Abstract
Background: Promoting catch-up growth in malnourished children has health benefits, but recent evidence suggests that accelerated child weight gain increases adult chronic disease risk.
Objective: We aimed to determine how birth weight (BW) and weight gain to midchildhood relate to blood pressure (BP) in young adults.
Design: We pooled data from birth cohorts in Brazil, Guatemala, India, the Philippines, and South Africa. We used conditional weight (CW), a residual of current weight regressed on prior weights, to represent deviations from expected weight gain from 0 to 12, 12 to 24, 24 to 48 mo, and 48 mo to adulthood. Adult BP and risk of prehypertension or hypertension (P/HTN) were modeled before and after adjustment for adult body mass index (BMI) and height. Interactions of CWs with small size-for-gestational age (SGA) at birth were tested.
Results: Higher CWs were associated with increased BP and odds of P/HTN, with coefficients proportional to the contribution of each CW to adult BMI. Adjusted for adult height and BMI, no child CW was associated with adult BP, but 1 SD of BW was related to a 0.5-mm Hg lower systolic BP and a 9% lower odds of P/HTN. BW and CW associations with systolic BP and P/HTN were not different between adults born SGA and those with normal BW, but higher CW at 48 mo was associated with higher diastolic BP in those born SGA.
Conclusions: Greater weight gain at any age relates to elevated adult BP, but faster weight gains in infancy and young childhood do not pose a higher risk than do gains at other ages.
INTRODUCTION
Stunting and underweight are related to increased morbidity, mortality, and poor cognitive outcomes during childhood (1–5). The promotion of compensatory or “catch-up” growth in malnourished children, a well-established health care practice designed to ameliorate these problems, has recently been questioned because evidence suggests that rapid weight gain in the first 2 y of life is associated with an increased risk of being overweight or obese in later life (6–9). Furthermore, the risk of certain chronic diseases and related risk factors is increased in individuals who are relatively small at birth, but relatively large as adults (6, 10–12), which suggests that postnatal weight gain contributes to the development of disease (13).
The long-term consequences of rapid weight gain in infancy and early childhood in populations with a high prevalence of early childhood undernutrition are unknown. It is critical to determine whether any long-term deleterious effects depend on the timing of rapid weight gain. Evidence from India (14), Guatemala (15), and Brazil (16) suggests that timing of weight gain affects adult body composition, which, in turn, is related to chronic disease risk. These studies show that faster infant and early childhood weight gain relates more strongly to adult lean mass than to adiposity, whereas weight gain in later childhood and adolescence contributes more to adult adiposity.
We used data from 5 low- and middle-income countries to examine how birth weight (BW) and weight gain into midchildhood relate to blood pressure (BP) in young adults. We study BP because it tracks into adulthood (17, 18) and is a significant risk factor for cardiovascular disease. Our objective was to address the following questions: 1) to what degree are BW and greater than expected weight gain in early to midchildhood associated with adult BP; 2) among adults who are the same height and weight, does it matter when a period of higher than expected weight gain occurred; and 3) does the association of early childhood weight gain with later BP differ between those who were born small and those adequate for gestational age (AGA).
SUBJECTS AND METHODS
Study populations
We used data from 5 birth cohorts in low- and middle-income countries, united in the Consortium on Health Orientated Research in Transitional Societies (COHORTS). The 5 cohorts include the 1982 Pelotas (Brazil) Birth Cohort (19), the Institute of Nutrition of Central America and Panama Nutrition Trial Cohort (INTC; Guatemala) (20), the New Delhi Birth Cohort (India) (14, 21), the Cebu Longitudinal Health and Nutrition Survey cohort (CLHNS; Cebu, Philippines) (22, 23), and the Birth-to-Twenty (Bt20; Soweto-Johannesburg, South Africa) cohort (24) (Table 1). We refer to these studies subsequently as Pelotas, Guatemala, New Delhi, Cebu, and Bt20, respectively. All studies were reviewed and approved by an appropriate ethics committee or Institutional Review Board.
TABLE 1.
Characteristics of the 5 COHORTS (Consortium on Health Orientated Research in Transitional Societies) studies1
| Study | Design | Cohort inception | Initial sample | Number examined in the last visit | Comments |
| Pelotas Birth Cohort, Brazil (19) | Prospective cohort | 1982 | 5914 | 4297 | Enrolled all children born in the city's maternity hospitals (>99% of all births) during 1982. All social classes included. |
| INTCS, Guatemala (20) | Community trial | 1969–1977 | 2392 | 1571 | Intervention trial of a high-energy and high-protein supplement. All children aged <7 y in 1969 and all born between 1969 and 1977 were enrolled and followed until age 7 y or until the study ended in 1977. Data were collected from mothers during pregnancy and breastfeeding periods. |
| New Delhi Birth Cohort Study, India (14, 21) | Prospective cohort | 1969–1972 | 8181 | 1583 | Pregnancies were identified in a population of married women living in a defined area of Delhi, and the newborns were enrolled and followed. Primarily middle-class sample included. |
| CLHNS, Cebu, Philippines (22, 23) | Prospective cohort | 1983–1984 | 3080 | 2032 | Pregnant women living in 33 randomly selected neighborhoods were included; 75% urban. First data collection at 30 wk gestation. All social classes included. |
| Bt20 cohort, Soweto-Johannesburg, South Africa (24) | Prospective cohort | 1990 | 3273 | 2100 | Pregnant women with a gestational age of 26–32 wk living in a delimited urban geographic area were included. Predominantly poor blacks included. |
INTCS, Institute of Nutrition of Central America and Panama Nutrition Trial Cohort; CLHNS, Cebu Longitudinal Health and Nutrition Survey; Bt20, Birth-to-Twenty.
We pooled individual data from the 5 cohorts. Our main analysis sample (n = 4335) included participants who were not pregnant and had height, weight, and BP measured during the most recent follow-up and weight measured at 0, 12, and 24 mo and during midchildhood. Except for the Bt20 participants, who were adolescents (mean age: 15 y), all others were young adults. However, for simplicity, we call these “adult” measures. Our analytic method required complete child data, so participants missing one or more child weight measures were excluded (n = 5266). The large size of the excluded group primarily reflected study designs in Pelotas, where 33% of the birth cohort was sampled in the first follow-up by selecting infants born between January and April, and in Bt20, where a subgroup of those enrolling in the birth cohort was sampled at 12 mo of age. For selected analyses, the sample is further reduced because of missing gestational age (n = 280) or more detailed body-composition data (n = 729).
Outcome variables
BP, the main outcome of interest, was measured with an aneroid sphygmomanometer in Pelotas, with a mercury sphygmomanometer in Cebu, and with digital devices in Guatemala (model UA-767; A&D Medical, San Jose, CA), for the Bt20 (Omron M6; Omron, Kyoto, Japan) and in New Delhi (Omron 711). Appropriate cuff sizes were used, and the participants were measured while seated after a 5–10 min rest. For Pelotas, New Delhi and Bt20, we used the mean of 2 measurements (for Bt20, 3 measurements were taken but the first was discarded). For Cebu and Guatemala, 3 measurements were averaged. BP was represented as a continuous variable [focus on systolic BP (SBP), but diastolic BP (DBP) also reported] or categorized to represent prehypertension and hypertension (P/HTN), defined as SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg for adults. Because Bt20 participants were adolescents, we defined P/HTN for them as SBP or DBP greater than or equal to the 90th percentile of age-, sex-, and height-specific cutoffs as recommended by the National High Blood Pressure Education Program Working Group (25). Antihypertensive medications were used by <0.5% of the participants. We included prehypertension in our outcome because of the young age of the study participants.
Infant and child anthropometric measures
BW was measured by research teams in Pelotas, New Delhi, and Guatemala. In Cebu, BW was measured by birth attendants who had been provided with mechanical scales for home births (60%) or was obtained from hospital records for the remainder. In Bt20, weight was obtained from reliable birth records (26). Subsequent weights were measured by research teams using standard techniques (14, 19, 20, 22, 24) and then converted to weight-for-age z (WAZ) scores using the WHO Growth Standards (27). The New Delhi weight data contributed to the pooled data set as values interpolated to exact ages of 12, 24, and 48 mo by using individual weight curves. Midchildhood weight was measured at a mean age of 48 mo in Pelotas, New Delhi, and Guatemala; at 60 mo for Bt20; and at 102 mo in Cebu. To make midchildhood weight comparable across sites, we imputed 48-mo z scores for Bt20 and Cebu participants, assuming a linear change in z score from 24 to 60 or 102 mo respectively, and back-transformed the resulting z scores into weight (in kg).
Gestational age
Gestational age for most participants was based on a mother's report of the date of her last menstrual period and infant birth date. For Cebu participants with low BW, or whose mothers had pregnancy complications, Ballard scores obtained by clinical assessment were used instead. Small for gestational age (SGA) was defined as a BW below the age- and sex-specific 10th percentile of the BW distribution published by Williams et al (28).
Anthropometric measures at follow-up
Body mass index (BMI; in kg/m2) was calculated from measured weight and height at age 15 y in Bt20 and in young adulthood for the other sites.
Other covariates
Socioeconomic status at birth and in young adulthood was represented by maternal education (or paternal occupation in New Delhi) and/or by ownership of various household assets. An assets score was created for each site (29), and study participants were characterized by quintiles of these scores. Site-specific variables considered as potential confounders included race-ethnicity for Pelotas and Bt20, urban-rural residence for Cebu, and village of residence for Guatemala (to represent village size and nutrition intervention study design).
Conditional weight
To eliminate some statistical problems associated with modeling highly correlated weight measures, we used conditional weight (CW) variables to represent the component of weight at a given age that is uncorrelated with earlier weight measures (30, 31). CWs were calculated as the residuals from site- and sex-stratified linear regressions of weight (kg) at a given age on BW and any prior weights. The regression models also included exact age at measurement, and squared prior weight terms to account for nonlinearities. CW is thus the deviation in an individual's weight from its expected value, given his or her prior weights. CWs are estimated by using an individual's own prior weight data, but age- and sex-specific population data are used to generate the estimation equation. When a CW variable is included in a multiple regression with the variables it is conditioned on (BW and any prior weights), it can be interpreted as change in weight over the prior interval.
The CW residuals were standardized to allow comparisons across ages. For comparability in analyses that include CW, we also expressed BW as an internal sex- and site-specific z score. At 12, 24, and 48 mo, 1 SD of CW at the median corresponded to about 1.0, 0.7, and 0.9 kg, respectively, and 1 SD of BW corresponded to 0.5 kg.
Body composition
We calculated percentage body fat at follow-up using site-specific methods: bioimpedance and estimated percentage body fat with a deuterium-validated equation in Pelotas (32); weight, height, and abdominal or waist circumferences with an equation validated by hydrostatic weighing in Guatemala (33); dual-energy X-ray absorptiometry (Hologic Delphi) for the Bt20; and skinfold-thickness equations based on published conversion tables (34) validated for Asian populations (35) in Cebu and New Delhi. Fat mass (kg) was calculated as percentage body fat × weight, and lean mass (kg) was calculated as adult weight minus fat mass.
Analysis
We first assessed unadjusted differences in mean weight at birth and during childhood in groups with and without P/HTN. Differences by P/HTN status, stratified by sex and site, were evaluated by t test. We then estimated linear (for continuous SBP and DBP) or logistic (for P/HTN) regression models. All models included age at follow-up, sex, and site. We found no strong evidence of heterogeneity by sex or site. Gestational age, socioeconomic status, and site-specific potential confounders were omitted because they were not associated with adult BP and did not change the coefficients for the variables of primary interest. We developed a series of models. The first included the BW z score calculated from the WHO reference and is presented for comparison with other studies. The second included a site- and sex-specific internal BW z score and CW at 12, 24, and 48 mo, similar to the approach used by others (30, 31). We compared models with 1) no adjustment for adult size, 2) adjustment for adult BMI, and 3) adjustment for adult BMI and height. Adjustment for adult measures addressed whether higher than expected weight gain at specific ages in childhood related to BP or odds of P/HTN among adults with the same BMI (or BMI and height). We adjusted for adult height because it is a strong predictor of BP in healthy individuals, particularly in adolescents (36), and it is highly related to lean body mass.
The third set of models included adult CW but not BMI, because adult CW and BMI are very highly correlated. These models addressed a different question, namely, whether higher than expected weight at any age (including adulthood) related to adult BP and odds of P/HTN. To aid in the interpretation of these models (given the strong association of BMI with BP), we also estimated a linear regression model to determine how each CW predicted adult BMI.
We assessed whether CW related differently to BP or odds of P/HTN in adults who were born SGA compared with those born AGA by adding to model 2 a binary variable (=1 if born SGA) and terms for the interaction of SGA with BW and of SGA with CW at each age. We tested whether the association of childhood CW with adult BP differed across the full range of BW by including an interaction of BW with each CW.
In a separate analysis, we aimed to isolate possible pathophysiologic effects of excess fatness on BP from physiologic variation in BP related to height and lean body mass. We first estimated site- and sex-specific residuals of SBP and DBP predicted from age, height, and lean mass (representing a deviation from what would be expected based on these variables) and then used these residuals as outcomes. This analysis could not be conducted for Pelotas females because body-composition data were not available for them.
To assess potential selection bias related to the exclusion of the large number of Pelotas and Bt20 participants with missing 12-mo weight measures, we created a 24-mo CW variable conditional only on BW. We tested whether BW and CW at 24 and 48 mo had similar associations with adult SBP and risk of P/HTN in our main analysis sample (n = 4335) and those excluded only because of missing 12-mo data (n = 3842). Results were considered to differ if the P value for the interaction of being in the analytic sample with BW or CW was <0.10.
RESULTS
Characteristics of participants in the 5 cohorts
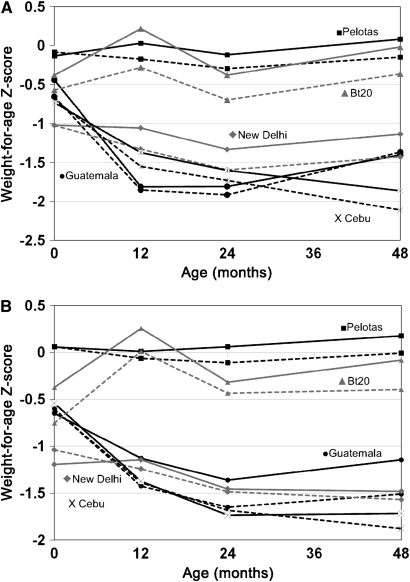
Mean age was near 30 y for the Guatemala and New Delhi cohorts, whereas participants from Pelotas and Cebu were in their early 20s, and the Bt20 participants were adolescents. Mean BW was highest in Pelotas and lowest in New Delhi (Table 2). WAZ scores varied little by age in Pelotas, whereas Bt20 children showed initial catch-up from lower WAZ at birth, but thereafter both cohorts had weight gain patterns that parallelled the WHO Growth Standard 50th percentile (Figure 1). In contrast, New Delhi, Guatemala, and Cebu children had declining WAZ scores in the first year of life. Young adults in Pelotas were the tallest, whereas Guatemala and Cebu participants were the shortest. Adult BMI was lowest in the Cebu and Bt20 cohorts (the latter likely reflected their young age). P/HTN prevalence was higher among males than among females. Within sites, P/HTN was lowest among males and females in Guatemala and females in Cebu.
TABLE 2.
Characteristics of the COHORTS (Consortium on Health Orientated Research in Transitional Societies) blood pressure analytic sample, by site1
| Infant and child |
|||||||||||
| Weight |
Adult |
||||||||||
| Site | SGA | Birth | 12 mo | 24 mo | 48 mo | Age | Weight | BMI | Height | SBP | P/HTN |
| kg | y | kg | kg/m2 | cm | mm Hg | ||||||
| Males | |||||||||||
| Pelotas Birth Cohort, Brazil (n = 487) | 0.16 | 3.30 ± 0.53 | 9.70 ± 1.22 | 12.16 ± 1.42 | 16.51 ± 2.19 | 23.07 ± 0.25 | 72.76 ± 14.22 | 24.13 ± 4.28 | 173.52 ± 6.38 | 123.56 ± 13.93 | 0.43 |
| INTCS, Guatemala (n = 115) | 0.35 | 3.09 ± 0.56 | 7.93 ± 1.06 | 9.85 ± 1.15 | 13.82 ± 1.36 | 31.46 ± 1.20 | 64.09 ± 10.66 | 24.12 ± 3.47 | 162.86 ± 6.17 | 116.59 ± 9.86 | 0.23 |
| New Delhi Birth Cohort Study, India (n = 591) | 0.41 | 2.89 ± 0.44 | 8.50 ± 1.06 | 10.33 ± 1.25 | 13.96 ± 1.58 | 29.40 ± 1.26 | 71.65 ± 13.78 | 24.92 ± 4.23 | 169.40 ± 6.27 | 118.14 ± 11.13 | 0.42 |
| CLHNS, Cebu, Philippines (n = 973) | 0.26 | 3.03 ± 0.42 | 8.27 ± 0.99 | 10.11 ± 1.13 | 13.20 ± 1.75 | 21.27 ± 0.78 | 56.01 ± 9.25 | 21.03 ± 3.04 | 163.03 ± 5.87 | 111.75 ± 10.93 | 0.44 |
| Bt20 cohort, Soweto-Johannesburg, South Africa (n = 168) | 0.16 | 3.14 ± 0.54 | 9.68 ± 1.42 | 11.64 ± 1.71 | 16.13 ± 1.80 | 15.62 ± 0.26 | 55.44 ± 12.00 | 19.94 ± 3.61 | 166.39 ± 7.74 | 116.64 ± 13.72 | 0.40 |
| Total (n = 2334) | 0.28 | 3.06 ± 0.49 | 8.71 ± 1.26 | 10.69 ± 1.53 | 14.32 ± 2.25 | 23.80 ± 4.38 | 63.82 ± 14.30 | 22.73 ± 4.15 | 167.06 ± 7.53 | 116.42 ± 12.66 | 0.42 |
| Females | |||||||||||
| Pelotas Birth Cohort, Brazil (n = 482) | 0.14 | 3.19 ± 0.49 | 9.10 ± 1.23 | 11.75 ± 1.47 | 16.20 ± 2.52 | 23.05 ± 0.25 | 61.42 ± 13.38 | 23.57 ± 4.86 | 161.34 ± 5.91 | 111.50 ± 12.34 | 0.22 |
| INTCS, Guatemala (n = 100) | 0.26 | 2.98 ± 0.44 | 7.65 ± 1.04 | 9.53 ± 1.09 | 13.31 ± 1.43 | 31.23 ± 1.22 | 60.48 ± 10.58 | 26.48 ± 4.36 | 151.09 ± 5.22 | 109.25 ± 10.57 | 0.14 |
| New Delhi Birth Cohort Study, India (n = 423) | 0.39 | 2.78 ± 0.38 | 7.80 ± 1.06 | 9.70 ± 1.22 | 13.17 ± 1.51 | 29.39 ± 1.33 | 59.48 ± 13.08 | 24.65 ± 4.96 | 155.03 ± 5.64 | 107.03 ± 10.88 | 0.24 |
| CLHNS, Cebu, Philippines (n = 845) | 0.21 | 2.98 ± 0.42 | 7.64 ± 0.94 | 9.45 ± 1.09 | 12.85 ± 1.72 | 21.10 ± 0.97 | 46.28 ± 7.94 | 20.26 ± 3.11 | 151.04 ± 5.40 | 99.52 ± 10.00 | 0.12 |
| Bt20 cohort, Soweto-Johannesburg, South Africa (n = 151) | 0.17 | 2.96 ± 0.49 | 9.13 ± 1.30 | 11.18 ± 1.59 | 15.56 ± 1.76 | 15.60 ± 0.25 | 54.80 ± 10.16 | 21.74 ± 3.79 | 158.76 ± 6.45 | 108.81 ± 12.61 | 0.22 |
| Total (n = 2001) | 0.23 | 2.99 ± 0.45 | 8.14 ± 1.26 | 10.19 ± 1.60 | 13.95 ± 2.38 | 23.41 ± 4.29 | 54.07 ± 12.88 | 22.41 ± 4.60 | 154.95 ± 7.07 | 105.18 ± 12.12 | 0.18 |
All values are proportions or means ± SDs. SGA, small-for-gestational age (birth weight <10th percentile of reference population; 26); P/HTN, prehypertension or hypertension; INTCS, Institute of Nutrition of Central America and Panama Nutrition Trial Cohort; CLHNS, Cebu Longitudinal Health and Nutrition Survey; Bt20, Birth-to-Twenty; SBP, systolic blood pressure.
FIGURE 1.
Mean weight-for-age z scores in males (A) and females (B). The solid lines represent those without prehypertension or hypertension (P/HTN), and the dashed lines represent those with P/HTN, defined as a systolic blood pressure ≥130 mm Hg or a diastolic blood pressure ≥80 mm Hg, except for Birth-to-Twenty (Bt20) adolescents (defined as a systolic or diastolic blood pressure ≥90th percentile of age-, sex-, and height-specific cutoffs; 25). Sample sizes were as follows for those with or without P/HTN, respectively: Pelotas (n = 1208 and 279 for males and n = 107 and 375 for females), Guatemala (n = 27 and 88 for males and n = 14 and 86 for females), New Delhi (n = 246 and 345 for males and n = 103 and 320 for females), Cebu (n = 431 and 542 for males and n = 99 and 746 for females), and Bt20 (n = 68 and 100 for males and n = 33 and 118 for females).
Population mean weight-for-age and later P/HTN
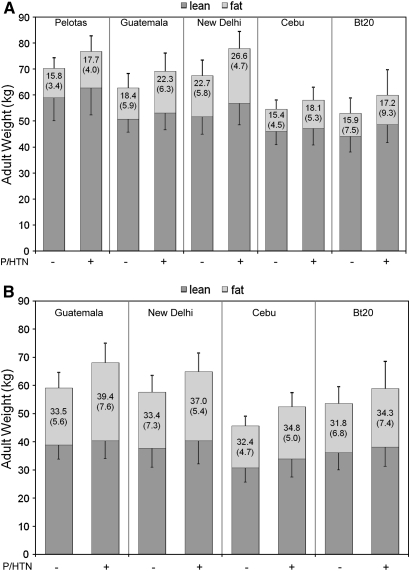
In all cohorts, WAZ scores tended to be higher in infancy and childhood among those who later had P/HTN (Figure 1). By 48 mo, WAZ scores were significantly higher in those with P/HTN in all cohorts except Guatemalan males and New Delhi females. In adults, BMI, on average, was 2.1 ± 0.14 (SE) units higher in those with P/HTN. Lean and fat mass were higher among those with P/HTN in all cohorts, and percentage body fat was higher in those with P/HTN in all but Bt20 males (Figure 2). The mean (±SE) age- and site-adjusted difference in fat mass in adults with and without P/HTN was 2.81 ± 0.16 kg in males and 3.85 ± 0.36 kg in females; whereas the mean difference in lean mass was 3.67 ± 0.23 kg in males and 2.44 ± 0.23 kg in females.
FIGURE 2.
Mean adult weight of males (A) and females (B) without (first bar for each site) or with prehypertension or hypertension (P/HTN; second bar for each site), stratified by lean and fat mass. The upper error bar represents the SD of the mean fat mass, and the lower error bar represents the SD of the mean lean mass. Numbers in the upper block of each column represent percentage body fat (fat mass/total body weight × 100 ± SD). Pelotas females were excluded because they had missing body-composition data. Sample sizes were as follows for those with or without P/HTN, respectively: Pelotas (n = 196 and 259 for males), Guatemala (n = 26 and 87 for males and n = 14 and 85 for females), New Delhi (n = 245 and 344 for males and n = 100 and 318 for females), Cebu (n = 411 and 485 for males and n = 92 and 638 for females), and Birth-to-Twenty (Bt20; n = 65 and 97 for males and n = 31 and 113 for females).
Multivariable models
BW was not associated with adult SBP, DBP, or odds of P/HTN without adjustment for adult size (Table 3 and Table 4; model 1A). After adjustment for BMI (model 1B), BW was inversely associated with adult SBP and odds of P/HTN, and the coefficient was larger and significant with additional adjustment for adult height (model 1C). BW was inversely related to DBP after adjustment for BMI alone (−0.39 mm Hg/SD; 95% CI: −0.68, −0.10) or BMI and height (−0.46 mm Hg/SD; 95% CI: −0.74, −0.18).
TABLE 3.
Association of birth weight and conditional weight (CW) at 12, 24, and 48 mo with adult systolic blood pressure: coefficients from multivariable linear regression models using pooled data from 5 birth cohorts (n = 4335)
| A. Adjusted for age, sex, site |
B. Also adjusted for adult BMI |
C. Also adjusted for adult BMI and
height |
|||||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Model 1 | |||||||||
| Birth weight (z score)1 | 0.11 | −0.22, 0.45 | 0.52 | −0.29 | −0.61, 0.04 | 0.08 | −0.52 | −0.85, −0.19 | <0.01 |
| Adult BMI (kg/m2) | 0.90 | 0.82, 0.99 | <0.01 | 0.90 | 0.82, 0.98 | <0.01 | |||
| Adult height (cm) | 0.16 | 0.11, 0.22 | <0.01 | ||||||
| Model 2 | |||||||||
| Birth weight (z score)2 | 0.13 | −0.22, 0.48 | 0.46 | −0.28 | −0.62, 0.06 | 0.11 | −0.57 | −0.93, −0.22 | <0.01 |
| CW3 12 mo | 1.14 | 0.79, 0.49 | <0.01 | 0.38 | 0.04, 0.73 | 0.03 | −0.04 | −0.42, 0.34 | 0.83 |
| CW 24 mo | 0.50 | 0.16, 0.85 | <0.01 | 0.06 | −0.27, 0.40 | 0.70 | −0.17 | −0.52, 0.17 | 0.32 |
| CW 48 mo | 1.23 | 0.89, 0.58 | <0.01 | 0.24 | −0.11, 0.59 | 0.17 | −0.03 | −0.39, 0.33 | 0.87 |
| Adult BMI (kg/m2) | 0.86 | 0.77, 0.96 | <0.01 | 0.91 | 0.82, 0.0 | <0.01 | |||
| Adult height (cm) | 0.17 | 0.11, 0.24 | <0.01 | ||||||
| Model 3 | |||||||||
| Birth weight (z score)2 | 0.13 | −0.21, 0.47 | 0.45 | 0.224 | −0.13, 0.574 | 0.224 | |||
| CW 12 mo | 1.13 | 0.79, 1.47 | <0.01 | 1.264 | 0.89, 1.634 | <0.014 | |||
| CW 24 mo | 0.51 | 0.18, 0.84 | <0.01 | 0.584 | 0.24, 0.924 | <0.014 | |||
| CW 48 mo | 1.25 | 0.91, 1.58 | <0.01 | 1.324 | 0.98, 1.664 | <0.014 | |||
| CW adult | 3.15 | 2.83, 3.48 | <0.01 | 3.204 | 2.87, 3.534 | <0.014 | |||
| Adult height (cm) | −0.064 | 0.084 | |||||||
z scores computed from the World Health Organization Growth Standard (27).
Internal site- and sex-specific z score.
CW standardized residual representing greater than expected weight gain in the prior interval.
Additionally adjusted for adult height only.
TABLE 4.
Association of birth weight and conditional weight (CW) at 12, 24, and 48 mo with adult prehypertension and hypertension: odds ratios (ORs) from logistic regression models using pooled data from 5 birth cohorts (n = 4335)
| A. Adjusted for age, sex, site |
B. Also adjusted for adult BMI |
C. Also adjusted for adult BMI and
height |
|||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Model 1 | |||||||||
| Birth weight (z score)1 | 0.99 | 0.93, 1.06 | 0.81 | 0.93 | 0.87, 1.00 | 0.04 | 0.91 | 0.85, 0.98 | 0.01 |
| Adult BMI (kg/m2) | 1.15 | 1.14, 1.18 | <0.01 | 1.16 | 1.13, 1.18 | <0.01 | |||
| Adult height (cm) | 1.01 | 1.00, 1.02 | 0.02 | ||||||
| Model 2 | |||||||||
| Birth weight (z score)2 | 1.00 | 0.93, 1.07 | 0.89 | 0.93 | 0.87, 1.00 | 0.05 | 0.91 | 0.84, 0.98 | 0.01 |
| CW3 12 mo | 1.20 | 1.12, 1.29 | <0.01 | 1.07 | 0.99, 1.15 | 0.09 | 1.03 | 0.94, 1.11 | 0.56 |
| CW 24 mo | 1.02 | 0.95, 1.09 | 0.54 | 0.95 | 0.89, 1.02 | 0.15 | 0.93 | 0.86, 1.00 | 0.05 |
| CW 48 mo | 1.18 | 1.10, 1.26 | <0.01 | 1.00 | 0.93, 1.08 | 0.99 | 0.98 | 0.90, 1.05 | 0.53 |
| Adult BMI (kg/m2) | 1.15 | 1.13, 1.18 | <0.01 | 1.16 | 1.14, 1.18 | <0.01 | |||
| Adult height (cm) | 1.02 | 1.00, 1.03 | 0.03 | ||||||
| Model 3 | |||||||||
| Birth weight (z score)2 | 0.99 | 0.93, 1.07 | 0.85 | 1.034 | 0.95, 1.114 | 0.474 | |||
| CW 12 mo | 1.21 | 1.13, 1.30 | <0.01 | 1.274 | 1.18, 1.384 | <0.014 | |||
| CW 24 mo | 1.02 | 0.95, 1.10 | 0.53 | 1.054 | 0.98, 1.134 | 0.174 | |||
| CW 48 mo | 1.18 | 1.10, 1.27 | <0.01 | 1.224 | 1.13, 1.314 | <0.014 | |||
| CW adult | 1.63 | 1.52, 1.75 | <0.01 | 1.664 | 1.55, 1.794 | <0.014 | |||
| Adult height (cm) | 0.984 | 0.96, 0.994 | <0.014 | ||||||
z score computed from the World Health Organization Growth Standard (27).
Internal site- and sex-specific z score.
CW standardized residual representing greater than expected weight gain in the prior interval.
Additionally adjusted for adult height only.
All CWs through midchildhood were strongly associated with adult SBP without adjustment for adult BMI or height (Table 3; model 2A). The 12- and 48-mo CW coefficients were >2 times the 24-mo CW coefficient. Similarly, higher CWs at 12 and 48 mo were associated with an increased odds of P/HTN (Table 4; model 2A). After adjustment for adult BMI and height (Table 3; model 2C), BW was inversely associated with SBP, whereas the CW measures were unrelated to SBP. Higher BW and CW at 24 mo were associated with reduced odds of P/HTN (Table 4; model 2C). Overall, a 1-SD (≈0.5 kg) increase in BW was associated with a 0.5–0.6-mm Hg decrease in SBP and a 9% reduction in odds of P/HTN. In a DBP model adjusted for adult BMI and height (comparable with that of model 2C in Table 3), BW was inversely associated with DBP (−0.51 mm Hg/SD; 95% CI: −0.82, −0.21), but CW measures were unrelated to DBP.
In models that included adult CW with or without adjustment for adult height (model 3C compared with model 3A in Tables 3 and 4), BW was unrelated to SBP or P/HTN, but all CW variables were strongly and positively associated with SBP and P/HTN. Adjustment for adult height (model 3C) increased the coefficients for the childhood CW terms. Taller adult stature was related to a lower odds of P/HTN in model 3C. The pattern of results for DBP was similar.
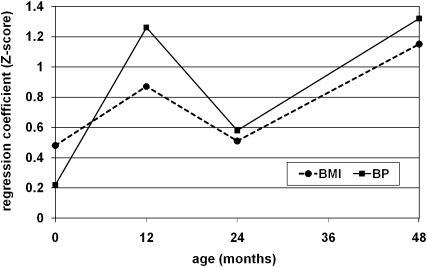
BW was more highly correlated with adult height (r = 0.25) than with adult BMI (r = 0.12), with correlations based on site- and sex-specific z scores. CW at all ages strongly predicted adult BMI. The sizes of the coefficients relating BW and CW to adult SBP in model 3C were roughly proportional to the coefficients relating BW and CW to adult BMI (Figure 3).
FIGURE 3.
Coefficients estimated from regressing adult systolic blood pressure (BP; solid line) or BMI (dotted line) on birth weight and conditional weight at 12, 24, and 48 mo by using data pooled from the 5 birth cohort studies (n = 4335).
Modeling the sex- and site-specific SBP residual as the outcome, we omitted age, site, and height (because the residual is uncorrelated with these variables by definition) but included BW and all CW measures (comparable with model 3A in Table 3). The SBP residual was inversely related to BW (−0.56 mm Hg/SD; 95% CI: −0.92, −0.21); unrelated to CW at 12, 24, and 48 mo; but positively related to adult CW (0.65 mm Hg/SD; 95% CI: 0.30, 1.00; P < 0.01). When added to this model, fat mass was strongly related to the residual (0.19 mm Hg per kg fat mass; 95% CI: 0.19, 0.25). Results for the DBP residual were very similar for BW (−0.58 mm Hg/SD; 95% CI: −0.88, −0.27) and adult CW (0.77 mm Hg/SD; 95% CI: 0.48, 1.07).
To determine whether the association of BW and CW with BP or risk of P/HTN differed according to whether an adult was born SGA, we specified model 2C to include a main effect of SGA and interactions of SGA with each CW (Table 5). BW was not included in these models because it is highly related to SGA. Being born SGA was associated with higher SBP and an increased odds of P/HTN, but there were no significant interactions of SGA with CW at any age. For DBP, there was no main effect of SGA, but higher CW was associated with higher DBP at 48 mo in those who were born SGA. In the alternate analysis designed to test whether CW had the same effect across the full BW distribution, no BW by CW interaction term was significant for SBP or DBP.
TABLE 5.
Coefficients from models estimating adult blood pressure, accounting for being born small-for-gestational age (SGA) and interactions of SGA with conditional weight (CW) at 12, 24, and 48 mo, males and females combined (n = 4055)1
| Systolic blood pressure |
Diastolic blood pressure |
Prehypertension and
hypertension |
|||||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | Odds ratio | 95% CI | P value | |
| SGA | 0.80 | 0.00, 1.60 | 0.05 | 0.38 | −0.30, 1.06 | 0.27 | 1.21 | 1.02, 1.43 | 0.03 |
| CW2 at 12 mo | 0.04 | −0.40, 0.49 | 0.85 | 0.07 | −0.31, 0.44 | 0.73 | 1.05 | 0.95, 1.15 | 0.36 |
| CW at 24 mo | −0.06 | −0.47, 0.34 | 0.76 | −0.07 | −0.42, 0.27 | 0.67 | 0.92 | 0.85, 1.01 | 0.08 |
| CW at 48 mo | −0.07 | −0.49, 0.36 | 0.76 | −0.39 | −0.75, −0.02 | 0.04 | 0.94 | 0.85, 1.03 | 0.17 |
| SGA × CW at 12 mo | −0.26 | −1.06, 0.55 | 0.53 | −0.23 | −0.91, 0.45 | 0.51 | 0.89 | 0.75, 1.05 | 0.18 |
| SGA × CW at 24 mo | −0.36 | −1.16, 0.43 | 0.37 | −0.05 | −0.72, 0.62 | 0.89 | 1.05 | 0.89, 1.24 | 0.55 |
| SGA × CW at 48 mo | 0.20 | −0.57, 0.97 | 0.62 | 0.73 | 0.08, 1.39 | 0.03 | 1.16 | 1.16, 1.37 | 0.08 |
| Wald test of interactions | F(3, 4039) = 0.47, P > F = 0.704 | F(3, 4039) = 1.73, P > F = 0.157 | χ2 = 5.18, P = 0.16 | ||||||
Models were adjusted for age at adult measurement, sex, site, adult height, and BMI.
CW standardized residual representing greater than expected weight gain in the prior interval.
Sample selectivity
The association of BW and CW with adult SBP and odds of P/HTN was attenuated in our analysis sample compared with the sample excluded because of a missing weight measurement at 12 mo (Table 6). The BW coefficient was about half as large in the analysis sample, and CW at 24 mo and CW at 48 mo estimated without inclusion of weight at 12 mo were not significantly related with SBP or of P/HTN in either sample.
TABLE 6.
Association of birth weight and conditional weight (CW) at 24 mo with adult systolic blood pressure (SBP) and risk of prehypertension and hypertension (P/HTN) in the analytic sample and in the sample missing weight data at 12 mo but no other covariates1
| Analytic sample (n
= 4335) |
Sample missing weight data at 12 mo
(n = 3926) |
|||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Linear regression SBP2 | ||||||
| Birth weight | −0.57 | −0.92, −0.21 | <0.01 | −1.40 | −1.86, −0.94 | <0.01 |
| CW3 | −0.13 | −0.50, −0.25 | 0.51 | −0.56 | −01.02, −0.10 | 0.02 |
| Logistic regression P/HTN4 | ||||||
| Birth weight | 0.91 | 0.84, 0.98 | 0.01 | 0.82 | 0.76, 0.89 | <0.01 |
| CW | 0.99 | 0.91, 1.07 | 0.73 | 0.91 | 0.84, 0.98 | 0.02 |
Models were adjusted for age at adult measurement, sex, site, adult BMI, and adult height.
F test for interactions of sample with birth weight and sample with CW, P = 0.05.
Conditional weight standardized residual representing greater than expected weight gain in the prior interval.
F test for interactions of sample with birth weight and sample with CW, P = 0.05.
DISCUSSION
Among healthy young adults, body size is the strongest determinant of BP. Thus, childhood weight gain is expected to predict adult BP to the extent that it determines adult body size. We wanted to determine how rapid childhood weight gain at specific age intervals through midchildhood related to adult BP and whether rapid weight gain was important independent of its contribution to adult size. We used CW to address these questions because each CW measure is uncorrelated with prior weight, which allowed for the assessment of the unique contribution of weight at each age.
In our sample, the associations of BW and CW at 12, 24, and 48 mo with adult BP roughly reflected the relative contribution of weight gain in these time periods to adult BMI. For example, children gained about twice as much weight in the first than in the second year of life. CW at 12 mo had a coefficient ≈2 times that of CW at 24 mo (Table 3). Adult CW (higher than expected gain from 48 mo to adulthood) had the largest BP coefficient.
When adult size (indexed by BMI and height) was held constant, there was no interval through midchildhood when greater than expected weight gain contributed to elevated BP. However, because models adjusted for adult height and BMI exclude adult CW (because of the high correlation of these variables), we concluded that weight gain after midchildhood is an important contributor to risk of elevated BP. This is consistent with other studies, which showed the importance of heterogeneous growth trajectories, including a pattern of relative thinness to age 2 y followed by more rapid growth to age 11 y (12) or excess weight gain after age 7 y (37). Further exploration of other weight and height trajectories is planned by the COHORTS group.
Consistent with a large body of research (38, 39) and with our prior metaregression analysis (29), we found significant inverse associations of BW with adult SBP and DBP and odds of P/HTN after adjustment for adult BMI and height. The size of these effects is consistent with previously published studies (equivalent to 1.1 mm Hg and a 19% reduction in odds of P/HTN per kg BW) (38, 40).
Concerns have been raised about the interpretation of BW associations in models that adjust for BMI measured concurrent with the BP outcome. Because of the positive association of BW with adult BMI and of BMI with BP, negative shifts in the BW coefficients after adjustment for BMI have been attributed to a bias resulting from “reversal paradox” (41), and the use of CW does not entirely free us of this concern (42). We opted to present unadjusted and adjusted results because, without adjustment for adult size, an independent effect of timing of weight gain cannot be estimated. Adjustment for adult BMI showed a significant inverse association of BW with adult SBP (models 2C and 3C), which was strengthened with further adjustment for adult height. This may reflect the higher correlation of BW with adult height and lean mass than with BMI or fat mass in our sample. We included adult height in our models because it is an important determinant of BP in healthy adolescents and was particularly relevant for the Bt20 cohort. Height is also an indicator of lean body mass; thus, adjustment for height may isolate the adverse effects of adult body fat on BP. It is interesting to note that in model 3C, which included CW through adulthood, taller stature was associated with lower odds of P/HTN. Ideally, we would like to have had complete length data for all of the cohorts, so that we could shed more light on the relative importance of weight gain and linear growth in childhood.
Given the particular importance in low- and middle-income countries of promoting early compensatory growth in SGA infants to reduce their risk of morbidity and mortality and to promote better cognitive outcomes, we tested whether higher CW related differently to BP in individuals who were SGA. Whereas SGA was related to higher SBP, the relation of CW to SBP was not different between adults who were born SGA and those born AGA, nor did this relation differ across the full range of BWs seen in our samples. Higher CW at 48 mo was associated with higher DBP and odds of P/HTN in adults who were born SGA. This could have been a chance finding or it may suggest that midchildhood growth is an important time for development of risk of elevated DBP in those with a history of prenatal growth restriction.
Several methodologic aspects of our study merit consideration. Integration of data from 5 cohorts for a pooled data analysis raises concerns about the comparability of measures across sites and whether the relations of interest vary substantially by site. Because of the variation in the timing of the midchildhood weight measurement, we imputed weight at 48 mo for the Bt20 and Cebu cohorts. CW coefficients through midchildhood were not substantially different when the actual 60-mo and 102-mo values were used for these sites, so we judged that the benefits of including these children in the analysis outweighed any potential biases related to imputation. Age at follow-up differed among the sites. Bt20 participants were adolescents, whereas the other cohorts included young adults. We addressed this by using a site-specific definition of P/HTN for Bt20 and adjusted for age in all models. We found no heterogeneity of effects by site or sex. Alternate models, which included additional potential confounders, including site-specific variables, produced no notable differences in the coefficients for BW or CW in childhood compared with our more parsimonious models. Despite substantial differences in infant and child weight, and adult age, height, BMI and BP, the similarity across sites of the relations of BW and CW to adult SBP enhances our confidence that we have identified biologically meaningful relations.
A final concern was with sample selection bias. Our analysis sample included a subset of participants with complete growth data in childhood (required to estimate the full set of CW variables) as well adult anthropometric and BP measurements. It differs from the full sample of cohort participants owing to attrition typical of longitudinal studies and to study design (eg, the loss of relatively more participants from the Pelotas and Bt20 cohorts). Results may be biased if BW and CW relate differently to adult BP in the included compared with excluded participants. Whereas it was not possible to estimate the effects of attrition, we used CW variables estimated without 12-mo data to compare selected models in our analysis sample to models run with the sample excluded owing to missing 12-mo data. BW coefficients were significantly smaller in our analysis sample, but CWs at 24 and 48 mo were unrelated to SBP or odds of P/HTN in both samples. The difference in the BW coefficient was accounted for primarily by the selectivity in the Pelotas sample. It is possible that seasonality played a role, because the Pelotas participants with data at 12 mo were those born between January and April.
Investigations of prenatal and early child growth effects on adult BP have been disproportionately carried out in high-income countries (39) and most have estimated the effects of weight gain without attention to the high level of correlation among weight measures at different ages (43–46). An exception to the latter is a recent study that used a linear spline random-effects model to show that higher weight gains in the first 5 mo and from 21 mo to 5 y were associated with higher BP (47).
Our study makes a unique contribution to the extant literature with its focus on samples from 5 low- and middle-income countries. In these settings, where chronic diseases of adulthood are rapidly emerging as major public health problems, the possible long-term risks of rapid child growth must be weighed against the well-established benefits of compensatory weight gain in growth-restricted children (4, 5). Evidence from our 5 birth cohorts suggests that higher weight gain in early life is only associated with elevated adult BP to the degree that early growth predicts adult BMI. However, at the same level of adult BMI, we found no association of weight gain from infancy to midchildhood to adult BP or risk of P/HTN. Furthermore, we confirmed prior studies showing that reduced fetal growth increases the risk of elevated BP in later life.
Because of its known association with height and BMI, BP may be more strongly affected by faster weight gain at any age than other chronic diseases and risk factors. This possibility will be tested by future analyses of the COHORTS data set addressing outcomes related to body composition, glucose concentrations, and lipid profiles. We will also look into how early growth might contribute to positive human capital outcomes, including school attainment and adult height. The evidence thus far suggests that the positive consequences of faster early weight gain in low- and middle-income countries outweigh its potential hazards (29). Nonetheless, prevention of overweight and obesity in children and young adults needs to be a priority to reduce the rising burden of cardiovascular disease in developing and transitional countries.
Acknowledgments
We especially thank the other contributors to the 5 studies: Guatemala (Rafael Flores and Ann DiGirolamo of the US Centers for Disease Control and Prevention; Usha Ramakrishnan; Kathryn Yount of Emory University; Ruben Grajeda, Paul Melgar, Humberto Mendez, and Luis Fernando Ramirez of INCAP; Jere Behrman of the University of Pennsylvania; John Hoddinott, Agnes Quisumbing, and Alexis Murphy of IFPRI; and John Maluccio of Middlebury College), Cebu (Barry Popkin of the University of North Carolina at Chapel Hill; Sororro Gultiano, Josephine Avila, and Lorna Perez of the Office of Population Studies Foundation, University of San Carlos, Cebu, Philippines; and Thomas McDade of Northwestern University), Pelotas (Fernando Barros of the Universidade Federal de Pelotas and Rosangela Lima of the Universidade Católica de Pelotas), Bt20 (Noel Cameron of Loughborough University and John Pettifor of the University of Witwatesrand), and New Delhi (Vinod Kapani of the Bureau of Labor Statistics, Washington, DC, and SK Dey Biswas of the Indian Council of Medical Research, New Delhi, India). Additional members of the COHORTS group were as follows: Fernando C Barros, Bernardo L Horta, and Denise P Gigante (Universidade Federal de Pelotas, Brazil); Caroline Fall and Clive Osmond (MRC Epidemiology Resource Centre, University of Southampton, Southampton, United Kingdom); Santosh K Bhargava (Sunder Lal Jain Hospital, New Delhi, India); Manuel Ramirez-Zea (Institute of Nutrition of Central America and Panama, Guatemala City, Guatemala); Daniel Lopez and Mathew Mainwaring (Department of Paediatrics, MRC Mineral Metabolism Research Unit, University of the Witwatersrand, Johannesburg; Bt20); Linda Richter (Human Sciences Research Council, Durban; Bt20); Christopher Kuzawa (Department of Anthropology, Northwestern University, Chicago, IL); and Judith Borja (Office of Population Studies Foundation, University of San Carlos, Cebu City, Philippines).
The authors' responsibilities were as follows—The COHORTS group was responsible for the development of the concept and methods; LSA: conducted the data analysis and prepared the first draft of the manuscript; each of the 5 studies was represented by one or more authors who participated in the design and/or implementation of the original study or follow-up surveys (Guatemala: RM and ADS; Pelotas: PCH and CGV; New Delhi: HSS and DP; Bt20: SAN; Cebu: LSA). All authors interpreted the results and helped revise the manuscript. None of the authors had any conflicts of interest.
REFERENCES
- 1.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr 1999;129:1555–62 [DOI] [PubMed] [Google Scholar]
- 2.Chang SM, Walker SP, Grantham-McGregor S, Powell CA. Early childhood stunting and later behaviour and school achievement. J Child Psychol Psychiatry 2002;43:775–83 [DOI] [PubMed] [Google Scholar]
- 3.Martorell R, Ho TJ. Malnutrition, morbidity and mortality. Popul Dev Rev 1984;10:49–68 [Google Scholar]
- 4.Victora CG, Barros FC. Commentary: the catch-up dilemma—relevance of Leitch's ‘low-high’ pig to child growth in developing countries. Int J Epidemiol 2001;30:217–20 [DOI] [PubMed] [Google Scholar]
- 5.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol 2001;30:1325–30 [DOI] [PubMed] [Google Scholar]
- 6.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteiro POA, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev 2005;6:143–54 [DOI] [PubMed] [Google Scholar]
- 8.Ong KK, Loos RJF. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006;95:904–8 [DOI] [PubMed] [Google Scholar]
- 9.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr 2005;82:456–66 [DOI] [PubMed] [Google Scholar]
- 10.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20 [DOI] [PubMed] [Google Scholar]
- 11.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 2004;363:1642–5 [DOI] [PubMed] [Google Scholar]
- 12.Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension 2007;49:1415–21 [DOI] [PubMed] [Google Scholar]
- 13.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease—the hypothesis revisited. BMJ 1999;319:245–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhargava SK, Sachdev HS, Fall CHD, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. Int J Epidemiol 2007;36:550–7 [DOI] [PubMed] [Google Scholar]
- 16.Victora CG, Sibbritt D, Horta BL, Lima RC, Cole T, Wells J. Weight gain in childhood and body composition at 18 years of age in Brazilian males. Acta Paediatr 2007;96:296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckett LA, Rosner B, Roche AF, Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol 1992;135:1166–77 [DOI] [PubMed] [Google Scholar]
- 18.Rosner B, Hennekens CH, Kass EH, Miall WE. Age-specific correlation analysis of longitudinal blood pressure data. Am J Epidemiol 1977;106:306–13 [DOI] [PubMed] [Google Scholar]
- 19.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol 2006;35:237–42 [DOI] [PubMed] [Google Scholar]
- 20.Stein AD, Melgar P, Hoddinott J, Martorell R. Cohort profile: the Institute of Nutrition of Central America and Panama (INCAP) Nutrition Trial Cohort Study. Int J Epidemiol 2008;37:716–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S, Bhargava SK, Moriyama IM. Longitudinal study of the survival and outcome of a birth cohort. New Delhi, India: Department of Paediatrics, Safdarjung Hospital, 1979 [Google Scholar]
- 22.Adair LS. Size at birth and growth trajectories to young adulthood. Am J Hum Biol 2007;19:327–37 [DOI] [PubMed] [Google Scholar]
- 23.Cebu Study Team. A child health production function estimated from longitudinal data. J Dev Econ 1992;38:323–51 [PubMed] [Google Scholar]
- 24.Richter L, Norris S, Pettifor J, Yach D, Cameron N. Cohort profile: Mandela's children: the 1990 Birth to Twenty study in South Africa. Int J Epidemiol 2007;36:504–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–76 [PubMed] [Google Scholar]
- 26.Ellison GTH, Richter LM, de Wet T, Harris HE, Griesel RD. The reliability of hand-written and computerised records of birth data collected at Baragwanath Hospital in Soweto. Curationis 1997;20:36–40 [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Multicentre Growth Reference Study Group WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76–85 [DOI] [PubMed] [Google Scholar]
- 28.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol 1982;59:624–32 [PubMed] [Google Scholar]
- 29.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol 2005;58:1320–4 [DOI] [PubMed] [Google Scholar]
- 31.Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr 2003;77:1498–505 [DOI] [PubMed] [Google Scholar]
- 32.Wells JCK, Gigante D, Wright A, Hallal PC, Victora CG. Validation of leg-to-leg impedance for body composition assessment in male Brazilians aged 16-19 years. Int J Body Comp Res 2003;1:63–7 [Google Scholar]
- 33.Ramirez-Zea M, Torun B, Martorell R, Stein AD. Anthropometric predictors of body fat as measured by hydrostatic weighing in Guatemalan adults. Am J Clin Nutr 2006;83:795–802 [DOI] [PubMed] [Google Scholar]
- 34.Durnin J, Womersley J. Body fat assessed from total body density and its estimation from skinfolds thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77–97 [DOI] [PubMed] [Google Scholar]
- 35.Deurenberg P, Deurenberg-Yap M. Validation of skinfold thickness and hand-held impedance measurements for estimation of body fat percentage among Singaporean Chinese, Malay and Indian subjects. Asia Pac J Clin Nutr 2002;11:1–7 [DOI] [PubMed] [Google Scholar]
- 36.Voors AW, Webber LS, Frerichs RR, Berenson GS. Body height and body mass as determinants of basal blood pressure in children—the Bogalusa Heart Study. Am J Epidemiol 1977;106:101–8 [DOI] [PubMed] [Google Scholar]
- 37.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension 2000;36:790–4 [DOI] [PubMed] [Google Scholar]
- 38.Schluchter MD. Publication bias and heterogeneity in the relationship between systolic blood pressure, birth weight, and catch-up growth–a meta analysis. J Hypertens 2003;21:273–9 [DOI] [PubMed] [Google Scholar]
- 39.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 2000;18:815–31 [DOI] [PubMed] [Google Scholar]
- 40.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–9 [DOI] [PubMed] [Google Scholar]
- 41.Tu YK, Ellison GTH, Gilthorpe MS. Growth, current size and the role of the ‘reversal paradox’ in the foetal origins of adult disease: an illustration using vector geometry. Epidemiol Perspect Innov 2006;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu Y-K, Gilthorpe MS. Unexplained residuals models are not solutions to statistical modeling of the fetal origins hypothesis. J Clin Epidemiol 2007;60:318–9(author reply 319–20) [DOI] [PubMed] [Google Scholar]
- 43.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 2003;41:451–6 [DOI] [PubMed] [Google Scholar]
- 44.Cheung YB, Low L, Osmond C, Barker D, Karlberg J. Fetal growth and early postnatal growth are related to blood pressure in adults. Hypertension 2000;36:795–800 [DOI] [PubMed] [Google Scholar]
- 45.Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 2007;92:98–103 [DOI] [PubMed] [Google Scholar]
- 46.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol 2005;161:27–32 [DOI] [PubMed] [Google Scholar]
- 47.Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension 2008;52:638–44 [DOI] [PubMed] [Google Scholar]