Abstract
Fatty acid binding protein (FABP) 4 chaperones free fatty acids (FFA) in the adipocytes during lipolysis. Serum FFA relates to Metabolic Syndrome (METS) and serum FABP4 is emerging as a novel risk marker. In 36 overweight/obese women, serum FABP4 and FFA were measured hourly during 5-hour oral glucose tolerance test (OGTT). Insulin resistance was determined using frequently sampled intravenous GTT (FS-IVGTT). Serum lipids and inflammation markers were measured at fasting.
During OGTT, serum FABP4 decreased by 40%, reaching its nadir at 3h (from 45.3±3.1 to 31.9±1.6 ng/mL) and stayed below the baseline at 5 h (35.9±2.2 ng/mL) (p < 0.0001 for both, compared to the baseline). Serum FFA decreased by 10 fold, reaching a nadir at 2h (from 0.611±0.033 to 0.067±0.004 mmol/L), then rebounded to 0.816±0.035 mmol/ L at 5h (p < 0.001 for both, compared to baseline). Both fasting-FABP4 and nadir-FABP4 correlated with obesity. Nadir-FABP4 correlated also with insulin resistance parameters from FS-IVGTT and with inflammation. Nadir-FFA, but not fasting-FFA, correlated with the METS-parameters.
In conclusion, fasting-FABP4 related to metabolic risk markers more strongly than fasting-FFA. Nadir-FABP4 and nadir-FFA measured after glucose loading may provide better risk assessment than the fasting values.
Insulin resistance, hyperinsulinemia, impaired glucose tolerance or diabetes, hypertension, elevated triglyceride and low HDL concentrations comprised the original definition of metabolic syndrome (METS) (1). Since then, several other abnormalities of coagulation, vascular function and inflammation have been associated with this syndrome (2). New evidence from population studies and experimental animal models indicate that serum fatty acid binding protein 4 (FABP4) is a powerful new risk marker for predicting METS and atherosclerosis (3, 4). In Caucasian and Asian populations, serum FABP4 correlated directly with obesity, insulin resistance and dyslipidemia. In Asians, FABP4 levels prospectively predicted incidence of the METS developing over the next 5 years. This was independent of adiposity and insulin resistance (5, 6). In a predominantly Caucasian population, the T-87C polymorphism in the FABP4 gene was associated with lower plasma triglyceride concentrations and decreased risk of risk for coronary heart disease (7).
The exact role of FABP4 in METS is not clear. Experimental evidence supports that FABP4 plays mechanistic roles in obesity, insulin resistance and atherogenesis. FABP4 is expressed both in the adipose tissue and macrophages and thus may integrate the metabolic and inflammatory responses that lead to atherosclerosis (3, 8): Mice deficient of FABP4 were resistant to diet induced insulin resistance despite gaining weight (9). Treatment with a pharmacological inhibitor of FABP4 improved insulin sensitivity in both genetic and diet induced mouse models (10). Elimination of FABP4 from macrophages protected Apo-E deficient mice from diet induced atherosclerosis without improving insulin resistance (11).
This study was designed to test the null hypothesis that there will be no difference between the serum FABP4 and FFA as they relate to METS risk markers. This hypothesis was based on the evidence supporting that serum FFA correlates with cardiovascular disease (12), endothelial dysfunction (13), obesity, insulin resistance (14), dyslipidemia (12) and TNFα (15).
In addition, to gain information about regulation of serum FABP4 and FFA dynamically in vivo FABP4 response to oral glucose load was determined. It is known that administration of glucose orally or intravenously stimulates insulin secretion, inhibits lipolysis in the adipose tissue and consequently decreases serum FFA concentrations (16, 17). We anticipated that serum FABP4 may also decline simultaneously because FABP4 chaperones FFA from the hormone sensitive lipase to the adipocyte membrane during lipolysis to facilitate efflux (18, 19).
Study Design
Subjects
Thirty-six women between the ages of 18–45 years, with BMI 27–40 kg/m2 were recruited after signing informed consents approved by the Human Subjects Committee of the UC Davis. Subjects were not recruited based on fulfilling any of the METS criteria. All participants were examined by the principal investigator (SEK-K). Patients using oral contraceptives, insulin sensitizers, lipid lowering medications or any other medicines or supplements affecting weight or insulin sensitivity during the preceding two months, having diabetes mellitus, untreated hypothyroidism, and any other systemic illnesses such as renal, hepatic and gastrointestinal diseases, smoking, and drinking more than 2 servings of alcoholic drinks/ week were excluded. Pregnant women, women who delivered during the previous 12 months and lactating women were excluded. The studies were carried out at the Clinical and Translational Science Center: Clinical Research Center (CCRC) of the University of California, Davis. The subjects were on their habitual diets and weight stable.
Anthropometric measurements
Weight was determined in light clothing using Tanita BWB800-P Digital Medical Scale. Height without shoes was measured using an Ayrton Model S100 stadiometer. Body composition was determined using bioelectrical impedance (Biostat, British Isles, UK) (20).
Oral glucose tolerance test (OGTT)
The nutritional intake was assessed by analyzing the 7-day food records with Food Processor software (ESHA Research, Inc., OR). All subjects consumed adequate carbohydrate prior to testing. The studies were initiated after an overnight fast, between 7AM −8AM. An intravenous catheter was placed in the forearm. After obtaining the baseline blood samples, the participants ingested 75 g of glucose (Glucola™) at 0 min, and additional blood samples were obtained every 30 min thereafter for 5 hours. The subjects remained supine in bed throughout the testing to avoid confounding effects of physical activity on blood glucose. The samples for glucose were collected in sodium fluoride containing tubes on ice. Other samples were collected either in serum separation tubes, or in EDTA or heparin containing tubes. Glucose and insulin were assayed in all samples. Free fatty acids were assayed in hourly samples.
Frequently sampled- intravenous glucose tolerance test (FSI-VGTT)
This was conducted approximately one week after the OGTT. After an overnight fast, an intravenous catheter was placed in each forearm, one for blood sampling and the other for administering glucose and insulin. The catheters were kept open with normal saline drip. Heating pads were used in order to maximize the blood flow. Three blood samples were obtained at times −20, −10 and 0 minutes. Glucose (0.3u/kg as 25% dextrose) was given intravenously at time 0. Intravenous insulin 0.03 u/kg (Humulin Regular: Eli Lilly) was administered at time 20 min. Blood samples were obtained at times 0, 2, 3, 4, 5, 6, 8,10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160 and 180 min. The samples were analyzed for glucose and insulin. The results were entered to the MiniMod-Millenium for the calculations of the acute insulin response (AIR), β-cell function, insulin sensitivity index (SI) and disposition index (DI) (21).
Laboratory assays
Glucose was measured using YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI Life Sciences, Yellow Springs, OH), triglyceride, cholesterol, HDL-cholesterol, apo B and FFA were measured using Poly-Chem System clinical chemistry analyzer (Cortlandt Manor, NY). The coefficients of variations for these assays were: 1% for glucose, 3.5% for cholesterol, 4% for triglyceride, 3.6% for direct HDL, 4.7% for apo B and 4.1% for FFA. Insulin, leptin and adiponectin were measured using RIA kits (Linco Research, St. Charles, MO) with cvs of 8.2%, 4.3% and 6.5%, respectively. The homeostatic model assessment (HOMA) was calculated using the formula: [fasting insulin (µU/mL) x fasting glucose (mM)/22.5]; FABP4 was measured using an ELISA kit (BioVendor, LLC, Candler, NC) with a cv of 5%. Inflammatory markers were measured by the CTSC Laboratory under the supervision of Dr. S. Devaraj. The hs-CRP was measured with a highly sensitive (hs) latex-enhanced immunonephelometric assay as reported previously (22). Both interassay and intra-assay coefficients of variation were <5% (23). IL-6, IL-1β, TNFα were measured using the High Sensitivity Human Cytokine Panel-3 Plex (Milliplex) kit (Millipore, St. Charles, MO) with cv of 11%.
Statistical Methods
All statistical analyses were performed using SAS, Version 9.1 (SAS Institute, Cary, NC). Measurements were log-transformed as necessary to improve the normality of residuals and homoscedasticity of errors. Arithmetic means ± SD were calculated for levels of serum measures. Mean differences (with a 95% CI) in response between two time-points were compared using paired t-test. Separate analyses were performed for each variable. A significance level of 0.05 was used to determine statistical significance of observed differences. Pearson correlation coefficients were calculated to assess the magnitude and direction of a linear association between two given variables. Multiple comparisons were controlled by the Sidak method where appropriate. An adjusted p-value <0.05 using the Sidak method was considered as significant. Trajectory of 5-hour change in response levels was estimated by a repeated measures analysis of variance. Individual trajectories of FABP4 and FFA changes in response level over 6 time points, evenly spaced, were estimated from linear random-effects models. Each response level was entered as the dependent variable (Y) and time (in minutes) was entered as the independent variable. To account for between subject heterogeneity in the changes of response levels, time was modeled as random effect.
Results
Baseline characteristics of the subjects are shown in Table 1.
Table 1.
Baseline characteristics of the subjects, obtained after overnight fasting (n=36, Mean±SEM).
| Age (years) | 32.2±1 |
| Weight (kg) | 99.1±3.0 |
| BMI (kg/m2) | 35.2±0.9 |
| Fat mass (kg) | 45.3±2.2 |
| Lean mass (kg) | 53.8±1.0 |
| Glucose (mg/dL) | 100.0±1.9 |
| Insulin (mU/mL) | 19.1±1.5 |
| HgBA1 (%) | 5.7±0.1 |
| HOMA | 4.0±0.3 |
| Leptin (ng/mL) | 25.7±1.7 |
| Adiponectin (ng/mL) | 10.5±0.9 |
| FFA (mmol/L) | 0.624±0.043 |
| Triglyceride (mg/dL) | 106±10 |
| Cholesterol (mg/dL) | 182±6 |
| LDL-C (mg/dL) | 122±5 |
| HDL-C (mg/dL) | 39±1 |
| Apo B (mg/dL) | 77±4 |
| Hs-CRP (mg/L) | 7.5±1.3 |
| TNF-α (pg/mL) | 267±46 |
| IL-1b(pg/mL) | 176±33 |
| IL-6 (pg/mL) | 63±11 |
Changes in glucose, insulin, FFA and FABP4 during OGTT
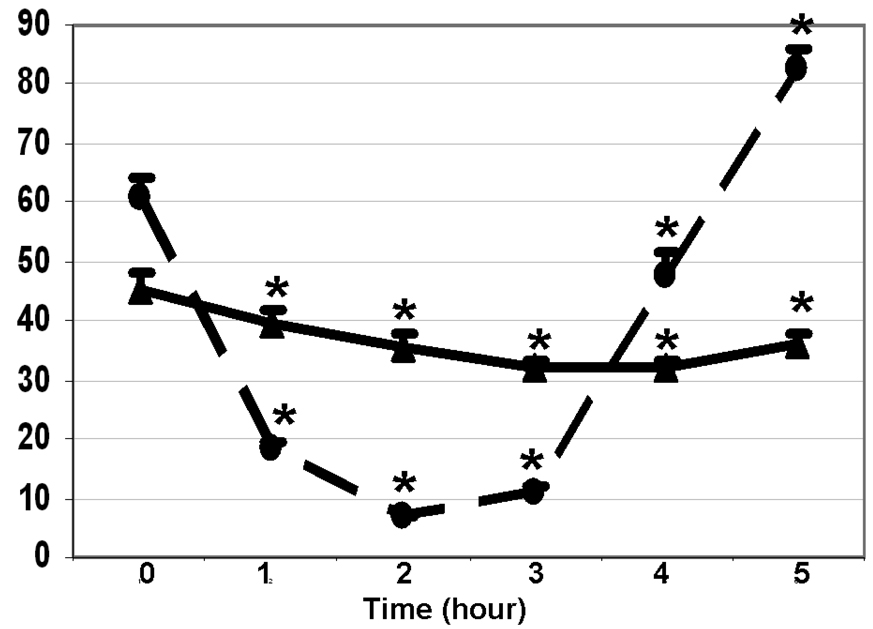
Serum glucose increased from 100±2 mg/dl to the peak value of 164±6 mg/dl in 1h (p < 0.0001), returned to the baseline (97±4 mg/dl) in 3 h. Serum insulin increased from the baseline of 19.3±1.5 µU/ml to 108.2±10.1 µU/ml within 30 min (0.0001), reached a peak of 123.8±11.8 µU/ml at 90 min, and returned to the baseline at 4h. As shown in Figure 1, serum FABP4 reduced from the baseline of 45.3±3.1 ng/mL to 39.3±2.8 ng/mL in 1h, to a nadir of 31.9±1.6 g/mL in 3h, and stayed below the baseline for the rest of the OGTT (p < 0.0001 for all time points). Most subjects had their lowest (nadir) FABP4 during the 2nd or the 3rd hour. Serum FFA reduced from the baseline of 0.611±0.033 mmol/L to 0.181±0.013 mmol/L in 1h, and to a nadir of 0.067±0.004 mmol/L in 2h (p < 0.0001 for both). Serum FFA rebounded to 0.816±0.035 mmol/L at 5h (p < 0.001, as compared to the baseline).
Figure 1.
Changes in FABP4 (ng /mL, ▲▬▬▲) and free fatty acids (100×mmol/L, ●- - -●) during oral glucose tolerance test. Significant changes (0.05< p < 0.0001) from the baseline are marked with *. (n = 36, Mean±SEM).
Relationships among FABP4 and FFA concentrations and other study parameters
Correlations among FABP4, FFA and various anthropometric, insulin resistance and inflammatory parameters were calculated as shown in Table 2. Fasting-FABP, nadir-FABP4 and the decrease (Δ) correlated directly with several of the anthropometric parameters and leptin. The nadir-FABP4 showed a significant correlation with IL-1β, and tended to correlate (p < 0.1) with hs-CRP, IL-6 and TNFα. Fasting-FABP4 also tended to correlate with hs-CRP and IL-6 (p< 0.1). The nadir-FABP4 correlated directly with pancreatic insulin secretion and inversely with sensitivity index (SI) measured by IVGTT. In general, nadir-FABP4 showed the strongest correlations with the insulin resistance and inflammation. None of the FABP4 fractions correlated with plasma lipids/lipoproteins (triglyceride, cholesterol, LDL-cholesterol, HDL-cholesterol or apoB).
Table 2.
Correlations among FFA, FABP4, anthropometric parameters, insulin resistance indicators and inflammatory markers. The “nadir” refers to the lowest FFA or FABP4 value reached during the 2nd or 3rd hour of the oral glucose tolerance test and Δ refers to the decrease between the fasting and nadir values. Coefficients of variations with p < 0.05-0.0001 are bolded (n = 36).
| FFA | FABP4 | |||||
|---|---|---|---|---|---|---|
| Fasting | Nadir | Decrease | Fasting | Nadir | Decrease | |
| Fasting-FFA | −0.1572 | 0.9929 | −0.0579 | 0.0059 | −0.0762 | |
| Anthropometric | ||||||
| Weight | −0.0219 | 0.5471 | −0.0871 | 0.4546 | 0.4186 | 0.3322 |
| BMI | −0.0469 | 0.4814 | −0.1036 | 0.5107 | 0.4178 | 0.4033 |
| Fat-mass | −0.0393 | 0.5240 | −0.1013 | 0.4349 | 0.3763 | 0.3316 |
| Lean-mass | 0.0243 | 0.4926 | −0.0356 | 0.3648 | 0.4099 | 0.2242 |
| Leptin | −0.0205 | 0.2205 | −0.0465 | 0.4441 | 0.2316 | 0.4262 |
| Insulin resistance | ||||||
| Fasting | ||||||
| Insulin | 0.0016 | 0.4727 | −0.0553 | 0.4773 | 0.5109 | 0.3791 |
| HOMA | 0.0476 | 0.3813 | 0.0005 | 0.3574 | 0.2896 | −0.0072 |
| Adiponectin | 0.1134 | −0.4007 | 0.1587 | −0.3185 | −0.2142 | −0.2781 |
| FS-IVGTT | ||||||
| AIRGlucose | −0.1329 | 0.3062 | −0.1663 | 0.3032 | 0.4718 | 0.1112 |
| β-cell func. | −0.0414 | 0.4092 | −0.0863 | 0.1655 | 0.3781 | −0.0085 |
| SI | −0.1703 | −0.2448 | −0.1364 | −0.3162 | −0.3405 | −0.2028 |
| Inflammation | ||||||
| *hs-CRP | 0.0154 | 0.1872 | −0.0075 | 0.2906+ | 0.3253+ | 0.1881 |
| *IL-6 | 0.0535 | 0.2479 | 0.0223 | 0.3081+ | 0.3101+ | 0.2100 |
| *IL-1β | −0.0064 | 0.1943 | −0.0296 | 0.2797 | 0.3484 | 0.1523 |
| *TNF-α | −0.0002 | 0.1851 | −0.0224 | 0.2824 | 0.3005+ | 0.1832 |
The data for the inflammatory markers were log transformed.
p < 0.1.
Fasting-FFA and the Δ-FFA did not correlate with FABP4 or any of the METS variables. On the other hand, nadir-FFA measured during the 2nd hour of OGTT correlated directly with weight, BMI, fat mass, fasting insulin, HOMA, pancreatic β-cell function measured by IVGTT, and inversely with adiponectin. None of the FFA fractions correlated with the inflammatory markers or serum lipids, although correlation of the nadir-FFA with HDL-cholesterol approached to statistical significance (r = −0.3061, p = 0.08).
Discussion
The study yielded the following new information: First, at fasting serum FABP4 related to several of the metabolic risk markers better than FFA. Second, FABP4 and FFA concentrations measured 2 to 3 hours after oral glucose loading related to obesity, inflammation and insulin resistance better than the fasting values.
The fasting-FABP4 correlated with obesity and fasting insulin. Similar relationships have been previously reported in type 2 diabetic patients (24). In this report FABP4 also collated directly with adiponectin and this finding was surprising because adiponectin increases insulin sensitivity while serum FABP4 is associated with insulin resistance (25–27). In our study, fasting-FABP4 showed a weak inverse correlation with adiponectin (r=-0.3185, p = 0.07). In HIV positive patients, serum FABP4 correlated with BMI, fasting glucose, insulin and CRP (28). In women with polycystic ovary syndrome, serum FABP4 correlated positively with BMI, and negatively with HDL-C (29). In hyperlipidemic patients, serum FABP4 correlated with fasting glucose, but not with lipids (30). In our study none of the FABP4 fractions correlated with plasma lipids or apoprotein B either.
It was clear that fasting-FABP4 related to the risk markers more than the fasting-FFA. Although literature indicates that fasting-FFA correlated with coronary artery disease (12), endothelial dysfunction (13), obesity, insulin resistance (14), dyslipidemia (12) and TNFα (15), we did not observe similar correlations. One explanation may be that the reports showing positive correlations with FFA involved large populations (12, 17), animal experiments (14) or case control studies (15).
A novel aspect of our study is that we measured dynamic changes in serum FABP4 and FFA after oral glucose loading. Serum FABP4 decreased by 40% and FFA decreased approximately 10 fold during the 2nd to 3rd hour of OGTT. Both the fasting-FABP4 and the nadir-FABP4 correlated with obesity, while the nadir-FABP4 showed stronger correlations with insulin resistance and inflammation. Interestingly, only the nadir-FFA correlated with the anthropometric and metabolic variables, whereas the fasting-FFA did not relate to any of these parameters. This finding is noteworthy, especially in the light of the recent report from Boston et al. (16) which described a minimal model of FFA kinetics using FS-IVGTT. In this model, fasting FFA concentrations did not relate to the rate of lipolysis and the nadir-FFA occurred when the rate of lipolysis equaled the rate of oxidation. It is conceivable that in obesity and insulin resistance lipolysis is not suppressed efficiently in the adipose tissue. Consequently obese and insulin resistant individuals exhibit higher nadir-FFA values (10, 18, 19). In agreement with this explanation, we found that the nadir FABP4 and FFA correlated with the anthropometric parameters and insulin resistance directly. We did not measure FABP4 during FS-IVGTT for several reasons: First, this test requires administration of glucose at the 0 min and insulin at the 20 min. Since our study was the first to measure FABP4 in a dynamic setting and the half-life of FABP4 is not known, we anticipated that 20 min may not be long enough to see a response to intravenous glucose. Second, the dose of intravenous glucose and insulin is calculated based on weight and varies from individual to individual. We anticipated that varying doses of glucose and insulin may modify FABP4 response. Third, each FS-IVGTT yields 31 samples and the cost the FABP4 assay would be prohibitive. Finally, OGTT is a widely used test and the findings obtained from OGTT can find wider clinical and research application.
In summary, the literature indicates that FABP4 may predict development of METS prospectively, and independently of adiposity and insulin resistance (5, 6). Our findings suggest that the predictive value may be improved by measuring FABP4 not only at fasting state but also after glucose loading.
Acknowledgements
This study was supported by grants to Dr. Karakas from the National Center for Complementary and Alternative Medicine (NCCAM, Grant number: AT002280) and the ALSAM Foundation, Los Angeles, CA. The clinical studies were partially supported by the UC Davis Clinical and Translational Science Center Grant (RR 024146). We are grateful to Drs. Gokhan Hotamisligil and Paul Davis for their input and critique and Ms. Elizabeth Garamendi for her diligent work.
ABREVIATIONS
- FABP
fatty acid binding protein
- METS
metabolic syndrome
- BMI
body mass index
- HOMA
homeostatic model assessment
- SI
sensitivity index
- AIR
acute insulin response
- DI
disposition index
- FS-IVGTT
frequently sampled intravenous glucose tolerance test
- OGTT
oral glucose tolerance test
- TG
triglyceride
- hs-CRP
highly sensitive C reactive protein
- IL
interleukin
- TNF
tumor necrosis factor
- FFA
free fatty acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
Institutional approval:
The study was approved by the Human Subjects Committee of the UC Davis and all subjects signed the approved informed consent.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 3.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Current opinion in lipidology. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden M. Blocking? fatty acids' mystery tour: a therapy for metabolic syndrome? Cell metabolism. 2007;6:89–91. doi: 10.1016/j.cmet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Stejskal D, Karpisek M. Adipocyte fatty acid binding protein in a Caucasian population: a new marker of metabolic syndrome? European journal of clinical investigation. 2006;36:621–625. doi: 10.1111/j.1365-2362.2006.01696.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clinical chemistry. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 7.Tuncman G, Erbay E, Horn X, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. The Journal of clinical investigation. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Science (New York, N.Y. Vol. 274. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein; pp. 1377–1379. [DOI] [PubMed] [Google Scholar]
- 10.Furuhashi M, Tuncman G, Gorgun CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature medicine. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirro M, Mauriege P, Tchernof A, et al. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec Cardiovascular Study. Atherosclerosis. 2002;160:377–384. doi: 10.1016/s0021-9150(01)00588-3. [DOI] [PubMed] [Google Scholar]
- 13.Shankar SS, Steinberg HO. FFAs: do they play a role in vascular disease in the insulin resistance syndrome? Current diabetes reports. 2005;5:30–35. doi: 10.1007/s11892-005-0064-6. [DOI] [PubMed] [Google Scholar]
- 14.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. The American journal of medicine. 2007;120:S3–S8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29-32. [DOI] [PubMed] [Google Scholar]
- 15.Ohmura E, Hosaka D, Yazawa M, Association of free fatty acids (FFA), and tumor necrosis factor-alpha (TNF-alpha), and insulin-resistant metabolicdisorder, et al. Hormone and metabolic research. Hormon- und Stoffwechselforschung. 2007;39:212–217. doi: 10.1055/s-2007-970421. [DOI] [PubMed] [Google Scholar]
- 16.Boston RC, Moate PJ. A Novel Minimal Model to describe NEFA Kinetics following an Intravenous Glucose Challenge. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00749.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Camastra S, Coppack SW, Fliser D, Golay A, Mitrakou A. Insulin action and non-esterified fatty acids. The European Group for the Study of Insulin Resistance (EGIR) The Proceedings of the Nutrition Society. 1997;56:753–761. doi: 10.1079/pns19970076. [DOI] [PubMed] [Google Scholar]
- 18.Shen WJ, Liang Y, Hong R, et al. Characterization of the functional interaction of adipocyte lipid-binding protein with hormone-sensitive lipase. The Journal of biological chemistry. 2001;276:49443–49448. doi: 10.1074/jbc.M104095200. [DOI] [PubMed] [Google Scholar]
- 19.Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5528–5532. doi: 10.1073/pnas.96.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierson RN, Jr, Wang J, Thornton JC. Measurement of body composition: applications in hormone research. Hormone research. 1997;48 Suppl 1:56–62. doi: 10.1159/000191271. [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN, Zaccaro DJ, Watanabe RM, et al. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;52:2168–2174. doi: 10.2337/diabetes.52.8.2168. [DOI] [PubMed] [Google Scholar]
- 22.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 23.Kasim-Karakas SE, Almario RU, Gregory L, Wong R, Todd H, Lasley BL. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2004;89:615–620. doi: 10.1210/jc.2003-030666. [DOI] [PubMed] [Google Scholar]
- 24.Cabre A, Lazaro I, Girona J, et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195:e150–e158. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature medicine. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 26.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of clinical endocrinology and metabolism. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 27.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 28.Coll B, Cabre A, Alonso-Villaverde C, et al. The fatty acid binding protein-4 (FABP4) is a strong biomarker of metabolic syndrome and lipodystrophy in HIV-infected patients. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Hu WH, Qiao J, Li R, Wang LN. [Relations of serum adipocyte fatty acid binding protein to sex hormone and lipoproteins in patients with polycystic ovary syndrome] Zhonghua yi xue za zhi. 2006;86:3186–3189. [PubMed] [Google Scholar]
- 30.Karpisek M, Stejskal D, Kotolova H, et al. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. European journal of clinical investigation. 2007;37:637–642. doi: 10.1111/j.1365-2362.2007.01835.x. [DOI] [PubMed] [Google Scholar]