6 questions Likert scale |
0–24 |
| PSS | Perceived stress | 4 questions Likert scale |
0–16 |
| SF-8 (MCS, PCS) | Quality of Life (Mental and Physical) | 8 questions Likert scale |
MCS 17.9–59.3 PCS 19.5–58.6 |
| WURSS-21 | Baseline Health | 21 questions Likert scale |
0–140 |
| “How Sick Today?” | Baseline Sickness | 1 question Likert scale |
0–7 |
| Feeling Thermometer | Assessment of Personal Health | 1 question Visual analog scale |
0–100 |
CARE (Consultation and Relational Empathy Questionnaire)
WURSS (Wisconsin Upper Respiratory Symptom Survey). AUC (Area-under-the-curve)
IL-8 (Interleukin-8)
LOT (Life Orientation Test)
PSS (Perceived Stress Scale)
Only two individuals were lost to follow-up. They were included in the analysis of baseline differences but were excluded from analysis of cold outcomes
Procedures
Members of the general public were recruited through multiple methods: advertisements in daily and weekly newspapers, flyers, e-mails, word-of-mouth, and a website. These materials noted the importance of telephoning study staff at the first sign of a cold. Study staff screened callers, scheduled appointments, distributed surveys, and performed nasal washes. Subjects came in three times: at the start of their colds, 48 hours later, and when their colds had ended. They saw a study clinician once—at the first visit.
Participants were randomized to: I) no practitioner-patient interaction, II) “standard” practitioner-patient interaction, and III) “enhanced” practitioner-patient interaction. Statistical staff prepared random assignments; clinicians learned which arm was assigned from the study packet s/he picked up upon entering an examining room. For the “no interaction” group, subjects saw study staff, but were not seen by a clinician. The “standard” visit included the basic ingredients of a clinical encounter including history and present illness, relevant past medical history, focused physical exam and diagnosis. The “enhanced” visit included the above with five added ingredients of the practitioner-patient interaction hypothesized to have a positive effect on health. These can be summarized using the PEECE mnemonic: (P) Positive prognosis, (E) Empathy, (E) Empowerment, (C) Connection and (E) Education. These domains were chosen because of their prominence in the practitioner-patient interaction literature. 21–41 For this paper, only the empathy scores for the “standard” or “enhanced” visits were evaluated. At the time of submission, the study was still blinded, and we were not able to show the distribution of CARE scores associated with each of these randomized groups.
Six clinicians were involved in the study (5 family physicians and 1 family nurse practitioner). Two were women (MD, FNP), and the rest were male. Each of the clinicians completed training from an acting coach to establish common reproducible behaviors assigned to a standard or an enhanced visit. The foundational mind-set that the clinician was taught before entering the room was to create a different degree of “connectedness,” depending on the participant’s assignment to a standard or enhanced visit. For the standard encounter, the practitioner tried to prevent a connection from forming by keeping the visit short, with limited touch and eye-contact. The enhanced visit focused on creating a connection or bond to enhance the interaction, with emphasis on the domains embodied in the (P.E.E.C.E) acronym described above. In short, the enhanced visit attempted to “stack the deck” in favor of utilizing non-specific variables to facilitate health and healing.
Data analyses
Students’ t-tests and chi square tests were used to assess potential differences between those reporting higher and lower scores on the CARE measure. Linear regression assessed the relationship between the dichotomous CARE measure and two of the outcomes: AUC cold severity and the changes in IL-8 value between baseline and the 48-hour follow-up visit. There was no graduated dose correlation between outcome measures and CARE scores except when the results were dichotomized to perfect (50) and non-perfect (<50) CARE scores. A Cox-proportional hazard model assessed the duration of the cold by looking at the rate at which colds are ending based on level of the CARE measure. All regression models were adjusted for potential confounders, specifically age, gender, race, education, optimism, perceived stress, and time from first symptom to enrollment. Further description of potential confounders can be found in Tables 1 and 2.
Table 2.
Group differences at enrollment based on perfect CARE score
| Perfect score n=84 | < Perfect score n=266 | ||
|---|---|---|---|
| mean (sd) or % | mean (sd) or % | p value | |
| Demographic | |||
| Age | 37.32 (16.17) | 33.69 (14.12) | 0.052 |
| % Male | 34.1 | 36.5 | 0.694 |
| % Caucasian | 84.7 | 93.6 | 0.011 |
| Education (%) | |||
| < High school | 6.49 | 6.00 | |
| High school | 20.8 | 5.60 | |
| Some college | 39.0 | 29.6 | |
| College/post- graduate | 33.8 | 58.8 | <0.001 |
| Health status | |||
| WURSS-21 | 45.39 (26.67) | 44.82 (23.38) | 0.859 |
| “How sick?” | 3.73 (1.48) | 3.56 (1.32) | 0.129 |
| Feeling Therm. | 61.72 (22.00) | 62.17 (18.33) | 0.851 |
| SF-8 (MCS) | 48.11 (9.54) | 48.18 (8.75) | 0.950 |
| SF-8 (PCS) | 43.64 (9.88) | 43.85 (7.88) | 0.840 |
| Psychosocial | |||
| Optimism | 22.98 (4.23) | 22.57 (3.82) | 0.415 |
| Perceived stress | 4.65 (3.33) | 5.26 (3.01) | 0.108 |
RESULTS
In evaluating the data, we found that patients who gave their clinical encounters perfect CARE scores at baseline subsequently had shorter duration and a trend to lower severity of their colds. Somewhat surprisingly, this predictive association did not seem to hold across CARE scores below the perfect score level. Based on these findings, we reviewed the data of “perfect-score” (84) and “non-perfect-score” (264) responders. (Figure 1) (Two “non-perfect-score” responders were lost to follow-up.)
Figure 1.
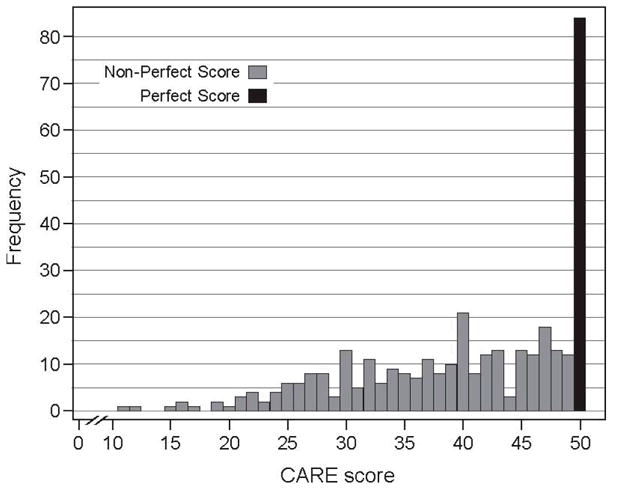
Graph of CARE score distribution (n=350): Perfect score (n=84) versus non-perfect score (n=266)
Perfect score individuals are typically older, report less than college/post graduate education, are more racially diverse, and tend to report lower perceived stress, although the latter is a non-significant trend. (See Table 2). Individuals reporting perfect CARE scores do not differ from other participants on several important baseline characteristics. These groups have a similar male/female ratio and rate themselves similarly on optimism as assessed via the Life Orientation Test (LOT)42 and on several baseline reports of health status including WURSS-21 Feeling Thermometer rating and the SF-8.™ (Table 2)
Because the larger PEP study remained blinded and we were unable to correlate CARE scores with the standard or enhanced groups, we evaluated the clinician ratings of “liking” and “feeling connected to” the patients to see if this data correlated with perfect CARE scores. Clinicians reported liking and feeling more connected to those patients who gave their encounters perfect CARE scores (77.7 versus 72.4, p 0.002 and 69.0 versus 59.8, p <0.001 respectively). Each of the six clinicians in the study saw a comparable proportion of individuals reporting perfect CARE scores, suggesting that a perfect score is not correlated with one clinician being naturally more empathetic than another.
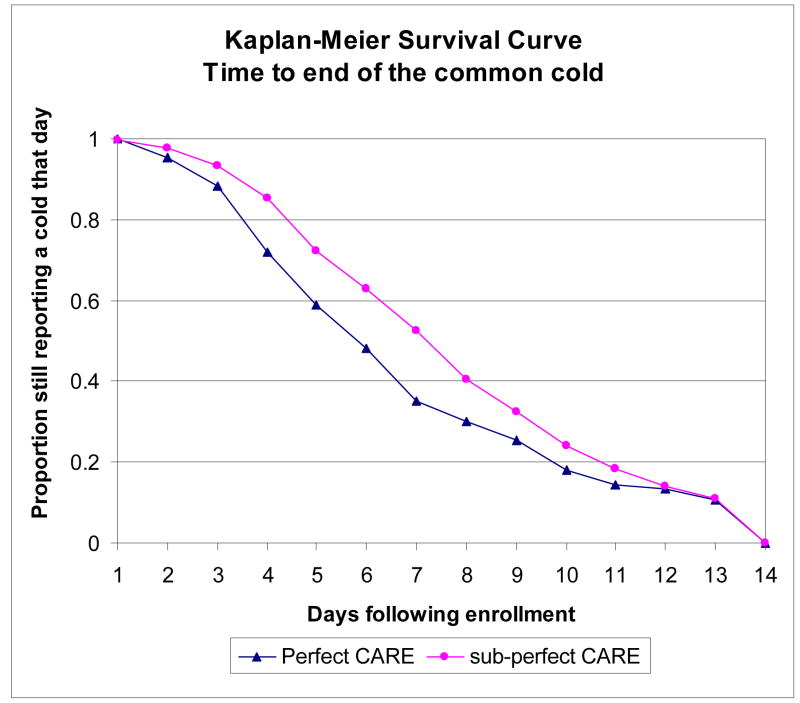
Cold outcomes are more favorable among individuals reporting perfect CARE scores in data that is unadjusted for differences (First 2 columns, Table 3). Analysis of AUC scores shows lower overall severity colds among those subjects giving perfect CARE scores (mean 240.40 versus 284.49), but the difference is not statistically significant. The mean cold duration from time of enrollment is almost one day shorter among this group (mean 7.10 versus 8.01, p 0.032). Figure 2 also highlights this difference in cold duration from enrollment to the end of their cold. The mean change in IL-8 from baseline to follow-up is higher in those reporting perfect scores (mean change 562.18 versus 220.14, p=0.180), though this difference is not statistically significant.
Table 3.
Group cold outcome differences based on perfect CARE score
| Perfect score n=84 | < Perfect score n=264 | |||||
|---|---|---|---|---|---|---|
| [1] | [2] | [3] | [4] | [5] | [6] | |
| Cold outcome | mean (sd) | mean (sd) | p value | Adjusted difference in meansa | p value adjusted for confounding variablesa | 95% CI |
| Duration | 7.10 days (3.50) | 8.01 days (3.36) | 0.032 | 0.375* | 0.017 | (0.068 – 0.682)† |
| Severity | 240.40 (226.31) | 284.49 (219.88) | 0.118 | − 66.58 | 0.037 | (−129.24 – −3.918)† |
| Change in IL-8 | 562.18 (2871.66) | 220.14 (1602.60) | 0.180 | 663.52 | 0.015 | (127.72 – 1199.32)† |
[ ] Column number
Cox proportional hazard model coefficient represents a % higher rate at which colds are ending
Adjusting for age, gender, race, education, optimism, perceived stress, hours since first symptom and squared hours since first symptom
p < 0.05
Figure 2.
Log rank test for equality of survivor functions p value 0.0803.
This unadjusted graphic of time post-enrollment until the end of the individual’s cold shows the proportion with a cold is typically less within the perfect CARE score group when compared to the non-perfect CARE score groups.
Analyses were adjusted for potential demographic and psychosocial confounders. Results indicate that AUC cold severity is milder (p value 0.037), and colds are ending sooner (p value 0.017) among those reporting perfect CARE scores (Columns 4 and 5, Table 3).
Adjusted analysis of change in IL-8 values between baseline and 48 hours post-enrollment also shows a significantly larger rise for those individuals reporting perfect CARE scores (p value 0.015). One IL-8 value determined too high for accurate measurement was winsorized43 to take the value of the next highest value in these analyses. When this outlier was included in a censored regression, study results did not differ.
DISCUSSION
A review of CARE scores measuring the effects of empathy in the clinical encounter found that perfect scores predicted the duration of subsequent illness. Although not statistically significant, perfect scores also showed a trend towards improved severity and the degree of rise of IL-8 levels from nasal wash. Including potential confounders did not reduce the strength of the observed predictive relationship.
A patient rating of a perfect score on the CARE instrument at the time of consultation was associated with a 0.91 day (7.10 vs. 8.01) shorter duration of illness, and trends in severity and IL-8: a 16% reduction in overall cold severity (AUC 240.4 vs. 284.5), and more than double the rise in the immune biomarker IL-8 (562.2 vs. 220.1).
To help determine if these associations are the result of clinician empathy or the unique characteristics of patients giving perfect scores, we looked at individual characteristics. Those who gave perfect scores were more likely to be older, non-white, and to have completed less education. However, these attributes did not predict illness severity or duration. We hypothesized that those who were more optimistic or reported less stress would be more likely to give perfect scores. When stress and optimism were included, a perfect CARE score was still associated with a shorter cold and less severe symptoms. Reporting a perfect CARE score did not significantly correlate with quality of life ratings (SF-8) or ratings of personal health (i.e. Feeling Thermometer, How sick are you?) at enrollment. We were unable to find unique characteristics of individuals giving perfect scores that could account for the associations with illness duration, severity and IL-8 levels. Even after controlling for possible confounders, the association of a perfect CARE score with reduced duration and severity remains (Table 3).
We therefore conclude that the empathetic interaction between patient and clinician may indeed influence subsequent illness.
Why did these changes only occur within patients who gave perfect CARE scores? We were not able to correlate sub-perfect scores with these outcomes, hence a graduated “dose-response relationship” was not seen. A possible explanation for this lack of dose-response is that if a “connection” happened in the consultation, it is likely that the patient gave a perfect score. Anything less than perfect may not correlate with a “connection” taking place where empathy was expressed or felt. This may suggest that the perception of empathy by patients may be more of an “on or off” phenomenon than a graduated response. We either feel empathy or we don’t.
The perfect CARE score group was also associated with a more robust IL-8 response at 48 hours after intake than those who gave a non-perfect score. Inflammatory/immune cytokines (i.e., IL-8) released in response to viral infections indicate an active immune response to the offending organism. 44–46 Our observation that IL-8 rises faster among those giving perfect CARE scores links the subjective rating of a positive emotion (empathy) with an objective measure (IL-8). Specific negative emotional states such as stress and depression can increase susceptibility to the common cold, suggesting that negative emotions may have a negative effect on immune function.47–49 Data presented here suggests that a consultation rated high in empathy, a positive emotion, is associated with an enhanced immune response and a shorter illness. These findings suggest that IL-8 holds promise for future research in relation to empathy’s effect on immune pathways.
The positive benefits of perceived empathy on the common cold can be put into perspective when compared to anti-viral studies showing similar modest effect sizes. In order for a drug to be beneficial for a self-limited illness such as the common cold, it needs to show efficacy, ease of dosing and few side effects.50 The best effect from a drug studied to date (pleconaril) also reduced the duration by about one day but worked only for picornavirus-associated colds and caused nausea and diarrhea.50,51 Empathy has a similar effect without side effects in all-cause colds and was found beneficial after only one dose of human empathy.
The findings here are tentative and in need of replication. Until then, including empathy in the clinical encounter has little potential for harm and has positive influences that extend beyond the medical consultation. A “connection” also enhances continuity and builds a foundation for relationship-centered primary care within the patient’s medical home.
LIMITATIONS
While the data presented here come from a randomized controlled trial, allocation remains concealed at the time of writing. Therefore, this study is best described as a prospective cohort, and hence while associations and predictive capacity can be assessed, we can in no way claim to assess causality. This study mainly looked at the CARE score that assesses the clinician-patient interaction and empathy. Since we can only interpret findings as associations, another interpretation is that the patients who feel better or have had less severe colds also perceive and transmit greater empathy and connectedness with their clinicians. A challenge in this work is disentangling the effects of physical status from reported empathy.
An additional limitation is that neither the clinical setting nor the provider was one with whom the patient already had a relationship. All clinicians were new to the patients. Prior relationships with practitioners who knew them well may have further enhanced the positive effects.
Acknowledgments
Financial support: This article describes the methods and rationale for an ongoing randomized controlled trial sponsored by the National Center for Complementary and Alternative Medicine at the National Institutes of Health (NIH NCCAM 1-R01-AT-1428). The University of Wisconsin School of Medicine and Public Health and the University of Wisconsin Department of Family Medicine have also invested substantially in this trial, particularly in the support of Drs. Rakel, Barrett, and Craig. Support for Dr. Barrett’s conception of the original trial came from a K-23 career development grant from NIH NCCAM and a career development grant from the Robert Wood Johnson Foundation Generalist Physician Scholars Program. The second author is supported by pre-doctoral training grant T32 HS000083 from the Agency for Healthcare Research and Quality (AHRQ).
Special thanks to Charlene Luchterhand in helping prepare the manuscript and co-investigators: Lucille Marchand, David Rabago, Randy Brown, Jo Sheder, Raandi Schmidt, Gay Thomas and Shari Barlow. Thanks also to Steward Mercer for reviewing the manuscript. Lastly, we appreciate the hundreds of trial participants who graciously gave of their time, energy, and good will to support medical research during a time of illness.
Abbreviations
- RCT
randomized controlled trial
- CARE
Consultation and Relational Empathy
- AUC
Area-under-the-curve
- WURSS-21
Wisconsin Upper Respiratory Symptom Survey
- IL-8
interleukin-8
- PEP trial
Physician, Echinacea and Placebo: A Randomized Controlled Trial in a Common Cold Model
- FNP
family nurse practitioner
- VAS
Visual analog scale
- LOT
Life Orientation Test
- PSS
Perceived Stress Scale
- SF-8
Short Form-8 Health Survey
Footnotes
Prior presentation: Brief presentation (“Practitioner Empathy and the Common Cold”) by the second author at the Wisconsin Research and Education Network (WREN) meeting on November 1, 2007, in Wisconsin Dells, WI.
Conflicts of interest: None of the authors have potential conflicts of interest.
References
- 1.Covington H. Caring presence. Delineation of a concept for holistic nursing. J Holist Nurs. 2003;21(3):301–17. doi: 10.1177/0898010103254915. [DOI] [PubMed] [Google Scholar]
- 2.Beach MC, Inui T. Relationship-Centered Care Research Network. Relationship-centered care. A constructive reframing. J Gen Intern Med. 2006;21 (Suppl 1):S3–8. doi: 10.1111/j.1525-1497.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrell-Carrio F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004;2(6):576–82. doi: 10.1370/afm.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357(9258):757–62. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]
- 5.Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract. 2002;52 (Suppl):S9–12. [PMC free article] [PubMed] [Google Scholar]
- 6.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–36. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 7.Novack DH. Realizing Engel’s vision: Psychosomatic medicine and the education of physician-healers. Psychosom Med. 2003;65(6):925–30. doi: 10.1097/01.psy.0000099944.98748.fc. [DOI] [PubMed] [Google Scholar]
- 8.Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237–51. doi: 10.1177/0163278704267037. [DOI] [PubMed] [Google Scholar]
- 9.Bikker AP, Mercer SW, Reilly D. A pilot prospective study on the consultation and relational empathy, patient enablement, and health changes over 12 months in patients going to the Glasgow Homoeopathic Hospital. J Altern Complement Med. 2005;11(4):591–600. doi: 10.1089/acm.2005.11.591. [DOI] [PubMed] [Google Scholar]
- 10.Price S, Mercer SW, MacPherson H. Practitioner empathy, patient enablement and health outcomes: a prospective study of acupuncture patients. Patient Educ Couns. 2006;63(1–2):239–45. doi: 10.1016/j.pec.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Barrett B, Muller D, Rakel D, Rabago D, Marchand L, Scheder JC. Placebo, meaning, and health. Perspect Biol Med. 2006;49(2):178–98. doi: 10.1353/pbm.2006.0019. [DOI] [PubMed] [Google Scholar]
- 12.Barrett B, Rakel D, Chewning B, et al. Rationale and methods for a trial assessing placebo, echinacea, and doctor-patient interaction in the common cold. Explore (NY) 2007;3(6):561–72. doi: 10.1016/j.explore.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Mercer SW, Maxwell M, Heaney D, Watt GC. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy-based consultation process measure. Fam Pract. 2004;21(6):699–705. doi: 10.1093/fampra/cmh621. [DOI] [PubMed] [Google Scholar]
- 14.Mercer SW, McConnachie A, Maxwell M, Heaney D, Watt GC. Relevance and practical use of the consultation and relational empathy (CARE) measure in general practice. Fam Pract. 2005;22(3):328–34. doi: 10.1093/fampra/cmh730. [DOI] [PubMed] [Google Scholar]
- 15.Barrett B, Brown R, Mundt M, et al. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58(6):609–17. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett B, Locken K, Maberry R, et al. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Fam Pract. 2002;51(3):265. [PubMed] [Google Scholar]
- 17.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol. 1994;67(6):1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 18.Cohen, Sheldon, Williamson . Perceived stress in a probability sample of the United States. In: Spacapan, Shirlynn, Oskamp, Stuart, editors. The Social Psychology of Health. Newbury Park, CA: Sage Publications; 1988. pp. 31–67. [Google Scholar]
- 19.Ware JE, Kosinski M, Dewey JE, Gandek B, editors. How to Score and Interpret Single-Item Health Status Measures: a Manual for Users of the SF-8 Health Survey. Lincoln, RI: Quality Metric; 2001. [Google Scholar]
- 20.Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St. George’s respiratory questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol. 2003;56(12):1170–76. doi: 10.1016/s0895-4356(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh Y. The Hawthorne experiments and the introduction of Jean Piaget in American industrial psychology, 1929–1932. Hist Psychol. 2002;5(2):163–89. doi: 10.1037/1093-4510.5.2.163. [DOI] [PubMed] [Google Scholar]
- 22.Starfield B, Wray C, Hess K, Gross R, Birk PS, D’Lugoff BC. The influence of patient-practitioner agreement on outcome of care. Am J Public Health. 1981;71(2):127–31. doi: 10.2105/ajph.71.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prev Control. 1999;3(1):25–30. [PubMed] [Google Scholar]
- 24.Suchman AL, Markakis K, Beckman HB, Frankel R. A model of empathic communication in the medical interview. JAMA. 1997;277(8):678–82. [PubMed] [Google Scholar]
- 25.Thomas KB. The consultation and the therapeutic illusion. Br Med J. 1978;1(6123):1327–28. doi: 10.1136/bmj.1.6123.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36(8):1138–61. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Roter DL, Hall JA. Doctors Talking with Patients: Patients Talking with Doctors: Improving Communication in Medical Visits. Westport CT: Auburn House; 1992. [Google Scholar]
- 28.Flood AB, Lorence DP, Ding J, McPherson K, Black NA. The role of expectations in patients’ reports of post-operative outcomes and improvement following therapy. Med Care. 1993;31(11):1043–56. doi: 10.1097/00005650-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–11. doi: 10.1136/bmj.323.7318.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. CMAJ. 2001;165(2):174–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Petrie KJ, Weinman J, Sharpe N, Buckley J. Role of patients’ view of their illness in predicting return to work and functioning after myocardial infarction: longitudinal study. BMJ. 1996;312(7040):1191–4. doi: 10.1136/bmj.312.7040.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryff CD. Positive mental health. In: Blechman EA, Brownell KD, editors. Behavioral Medicine and Women: a Comprehensive Handbook. New York: Guilford Publications, Inc; 1998. pp. 183–8. [Google Scholar]
- 33.Savage R, Armstrong D. Effect of a general practitioner’s consulting style on patients’ satisfaction: a controlled study. BMJ. 1990;301(6758):968–70. doi: 10.1136/bmj.301.6758.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roter DL, Hall JA. Patient-provider communication. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Health Education: theory, Research and Practice. San Francisco: Jossey-Bass, A Wiley Company; 1997. pp. 206–26. [Google Scholar]
- 35.Evans RG, Barer ML, Marmor TR. Why are some People Healthy and Others Not? the Determinants of Health of Populations. Hawthorne, NY: Aldine de Gruyter; 1994. [Google Scholar]
- 36.Marmot MG. Social differentials in health within and between populations. Daedalus: Journal of the American Academy of Arts and Sciences. 1994;123:197–216. [Google Scholar]
- 37.McKnight JL. Health and empowerment. Can J Public Health. 1985;76 (Suppl 1):37–8. [PubMed] [Google Scholar]
- 38.Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Ann N Y Acad Sci. 1999;896:96–115. doi: 10.1111/j.1749-6632.1999.tb08108.x. [DOI] [PubMed] [Google Scholar]
- 39.Guadagnoli E, Ward P. Patient participation in decision-making. Soc Sci Med. 1998;47(3):329–39. doi: 10.1016/s0277-9536(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 40.Suchman AL, Matthews DA. What makes the patient-doctor relationship therapeutic? Exploring the connexional dimension of medical care. Ann Intern Med. 1988;108(1):125–30. doi: 10.7326/0003-4819-108-1-125. [DOI] [PubMed] [Google Scholar]
- 41.Matthews DA, Suchman AL, Branch WT., Jr Making “connexions”: enhancing the therapeutic potential of patient-clinician relationships. Ann Intern Med. 1993;118(12):973–7. doi: 10.7326/0003-4819-118-12-199306150-00010. [DOI] [PubMed] [Google Scholar]
- 42.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4(3):219–47. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 43.Tsay JY, Chen IW, Maxon HR, Heminger L. A statistical method for determining normal ranges from laboratory data including values below the minimum detectable value. Clin Chem. 1979;25(12):2011–14. [PubMed] [Google Scholar]
- 44.Klemens C, Rasp G, Jund F, et al. Mediators and cytokines in allergic and viral-triggered rhinitis. Allergy Asthma Proc. 2007;28(4):434–41. doi: 10.2500/aap.2007.28.3017. [DOI] [PubMed] [Google Scholar]
- 45.Stoll D. Inflammatory acute rhinosinusitis. Presse Med. 2001;30(39–40 Pt 2):33–40. [PubMed] [Google Scholar]
- 46.Barrett B, Brown R, Voland R, Maberry R, Turner R. Relations among questionnaire and laboratory measures of rhinovirus infection. Eur Respir J. 2006;28(2):358–63. doi: 10.1183/09031936.06.00002606. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S. Keynote presentation at the Eight International Congress of Behavioral Medicine: the Pittsburgh common cold studies: Psychosocial predictors of susceptibility to respiratory infectious illness. Int J Behav Med. 2005;12(3):123–31. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Coe CL, Laudenslager ML. Psychosocial influences on immunity, including effects on immune maturation and senescence. Brain Behav Immun. 2007;21(8):1000–8. doi: 10.1016/j.bbi.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patick AK. Rhinovirus chemotherapy. Antiviral Res. 2006;71(2–3):391–6. doi: 10.1016/j.antiviral.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayden FG, Herrington DT, Coats TL, et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36(12):1523–32. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]