Abstract
Autoimmune attack on the heart is linked to host immune responses against cardiac myosin, the most abundant protein in the heart. Although adaptive immunity is required for disease, little is known about innate immune mechanisms. Here we report that human cardiac myosin (HCM) acted as an endogenous ligand to directly stimulate human toll-like receptors (TLR) 2 and 8 and activated human monocytes to release proinflammatory cytokines. In addition, pathogenic epitopes of HCM, S2-fragment peptides S2–16 and S2–28, stimulated TLRs directly and activated human monocytes. Our data suggest that cardiac myosin and its pathogenic T cell epitopes may link innate and adaptive immunity in a novel mechanism which could promote chronic inflammation in the myocardium.
Keywords: autoimmunity, inflammation, cell activation
Introduction
Myocarditis is an acute inflammatory disease of the heart which may lead to chronic inflammation of the myocardium and dilated cardiomyopathy (DCM) (1). In myocarditis and DCM, autoantibodies react with cardiac myosin (2). In animal models of cardiac-myosin induced myocarditis, pathogenic roles for CD4+ T cells (3, 4) and autoantibodies (5) have been described. While virus is important in acute myocarditis (3), the development of chronic myocarditis and cardiomyopathy may require exposure and release of cardiac myosin from damaged myocytes (6). However, little is known how an intracellular protein autoantigen like cardiac myosin activates inflammatory cells.
While studies of myocarditis over the past 20 years focused on adaptive immunity, activation of innate immunity in the induction of myocarditis and progression to DCM has only recently been appreciated (7, 8). Innate immune cells including macrophages, neutrophils, dendritic cells (9–11), and several cytokines including IL-6, IL-1, TNF-α are present in inflamed myocardium (9, 12, 13). In addition, IL-6 knockout mice are resistant to disease induction (14), and overexpression of TNF-α resulted in severe myocarditis in susceptible mice (15).
Although Toll-like receptors (TLRs) were initially discovered to play essential roles in innate immune recognition of pathogen-associated molecular patterns (PAMPs), increasing evidence indicates that TLR activation by endogenous ligands stimulates acute and chronic inflammatory responses (16). Endogenous ligands released from damaged tissue may activate TLRs leading to chronic inflammation.
The involvement of TLRs has been reported in myocarditis (7, 9, 17). Activation of TLRs in dendritic cells (DCs) through MyD88 signaling is critical since MyD88 deficiency rendered susceptible mice resistant to myocarditis (8). It was shown that several TLR ligands replaced complete Freund’s adjuvant in a mouse model of myocarditis where dendritic cells loaded with α-myosin heavy chain-derived peptide caused disease (7). Activation of innate immunity through TLRs appears to be essential for prolonged adaptive responses to cardiac myosin. We propose that cardiac myosin, released from damaged cardiomyocytes, may act as an endogenous ligand for TLRs and stimulate chronic inflammation leading to cardiomyopathy.
Our study shows that HCM and its pathogenic peptides directly stimulated human TLRs 2 and 8 and activated human monocytes to secrete proinflammatory cytokines in a TLR dependent manner. Thus, cardiac myosin is not only a major target of the adaptive immune response, but it may directly stimulate innate immune responses that promote chronic inflammation in the myocardium.
Materials and Methods
Reagents
HCM was purified as previously described (18). S2 peptides were previously described (13) and analyzed for purity by mass spectrometry. Rabbit skeletal myosin (SKM) and chloroquine were from Sigma. mAb to human TLR2 (TL2.1) was from Stressgen. LTA, LPS, CL075 were from InVivogen.
PBMC and human monocyte preparation
PBMCs were prepared on Histopaque-1077 (Sigma) from human peripheral blood of healthy volunteers and monocytes enriched by negative selection using a monocyte isolation kit (Dynal MPC-L). PBMCs and monocytes were plated in 24 well plates and cultured in RPMI 1640 medium supplemented with either 5 or 10% heat-inactivated FBS or human sera from AB blood group or monocyte donors, 2mM L-glutamine, 100 U/ml of penicillin, 100 µg/ml of streptomycin. In certain cases, monocytes were preincubated with gamma globulin to block Fc receptors followed by incubation with TL2.1 mAb (50 ng/ml) for 1h before treatment with HCM. Monocytes were treated with HCM in the presence of chloroquine (50 nM).
THP-1 cell culture
Human monocytic cell line, THP-1, was obtained from ATCC and maintained according to instructions.
FACS Analysis
Cells were stained with the following: PE-anti-human TLR2 (eBioscience), FITC-anti-human TLR8 (Imgenex), APC-anti-human CD14 (monocyte marker) (BD Biosciences) and appropriate isotype controls. Intracellular staining with anti-TLR8 was done using IC-Flow Kit (Imgenex). Samples were analyzed on a FACSCalibur™.
Cytokine detection
Human PBMC, monocytes and THP-1 cells were treated with TLR2 stimulants for 24 h. Supernatants were collected and assayed using ELISA kits (human IL-8 from BD Pharmingen; human IL-6, TNF-α from MABTECH USA).
HEK293-hTLR stably transfected cell lines
HEK293 cells stably transfected with human TLRs 2, 3, 4, 5, 7, 8, 9 and control untransfected HEK 293 cells were from InVivogen. Expression of TLRs was verified by Western blots (data not shown). HEK-TLR transfectants were maintained in DMEM, 4.5 g/L glucose, 10% heat-inactivated FBS or human AB serum, 10 µg/ml of Blastocidin and 100 µg/ml Normocin. Cells were treated with HCM, S2–16 peptide, SKM or known TLR ligands. Supernatants harvested after 72 h were assayed for IL-8 as indicator of TLR activation.
Silencing TLR2 and 8 on human monocytes using psiRNA-TLR plasmids
psiRNA-hTLR2 and psiRNA-TLR 8 (InVivogen) were used to silence TLR2 and TLR8 in human monocytes using nucleofector (AMAXA) according to manufacturer’s instruction. Cells (8 ×106) were subjected to nucleofection using Y001 program. Control cells were transfected with a control siRNA (psiRNA-LucGL3). Transfected cells were prewarmed in RPMI 1640 medium with 10% FBS. After 24h, transfection efficiency was monitored by GFP positive cells by immunofluorescence microscopy. Cells were treated with HCM and supernatants harvested 24h later and assayed for IL-8. Silencing efficiency was ~100 percent as determined by Western blotting.
Statistical analysis
Data are expressed as the mean ± s.e.m of triplicate wells in cytokine ELISA. Effect of multiple treatment groups was evaluated by one way ANOVA using GraphPad Prism4 software. Difference of the means was evaluated by Tukey test. For HCM S2–16 on HEK-hTLRs, mAb and chloroquine inhibition on monocyte IL-8 and siRNA data, comparison between treatment and media was performed by Student’s unpaired two-tailed t test.
Results
HCM activates TLRs 2 and 8
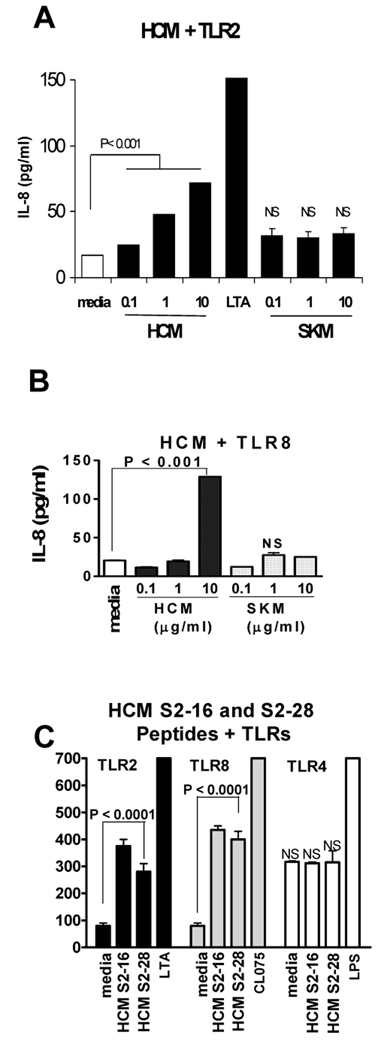
To determine TLR activation by HCM, we employed HEK 293 cells transfected with human TLR2, 3, 4, 6, 7, 8, or 9. HCM treated cells demonstrated a 2–5 fold increase in IL-8 secretion in HEK 293 cells transfected with TLR2 (Fig. 1A) and TLR8 (Fig. 1B). No increase of IL-8 secretion was observed over control in HEK 293 cells transfected with TLR3, 4, 6, or 9 when treated with HCM (data not shown). The data show HCM activates TLR2 and 8, but not other TLRs. Although not shown or studied further, TLR7 was activated by HCM but not SKM. Interestingly, SKM, which does not induce myocarditis in animal models (18, 19), did not significantly activate TLRs 2 and 8 (Fig. 1A and 1B). HCM peptides S2–16, a cryptic pathogenic peptide of HCM and S2–28, a dominant epitope in experimental autoimmune myocarditis (13), activated TLR2 and 8, but not TLR4 (Fig. 1C) or other TLRs (not shown). Other HCM peptides such as S2-2 did not have the same stimulatory effect (not shown). FACS analysis of transfectants (293-hTLR2 and 293-hTLR8) confirmed surface expression of TLR2 and intracellular expression of TLR8 (data not shown). Untransfected HEK-293 cells did not express TLR2 or TLR8.
FIGURE 1.
HCM activates TLR2 and TLR8. (A) HEK293-hTLR2 cells were stimulated with HCM or skeletal myosin (SKM). Supernatants harvested at 24 h were assayed for IL-8. LTA, lipoteichoic acid, TLR2 ligand. (B) HCM stimulated IL-8 secretion from HEK293-hTLR8 cells, P < 0.001. (C) HCM S2–16 and S2–28 peptides activated TLR2 (P < 0.0001) and 8 (P < 0.0001). S2–16 is cryptic pathogenic HCM peptide; and S2–28 is dominant epitope of cardiac myosin in EAM (13). CL075, TLR7/8 ligand. LPS, lipopolysaccharide, TLR4 ligand. Values are means ± s.e.m. Representative results from three to six independent experiments are shown. NS, not significant. P value obtained by Tukey’s test using one way ANOVA.
HCM activates human monocytes
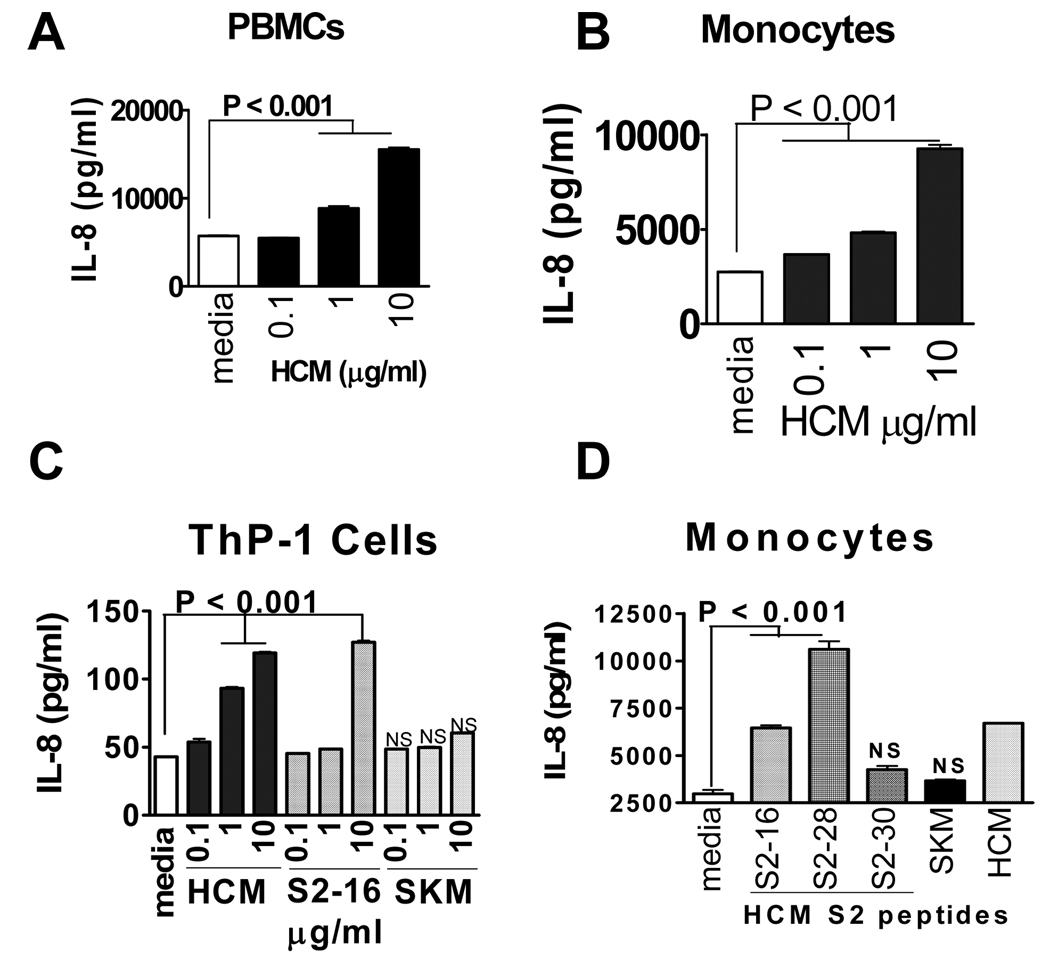
Our discovery that HCM activated TLRs, led us to study human monocytes which express TLRs 2 and 8. Treatment of PBMCs with HCM resulted in a dose-dependent 2.5-fold increase in IL-8 secretion (Fig. 2A). We isolated human monocytes and found that HCM stimulated IL-8 secretion from human monocytes in a dose-dependent manner up to 4.3-fold increase (Fig. 2B). Importantly, monocytes cultured with HCM peptide S2–16, led to a dose-dependent increase of 2.5 fold in IL-8 secretion. Stimulation of IL-8 by HCM and S2–16 peptide was also observed in human monocytic THP-1 cells. No stimulation was observed with SKM (Fig. 2C). HCM S2 peptides 16 and 28 stimulated IL-8 secretion from human monocytes with S2–16 moderate and S2–28 highly stimulatory (Fig. 2D). S2–30 peptide and SKM were not stimulatory. In further studies of human monocytes, S2–28 (10 ug/ml) consistently stimulated IL-8 in PBMCs to high levels (55,000 pg/ml) above that seen for HCM peptide S2–16 (20,000 pg/ml). Our findings suggest pathogenic epitopes for T cells may link adaptive and innate immunity.
FIGURE 2.
HCM activates human monocytes and induces IL-8 from (A) PBMCs (p<0.001); (B) monocytes (p<0.001); and (C) human monocytic THP-1 cells (p<0.001); treated with HCM, S2–16 HCM peptide, or SKM for 24h. Supernatants assayed for IL-8. (D) HCM peptides S2–16 and S2–28 stimulated IL-8 secretion from human monocytes. Values are means ± s.e.m. NS, not significant. P value obtained by Tukey’s test using one way ANOVA.
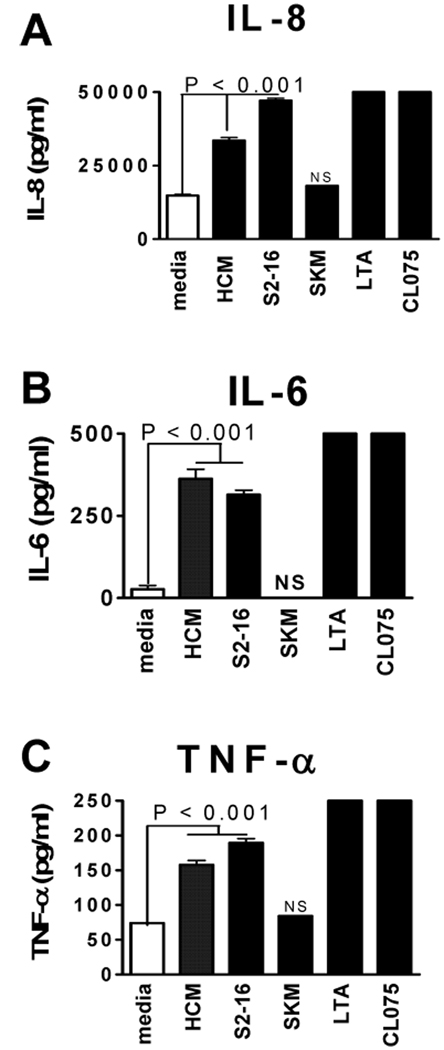
To further confirm that HCM activates human monocytes, we analyzed several proinflammatory cytokines besides IL-8 (Fig. 3A). We found that HCM also stimulated IL-6 (Fig. 3B) and TNF-α (Fig. 3C). SKM did not have a stimulatory effect on secretion of proinflammatory cytokines from human monocytes. In further studies, we found that S2–28 (10 ug/ml) stimulated production of more IL-6 (1500 pg/ml) from PBMCs than seen with S2–16 (500 pg/ml).
FIGURE 3.
HCM and peptide S2–16 stimulated proinflammatory cytokines from human monocytes. (A) IL-8; (B) IL-6 and (C) TNF-α as measured by ELISA while monocytes treated with SKM did not (A–C). Values are means ± s.e.m. NS, not significant. P value obtained by Tukey’s test using one way ANOVA.
HCM activation of human monocytes is dependent on TLR2 and TLR8
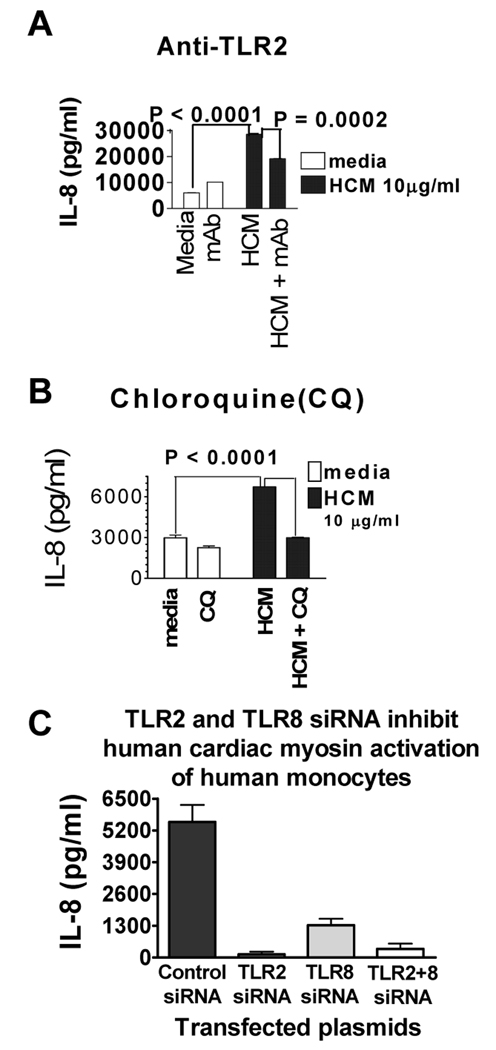
Human monocytes are known to express several TLRs, with TLR2 and TLR8 the most dominant (20). We hypothesize that HCM activates human monocytes in a TLR-dependent manner. To demonstrate that activation of human monocytes by HCM was in part dependent on TLR2, we preincubated monocytes with a mAb to TLR2 for 1 h before HCM treatment. The mAb inhibited HCM stimulated-IL-8 secretion by 40% (Fig. 4A). To determine if HCM activated monocytes in part through TLR8, we cultured monocytes with HCM in the presence of chloroquine, a chemical inhibitor of endosome acidification which interferes with activation of TLR8. We found that chloroquine inhibited HCM stimulated-IL-8 production by 60% (Fig. 4B).
FIGURE 4.
Anti-TLR2, chloroquine and siRNA inhibit HCM stimulation of TLR2 or TLR8. (A) Human monocytes preincubated with mAb to human TLR2 and treated with HCM 24 h. Supernatants assayed for IL-8. Anti-TLR2 inhibited HCM-stimulation (p = 0.0002). (B) Chloroquine inhibited HCM stimulation of IL-8 secretion from human monocytes (P < 0.0001). (C) Silencing TLR2 and TLR8 RNA inhibited HCM-stimulated human monocyte activation. Human monocytes were transfected with siRNA against TLR2 or TLR8 or both, then stimulated with HCM. Bar graphs show pg/ml of IL-8 above media control for human monocytes containing control plasmid (psiRNA-LucGL3) or TLR siRNA plasmids after HCM activation of human monocytes. Representative results from three independent experiments shown. Values are means ± s.e.m.
HCM treatment of human monocytes did not alter TLR expression on the surface of monocytes
FACS analysis of PBMCs and human monocytes from three different individuals showed little difference in TLR expression before and after treatment with HCM and S2 peptides. Percent difference ranged from 1–4% (TLR2) and 1–9% (TLR8).
siRNA inhibited TLR2 and 8 activation by HCM
To further confirm that stimulation of monocyte IL-8 by HCM was dependent upon activation of TLR2 and TLR8, we transfected human monocytes with siRNA against TLR2, TLR8, or both. Control siRNA did not affect HCM stimulation of monocyte IL-8 (Fig. 4C). Knocking down TLR2 in monocytes resulted in complete inhibition of stimulation of IL-8 by HCM (Fig. 4C). Knocking down TLR8 resulted in almost complete inhibition of HCM stimulated increase of IL-8 (Fig. 4C). However, when knocking down both TLR2 and TLR8, the stimulation of IL-8 by HCM was again strongly inhibited (Fig. 4C), suggesting cooperation of TLR2 and TLR8 in the activation of human monocytes by HCM.
Discussion
Our results show for the first time that cardiac myosin, the major autoantigen in myocarditis, activated TLRs 2 and 8 while skeletal myosin did not, and HCM directly activated human monocytes to release proinflammatory cytokines. It is currently well accepted that endogenous TLR ligands may play an important role in pathogenesis of some autoimmune diseases(16).
The specificity of TLRs for cardiac myosin is novel and intriguing. The structural similarity of cardiac myosin with virulence factors of pathogens may be important in its overall ability to activate innate immunity. Most known endogenous TLR ligands have been shown to activate TLR2 and/or TLR4 (16, 21). Only nucleic acid self-antigens have been reported to activate TLR7, TLR8 and TLR9 (21). Cardiac myosin may function as a TLR ligand by mimicking epitopes on pathogens or PAMPs. To support this hypothesis, immunological mimicry has been shown between streptococcal M protein, coxsackievirus and cardiac myosin (22, 23) indicating that cardiac myosin shares structural similarites with pathogens. Evidence has demonstrated mimicry between alpha-helical molecules such as streptococcal M protein and myosin and DNA (24). Recently it was shown that the alpha-helical coiled-coil streptococcal M protein activated TLR2 on monocytes and was a potent inducer of inflammatory cytokines (25). The strong similarity of cardiac myosin and streptococcal M protein supports the recognition of TLR2 by both molecules (25). Crystal structure of streptococcal M protein reveals that non-idealities of the α-helices may interact with the immune system (26). This may be the case for cardiac myosin.
Microbial PAMPS did not appear to be in our purified HCM preparations and peptides because they did not activate TLR4 and several other TLRs, which are activated by bacterial LPS or other microbial products. ssRNA which activates TLR7 and TLR8, would be rapidly degraded. SKM was negative with all TLRs, and analysis of HCM preparations and peptides for 260nm/280nm ratio < 0.57 indicated that nucleic acids were not present. All peptides were tested for purity by mass spectrometry.
Stimulation of TLRs on monocytes was observed not only with intact HCM but also with peptides from the S2 region of cardiac myosin, in particular peptide S2–16 and dominant HCM epitope S2–28. Our findings suggest that two dominant T cell epitopes of HCM have the ability to directly activate innate immune cells, thus linking adaptive and innate immunity.
It is well known that patients with myocarditis or dilated cardiomyopathy have elevated levels of autoantibodies against cardiac myosin (2) and that antibodies against cardiac myosin deposit in hearts of animals with myocarditis (5). Immune complexes containing HCM or its peptides may target TLR7 or TLR8 on endosomes after internalization through Fc receptors on immune cells. Cooperation of Fc receptors and TLRs might be important in tissue-specific autoimmune diseases which display elevated levels of autoantibodies to tissue-specific antigens (27, 28)
Although the classic view of immune recognition was based on discrimination of self-nonself, more and more evidence suggests that self molecules which have hydrophobic regions exposed to the immune system are perceived as a “danger signal” and have the ability to activate the immune response in a similar way as non-self molecules (29). Our hypothesis is supported by our data which suggests that cardiac myosin is not only a major target of the adaptive immune response, but it may serve as a “signal” from within the heart to crosstalk with the immune system.
Footnotes
This study was supported by NHLBI R01 grant HL56267. MWC is the recipient of a NHLBI merit award HL35280.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Brown CA, O'Connell JB. Myocarditis and idiopathic dilated cardiomyopathy. Amer J Med. 1995;99:309–314. doi: 10.1016/S0002-9343(99)80164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caforio ALP, Goldman JH, Haven AJ, Baig KM, McKenna WJ. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54:157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- 3.Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsackieviral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- 4.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- 5.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 6.Rose NR. Viral damage or 'molecular mimicry'-placing the blame in myocarditis. Nat Med. 2000;6:631–632. doi: 10.1038/76199. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 8.Marty RR, Dirnhofer S, Mauermann N, Schweikert S, Akira S, Hunziker L, Penninger JM, Eriksson U. MyD88 signaling controls autoimmune myocarditis induction. Circulation. 2006;113:258–265. doi: 10.1161/CIRCULATIONAHA.105.564294. [DOI] [PubMed] [Google Scholar]
- 9.Fairweather D, Frisancho-Kiss S, Njoku DB, Nyland JF, Kaya Z, Yusung SA, Davis SE, Frisancho JA, Barrett MA, Rose NR. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J Immunol. 2006;176:3516–3524. doi: 10.4049/jimmunol.176.6.3516. [DOI] [PubMed] [Google Scholar]
- 10.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 11.Shioji K, Kishimoto C, Sasayama S. Fc receptor-mediated inhibitory effect of immunoglobulin therapy on autoimmune giant cell myocarditis: concomitant suppression of the expression of dendritic cells. Circulation Res. 2001;89:540–546. doi: 10.1161/hh1801.096263. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather D, Rose NR. Inflammatory heart disease: a role for cytokines. Lupus. 2005;14:646–651. doi: 10.1191/0961203305lu2192oa. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat. J Immunol. 2004;172:3225–3234. doi: 10.4049/jimmunol.172.5.3225. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 15.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circulation Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 16.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 18.Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 20.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 21.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krisher K, Cunningham MW. Myosin: a link between streptococci and heart. Science. 1985;227:413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham MW, Swerlick RA. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986;164:998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahlman LI, Morgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, Soehnlein O, Lindbom L, Norrby-Teglund A, Schumann RR, Bjorck L, Herwald H. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177:1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- 26.McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science. 2008;319:1405–1408. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Riesbeck K. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nature Rev. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]