Abstract
Objective
Androgen deficiency is common in HIV infected women. We investigated the long-term effects of transdermal testosterone on body composition, bone density, quality of life, and safety.
Design
Twenty-five HIV-infected women with free testosterone below the median (≤ 3 pg/mL) of the female normal range were randomized to receive transdermal testosterone (300 mcg twice weekly) or identical placebo over 18 months.
Results
Women demonstrated low androgen levels (1.3±0.1 pg/mL), with relatively low weight (22.8±0.6 kg/m2) and low bone density (−0.61±0.17 SD hip T score) at baseline. No statistically significant differences were seen between the groups at baseline. The discontinuation rate was 16% and did not differ between treatment groups (p=0.24). Free testosterone by equilibrium dialysis increased over 18 months (7.9±1.8 pg/mL vs. 0.3±0.4 pg/mL; p=0.002, testosterone vs. placebo). Testosterone was well tolerated and did not effect lipids, liver, or safety indices. Lean mass (1.8±0.5 vs. 0.8±0.9 kg; p=0.04), and BMI (1.6±0.4 vs. 0.8±0.6 kg/m2; p=0.03, testosterone vs. placebo) increased in response to testosterone, whereas fat mass remained unchanged. Testosterone increased BMD at the hip (0.01±0.01 vs. −0.01±0.01 g/cm2; p=0.02) and trochanter (0.01±0.01 vs. −0.02±0.01 g/cm2; p=0.01, testosterone vs. placebo). Testosterone significantly improved depression indices (−6.8±2.2 vs. −1.9±3.1; p=0.02), and problems affecting sexual function (−1.8±0.8 vs. 0.5±0.5; p=0.01, testosterone vs. placebo).
Conclusions
Long-term testosterone administration was well tolerated in HIV-infected women and resulted in significant improvements in body composition, bone density, and quality of life indices. Further evaluation of the safety and efficacy of testosterone use among HIV-infected women is warranted.
Keywords: HIV, AIDS, Women, Testosterone
Introduction
Prior studies have demonstrated that androgen deficiency is prevalent among women with HIV 1, 2, 3, 4 and associated with reduced lean body mass, functional status and bone density in this population 1, 3, 5. The effects of short-term testosterone have been reported in a limited number of studies of HIV-infected women 1, 3, 6. However, little is known regarding the long-term effects of testosterone administration in this population.
An initial 12 week, dose-ranging, pilot study investigated the safety and efficacy of testosterone replacement, and concluded that testosterone was well tolerated, and associated with a trend towards improvement in weight and quality of life 3. In a 6 month randomized, placebo-controlled study of 150 mcg of testosterone twice weekly, a trend toward improvement in lean muscle mass was demonstrated 1. A 24 week randomized placebo-controlled study determined that a higher testosterone dose of 300 mcg twice weekly was safe, but did not significantly effect body composition 6.
The purpose of the current study was to determine the effects of long-term testosterone administration, 300 mcg twice weekly versus placebo, over 18 months. Primary study endpoints included lean body mass and bone mineral density. Quality of life parameters of depression, sexual function and safety were investigated.
Methods
Recruitment and Enrollment
Women were recruited from 2004–2006 via community and newspaper advertisements and provider referral. Eligibility was established based on age 18–55, body mass index (BMI) ≤ 26 kg/m2, HIV infected, relative reduction in androgen levels (free testosterone level < 3.0 pg/mL, median of the normal range for women by equilibrium dialysis), creatinine < 1.5 mg/dL, normal serum calcium, and FSH < 25 if oligomenorrheic. The specific age range was chosen to exclude younger women < 18 in whom effects on growth might be seen and in women > 55 years of age who were more likely to be postmenopusal. Subjects were excluded for any change in ARV regimen or use of an anabolic agent including testosterone, growth hormone, or other preparations within 3 months of study participation. Subjects were also excluded for current use of estrogen or any preparation known to affect bone density or bone turnover including oral contraceptives, trans-dermal contraceptive patches, depo provera, and combined progesterone-estrogen injections, or history of congestive heart failure, unstable angina, deep vein thrombosis, breast cancer, or sleep apnea. Pregnancy testing was performed at baseline and each subsequent visit and subjects were excluded for a positive pregnancy test. Subjects were required to demonstrate understanding of appropriate barrier contraceptive methods, and/or have a history of a tubal ligation, or hysterectomy prior to study entry. Stable use of lipid lowering and antidiabetic medications were permitted.
Protocol and Randomization
This study was approved by the IRB at MGH and MIT. All subjects gave written and informed consent. Free testosterone, calcium, 25-hydroxyvitamin D level, urine pregnancy testing, medical history including menstrual history, weight and bone density by dual energy X-ray absorptiometry (DEXA) were obtained at the screen visit.
The primary care physician for each eligible participant was contacted prior to the baseline visit to confirm safety of study enrollment, and to provide details on the study procedures. Subjects greater than 40 years of age were required to have a mammogram performed within one year of study enrollment, and to provide a copy of these results prior to the baseline visit.
Eligible subjects attended a baseline study visit in the morning following a 12 hour fast. Fasting blood was drawn for testosterone, free testosterone, SHBG, estradiol, LH, FSH, lipids, LFT’s, glucose, insulin, CD4 and viral load. Subjects also underwent a standard oral glucose tolerance test. Subjects provided a four day food record to determine caloric intake, and a menstrual history. A history and physical exam were performed and acne and degree of hirsutism were assessed. Subjects underwent DEXA scanning for assessment of bone density and body composition, and completed surveys evaluating mood and sexual function. All study subjects were seen one month after the baseline visit for a safety visit and then every 6 weeks for a urine pregnancy test, and every 3 months for blood work, urine pregnancy testing, history and physical exam. Visits identical to the baseline visit occurred at 9 and 18 months. Compliance was assessed at each visit by count of returned patches. A supply of study medication and a menstrual diary were distributed to subjects at each visit after confirmation of a negative pregnancy test, and safety and compliance assessment. After the 18 month visit, all patients were offered open label testosterone for an additional 12 months, as a further incentive to enter the study and to collect longer-term safety data. Patients remain enrolled in this second open-label phase of the study, the results of which will be reported at a later date after all subjects complete the study.
Subjects were randomly assigned to receive either active testosterone transdermal delivery system (estimated delivery dose 300 mcg/day, changed twice weekly; 8.4 mg patch; Proctor and Gamble Pharmaceuticals), or an identical placebo patch. The dose was chosen based on dose-ranging pilot studies suggesting that the 300 mcg dose was safe 3 and would be more effective than a 150 mcg dose 1 in HIV-infected women. Study investigators and subjects were blinded to drug assignment, and randomization was performed by the Research Pharmacy at the MGH. Randomization was stratified by bone density (lumbar T score < or ≥ −1.0 SD by DEXA at screen visit) using a permuted block algorithm with randomly generated numbers. Subjects were instructed on proper patch application technique and were told to return all used and unused patches to the investigator at subsequent study visits for assessment of compliance. Subjects applied their first patch upon completion the baseline visit.
Laboratory Assessment
Testosterone and Immunologic Assays
Free testosterone was measured by equilibrium dialysis (Esoterix, CV 6.6%–9.4%) with a normal range for females of 1.1–6.3 pg/mL. HIV RNA was quantified from 400 to 750,000 copies/mL (Roche Diagnostics).
Nutritional Assessment and Body Composition
Height, fasting weight and BMI were determined 7. Food records were analyzed using Nutrition Data System, version 2006.
Total body lean mass and fat, in addition to bone mineral density at the lumbar spine, total hip, and greater trochanter were measured using DEXA (Hologic 4500). Precision for total body lean and fat mass is 3% and for bone > 1.5% 1, 5.
Mood State and Sexual Function
Mood and sexual function were evaluated by questionnaires completed by study subjects. Depression was evaluated with the Beck’s Depression Inventory (BDI) 8, and sexual function was assessed with the Brief Index of Sexual Function–Women (BISF-W) 9. Subjects were instructed to answer all questions. The BISF-W consists of 22 items with seven domains including: Thoughts and Desires, Arousal, Frequency of Sexual Activity, Receptivity/Initiation, Pleasure, Relationship Satisfaction, and Problems Affecting Sexual Function. Data from one patient who did not complete the BDI were excluded.
Safety
Subjects were counseled on appropriate barrier contraception methods at each visit, and a urine pregnancy was performed on all subjects, every six weeks. Subjects who experienced increased hair growth (e.g. facial hair) could remain in the study on a lower dose of testosterone (1 patch per week), but dose reductions were not necessary and full dosing was continued throughout the study for all subjects. Changes in menstrual status, missed periods and/or irregular bleeding, were noted and reported back to the primary care physician if significant (see Safety Assessment). One woman in the placebo group discontinued due to irregular menstrual bleeding. A data and safety monitoring board met every three months to monitor the adverse events in the study.
Statistical Analysis
Baseline comparisons between the two groups were analyzed using the Student’s t test for continuous variables, and Chi-square analyses for categorical variables. Summary statistics are presented as mean ± SEM for continuous outcomes and frequency (%) for categorical outcomes. A longitudinal linear mixed effects model was applied to evaluate the effect of 300 mcg twice weekly transdermal testosterone patch over 18 months in which the intercept and effect of time were random. Exploratory data analysis revealed that the response after 18 months was not different from the response at 9 months. Responses at 9 and 18 months were pooled as the post treatment repeated measures. All data were included in the analysis, including 9 month data from the 4 subjects who discontinued after the 9 month visit. For immune parameters CD4 and viral load, non parametric comparisons were made by the Wilcoxon test between treatment groups at baseline, 9 and 18 months during the study. The study was powered at 80% to detect a significant treatment difference of 2.7 kg in lean body mass between randomization groups, with 25 randomized patients and a 15% assumed dropout rate, using a two sided 5.0 percent significance level. Power calculations were based on the shorter-term study of Choi et al, demonstrating a 2.3 kg SD for the change in lean body mass over 24 weeks in response to a similar dose of testosterone in HIV-infected women 6. Data were analyzed using JMP statistical software (SAS Institute), and SAS (Version 9).
Results
Recruitment and Enrollment
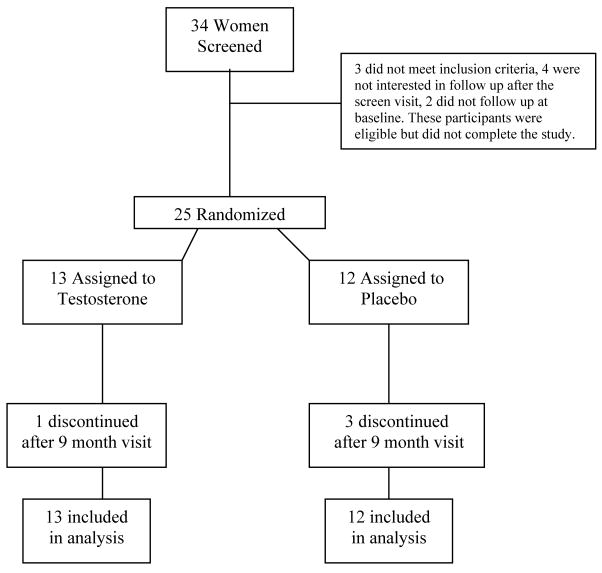
Thirty four women screened for the study, and twenty-five were randomized (Figure 1). Twenty five women were randomized to receive testosterone (n = 13) or placebo (n = 12) and received study drug. Four subjects discontinued study participation after the 9 month visit (3 in the placebo arm and 1 in the testosterone arm). Three of these subjects discontinued for non-medical issues unrelated to the study, and 1 discontinued after experiencing dysfunctional menstrual bleeding for which she requested the blind be broken. She was receiving placebo and did not wish to continue in the study. The overall dropout rate was 16% and was not different between the treatment groups (p=0.24).
Figure 1.
Schematic overview of study enrollment
Baseline Demographics & Clinical Characteristics
No significant differences were seen between the two groups for any of the baseline variables (Tables 1 and 2). One subject in the testosterone treatment group had a bilateral oophorectomy. Subjects were low weight (BMI = 22.4 ± 1.0 vs. 23.2 ± 0.7 kg/m2, p=0.53, testosterone vs. placebo) and had low androgen levels (1.2 ± 0.1 vs. 1.4 ± 0.2 pg/ml, p=0.45; testosterone vs. placebo; normal range for females 1.1–6.3 pg/ml; Table 2). Subjects also demonstrated low bone density at baseline with reduced T scores at the hip, trochanter, and lumbar spine (Table 1). Twenty five hydroxy-vitamin D (28 ± 2 vs. 28 ± 3 ng/mL, p=0.91) and calcium levels (9.3 ±0.1 vs. 9.2 ± 0.1 mg/mL, p=0.38, were not different between the groups at baseline.
Table 1.
Baseline Demographic Characteristics & Immune Function
| Placebo (n = 12) | Testosterone (n = 13) | P Value | |
|---|---|---|---|
| Age (years) | 43 ± 1 | 45 ± 2 | 0.32 |
| Race/ Ethnicity (%) | 0.25 | ||
| White | 25% (3) | 46% (6) | |
| Black | 50% (6) | 15% (2) | |
| Hispanic | 25% (3) | 31% (4) | |
| Native American | 0 | 8% (1) | |
| Caucasian vs. Non-Caucasian | - | - | 0.27 |
| Menstrual Status (%) | 0.16 | ||
| Amenorrehic | 17% (2) | 15% (2) | |
| Eumenorrheic | 75% (9) | 54% (7) | |
| Oligomenorheic | 8% (1) | 0 | |
| Hysterectomy | 0 | 31% (4) | |
| Eumenorrheic or Not | - | - | 0.27 |
| Current Smoker (%) | 50% (6) | 54% (7) | 0.85 |
| Lipid Lowering Medication Use (%) | 17% (2) | 15% (2) | 0.93 |
| Anti-diabetic Medication Use (%) | 8% (1) | 0% | 0.29 |
| Immune Function | |||
| Viral Load (log 10) | 3.0 ± 0.3 | 2.7 ± 0.1 | 0.23 |
| HIV Viral load % ≤ 400 copies | 75% (9) | 77% (10) | 0.91 |
| CD4 Count cells/mm3 | 509 ± 83 | 507 ± 72 | 0.98 |
| Duration of HIV (years) | 13 ± 1 | 15 ± 1 | 0.34 |
| Antiretroviral Therapy Use (%) | 83% (10) | 100% (13) | 0.12 |
| Current PI use (%) | 58% (7) | 69% (9) | 0.49 |
| Duration of PI use (years) | 3 ± 1 | 3 ± 1 | 0.90 |
| Current NRTI use (%) | 83% (10) | 92% (12) | 0.56 |
| Duration of NRTI use (years) | 7 ± 1 | 6 ± 2 | 0.73 |
| Current NNRTI use (%) | 27% (3) | 25% (3) | 0.98 |
| Duration of NNRTI use (years) | 2 ±1 | 2 ± 1 | 0.79 |
| Bone Mineral Density | |||
| Lumbar T-score | −0.64 ± 0.19 | −0.64 ± 0.29 | 0.99 |
| Hip T-score | −0.42 ± 0.18 | −0.79 ± 0.27 | 0.27 |
| Trochanter T-score | −0.34 ± 0.23 | −0.89 ± 0.31 | 0.18 |
Table 2.
Baseline Clinical Characteristics & Treatment Effects
| Placebo (n = 12) | Testosterone ( n = 13) | Baseline Comparison |
Change Between Groups |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Change at 9M | Change at 18M | Baseline | Change at 9M | Change at 18M | P Value | P Value |
| Body Composition | ||||||||
| Body Mass Index (kg/m²) | 23.2 ± 0.7 | −0.1 ± 0.4 | 0.8 ± 0.6 | 22.4 ± 1.0 | 1.2 ± 0.4 | 1.6 ± 0.4 | 0.53 | 0.03 |
| Weight (kg) | 59.9 ± 1.7 | −0.1 ± 1.0 | 2.0 ± 1.7 | 59.7 ± 3.2 | 3.0 ± 1.0 | 4.2 ± 1.2 | 0.95 | 0.03 |
| Total Lean Mass (kg) | 42.1 ± 0.9 | −0.3 ± 0.5 | 0.8 ± 0.9 | 44.1 ± 2.1 | 1.2 ± 0.4 | 1.8 ± 0.5 | 0.42 | 0.04 |
| Total Fat Mass (kg) | 17.7 ± 1.6 | 0.0 ± 0.7 | 0.6 ± 1.0 | 15.7± 1.3 | 1.6 ± 1.0 | 1.7 ± 1.0 | 0.36 | 0.16 |
| Metabolic Parameters | ||||||||
| Cholesterol (mg/dL)[mmol/L] | 180 ± 12 [4.66 ± 0.31] |
8 ± 6 [0.21 ± 0.16] |
−5 ± 10 [−0.13 ± 0.26] |
196 ± 17 [5.08 ± 0.44] |
−11 ± 12 [−0.28 ± 0.31] |
2 ± 9 [0.05 ± 0.23] |
0.49 | 0.66 |
| Low Density Lipoprotein (mg/dl)[mmol/L] | 114 ± 11 [2.95 ± 0.28] |
2 ± 6 [0.05 ± 0.16] |
−10 ± 10 [−0.26 ± 0.26] |
123 ± 14 [3.19 ± 0.36] |
−10 ± 9 [−0.26 0.23] |
−3 ± 7 [−0.08 ± 0.18] |
0.60 | 0.79 |
| High Density Lipoprotein (mg/dl)[mmol/L] | 47 ± 3 [1.22 ± 0.08] |
2 ± 2 [0.05 ± 0.05] |
−1 ± 3 [−0.03 ± 0.08] |
46 ± 3 [1.19 ± 0.08] |
−2 ± 3 [−0.05 ± 0.08] |
−6 ± 2 [−0.16 ± 0.05] |
0.90 | 0.18 |
| Triglycerides (mg/dl)[mmol/L] | 100 ± 16 [1.13 ± 0.18] |
22 ± 16 [0.25 ± 0.18] |
30 ± 16 [0.34 ± 0.18] |
132 ± 19 [1.49 ± 0.21] |
11 ± 17 [0.12 ± 0.19] |
105 ± 75 [1.19 ± 0.85] |
0.21 | 0.56 |
| 0 minute Glucose (mg/dL)[mmol/L] | 86 ± 2 [4.77 ± 0.11] |
7 ± 3 [0.39 ± 0.17] |
8 ± 5 [0.44 ± 0.28] |
93 ± 6 [5.16 ± 0.33] |
−7 ± 5 −0.39 ± 0.28 |
−4 ± 7 −0.22 ± 0.39 |
0.25 | 0.03 |
| 120 minute Glucose (mg/dL)[mmol/L] | 131 ± 18 [7.27 ± 1.00] |
6 ± 8 [0.33 ± 0.44] |
14 ± 9 [0.78 ± 0.50] |
114 ± 8 [6.33 ± 0.44] |
[−4 ± 8] [−0.22 ± 0.44] |
[18 ± 12] [1.00 ± 0.67] |
0.37 | 0.85 |
| 0 minute Insulin (mcg IU/mL)[pmol/L] | 4.8 ± 0.7 [33 ± 5] |
2.8 ± 1.2 [19 ± 8] |
−79.3 ± 2.4 [−551 ± 17] |
7.0 ± 1.4 [49 ± 10] |
−0.5 ± 0.6 [−3 ± 4] |
−86.1 ± 6.7 [−598 ± 47] |
0.23 | 0.21 |
| Serum glutamic pyruvic transaminase (U/L) | 24 ± 3 | 0 ± 4 | −4 ± 4 | 27 ± 7 | 5 ± 2 | −3 ± 5 | 0.67 | 0.52 |
| Caloric Intake (kcal/d) | 2148 ± 174 | −85 ± 185 | 466 ± 544 | 2088 ± 231 | −132 ± 218 | −194 ± 226 | 0.84 | 0.27 |
| Hormones | ||||||||
| Free Testosterone (pg/ml)[pmol/L] | 1.4 ± 0.2 [5 ± 1] |
0.0 ± 0.3 [0 ± 1] |
0.3 ± 0.4 [1 ± 1] |
1.2 ± 0.1 [4 ± 0] |
5.1 ± 1.5 [18 ± 5] |
7.9 ± 1.8 [27 ± 6] |
0.45 | 0.002 |
| Total Testosterone (ng/dl)[nmol/L] | 20 ± 2 [0.7 ± 0.1] |
3 ± 3 [0.1 ± 0.1] |
2 ± 4 [0.1 ± 0.1] |
21 ± 5 [0.7 ± 0.2] |
66 ± 19 [2.3 ± 0.7] |
104 ± 24 [3.6 ± 0.8] |
0.88 | 0.001 |
| Lutenizing Hormone (mIU/L)[IU/L] | 18 ± 6 | −7 ± 5 | −5 ± 8 | 20 ± 6 | −7 ± 3 | −6 ± 4 | 0.81 | 0.95 |
| Follicular Stimulating Hormone (mIU/mL)[IU/L] | 20 ± 7 | −2 ± 2 | −2 ± 5 | 23 ± 7 | −3 ± 3 | −1 ± 3 | 0.75 | 0.90 |
| Estradiol (pg/ml)[pmol/L] | 57 ± 11 [209 ± 40] |
−2 ± 18 [−7 ± 66] |
−7 ± 17 [−26 ± 62] |
64 ± 14 [235 ± 51] |
−15 ± 8 [−55 ± 29] |
−13 ± 15 [−48 ± 55] |
0.72 | 0.52 |
| SHBG (nmol/L)[nmol/L] | 144 ± 9 | 17 ± 8 | 1 ± 12 | 139 ± 24 | −6 ± 11 | −5 ± 12 | 0.87 | 0.18 |
| Bone Mineral Density | ||||||||
| Lumbar BMD (g/cm²) | 1.03 ± 0.03 | −0.01 ± 0.01 | −0.02 ± 0.01 | 0.98 ± 0.03 | 0.01 ± 0.01 | −0.02 ± 0.01 | 0.33 | 0.45 |
| Hip BMD (g/cm²) | 0.93 ± 0.03 | −0.02 ± 0.01 | −0.01 ± 0.01 | 0.86 ± 0.04 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.13 | 0.02 |
| Trochanter BMD (g/cm²) | 0.70 ± 0.03 | −0.02 ± 0.01 | −0.02 ± 0.01 | 0.62 ± 0.03 | 0.02 ± 0.01 | 0.01± 0.01 | 0.08 | 0.01 |
| Quality of Life | ||||||||
| Becks Depression Inventory | 11.8 ± 2.8 | 0.3 ± 2.4 | −1.9 ± 3.1 | 11.7 ± 2.8 | −2.7 ± 1.6 | −6.8 ± 2.2 | 0.98 | 0.02 |
| Brief Index of Sexual Function: | ||||||||
| D1: Thoughts/ Desires | 2.7 ± 0.6 | 0.5 ± 0.7 | −0.4 ± 0.6 | 3.9 ± 1.2 | 0.3 ± 1.1 | 1.4 ± 0.8 | 0.32 | 0.75 |
| D2: Arousal | 1.1 ± 0.5 | 0.9 ± 0.5 | 0.3 ± 0.5 | 2.7 ± 1.0 | 0.2 ± 1.2 | 0.8 ± 1.7 | 0.16 | 0.88 |
| D3: Frequency of Sexual Activity | 0.9 ± 0.3 | 0.6 ± 0.8 | −0.6 ± 0.2 | 1.9 ± 0.8 | 0.3 ± 0.7 | 0.0 ± 0.8 | 0.25 | 0.64 |
| D4: Receptivity/ Initiation | 3.2 ± 1.4 | −0.1 ± 0.6 | −0.3 ± 0.8 | 4.4 ± 1.4 | −0.9 ± 1.5 | 3.7 ± 2.1 | 0.57 | 0.62 |
| D5: Pleasure/ Orgasm | 1.9 ± 0.9 | −0.3 ± 0.9 | −0.9 ± 0.7 | 2.0 ± 0.9 | 0.5 ± 1.1 | 0.5 ± 1.1 | 0.91 | 0.26 |
| D6: Relationship Satisfaction | 5.0 ± 1.1 | −1.6 ± 1.0 | −1.8 ± 1.1 | 6.8 ± 1.2 | −1.1 ± 1.3 | −0.4 ± 1.9 | 0.28 | 0.59 |
| D7: Problems Affecting Sexual Function | 3.4 ± 0.7 | 0.4 ± 0.4 | 0.5 ± 0.5 | 4.4 ± 0.7 | −1.6 ± 0.6 | −1.8 ± 0.8 | 0.39 | 0.01 |
Ninety-two percent of the women were receiving antiretroviral therapy, and no significant difference was seen in baseline immune function or treatment status between the two groups (Table 1). 75% or more of the women in each group had undetectable viral load based on the assay used, p=0.91 (Table 1). The number of women receiving lipid lowering medications was 17% vs. 15% (p=0.93). One participant in the placebo group was receiving a stable metformin regimen upon study entry. No other patients were receiving antidiabetic medications.
Treatment Effects from Baseline to 18 Months
Hormone Levels
Testosterone treatment resulted in a significant increase in both free and total testosterone levels (change in free testosterone at 18 months; 7.9 ± 1.8 vs. 0.3 ± 0.4 pg/mL, p=0.002; change in total testosterone at 18 months; 104 ± 24 vs. 2 ± 4 ng/dL, p=0.001, testosterone vs. placebo; Table 2). Free testosterone levels achieved at 18 months were 6.8 (5.2, 15.5) pg/mL (median, interquartile range [IQR]; normal range 1.1 to 6.3 pg/mL). Estradiol, LH, FSH and SHBG did not change significantly between the treatment groups over 18 months (Table 2).
Weight and Body Composition
Lean body mass increased significantly among those in the testosterone treatment group compared to placebo (1.8 ± 0.5 vs. 0.8 ± 0.9 kg, p=0.04; Table 2), whereas no significant change was observed between the two groups for total body fat. Weight and BMI increased significantly in the testosterone treatment group compared to placebo (Table 2).
Bone Mineral Density
Bone mineral density increased among the testosterone treatment group at the total hip (0.01 ± 0.01 vs. −0.01 ± 0.01 g/cm2, p=0.02, testosterone vs. placebo), and trochanter (0.01 ± 0.01 vs. −0.02 ± 0.01 g/cm2, p=0.01, testosterone vs. placebo). A significant change in BMD was not observed at the lumbar spine (Table 2).
Immune Function
Neither HIV log 10 viral load nor CD4 count differed between the treatment groups at 9 months (2.6 [2.6, 3.1] vs. 2.6 [2.6, 2.8] copies (median[IQR]), p=0.61; 384 [262, 640] vs. 478 [352, 724] cells/mm3, p=0.28, testosterone vs. placebo) or 18 months (2.6 [2.6, 2.6] vs. 2.6 [2.6, 2.6] copies, p=0.41; 511 [248, 608] vs. 532 [416,792] cells/mm3, p=0.26, testosterone vs. placebo). The percentages with suppressed viral load at 9 months (64% vs. 73%, p=0.65) and 18 months (75% vs. 89%, p=0.42) testosterone vs. placebo, were not different between treatment groups.
Quality of Life
Testosterone treatment significantly improved depression indices, (−6.8 ± 2.2 vs. −1.9 ± 3.1, p=0.02 testosterone vs. placebo) and problems affecting sexual function compared to the placebo group, though changes were not observed for the other domains of the BISF-W (Table 2).
Compliance
All used and unused patches were collected every six weeks to measure study medication compliance. Compliance did not differ between the treatment groups (98% vs. 97%; testosterone vs. placebo, p=0.40).
Safety
Statistically significant differences in liver function and lipid levels were not observed between the treatment groups, and fasting glucose decreased in the testosterone vs. placebo patients (Table 2). Hirsutism scores did not differ significantly between the two groups at 18 months (0.1 ± 0.1 vs. 0.0 ± 0.4, p=0.81; testosterone vs. placebo).
No serious study-related adverse events occurred during the randomized portion of the study. The frequency of important adverse events potentially related to testosterone administration is shown in Table 3 and the frequencies of these events did not differ between treatment groups. Acne was reported in 4 patients receiving testosterone and 3 patients receiving placebo (p=0.75). None of the subjects required a dose reduction.
Table 3.
Adverse Events Potentially Related to Testosterone Administration
| Testosterone | Placebo | P Value*** | |
|---|---|---|---|
| Skin Reaction to patch | 4 | 1 | 0.16 |
| Change in Hair Pattern* | 2 | 4 | 0.29 |
| Acne | 4 | 3 | 0.75 |
| Change in Menstrual Status** | 6 | 9 | 0.14 |
Increased hair on chin, upper lip, chest, abdomen, fore arms, legs
Reported having more than 1 period in 1 month or missed a period during a monthly cycle
P value from Chi Square analyses
Discussion
This study is the first to investigate the effects of testosterone use over 18 months among HIV-infected women. Similar to other shorter-term studies 6, 10, 11, we now show that testosterone is well-tolerated over a long treatment period. In addition, we demonstrate that testosterone use among HIV-infected women with relatively low androgen levels, weight and bone density resulted in a significant increase in lean mass, weight, bone density at the hip and trochanter, and improvement in quality of life indices. Prior data demonstrate that low androgen levels are common among HIV-infected women 1, 2, 3, 4 suggesting a sizable population that might benefit from testosterone administration. Low androgen levels are seen among HIV-infected women even in the current era of HAART, as 31/35 women met the criterion for relative androgen deficiency in this study and at least 50% met a definition of relative androgen deficiency in a prior study 6.
Subjects randomized to testosterone experienced a significant increase in both free and total testosterone levels without simultaneous effects on estradiol. This suggests that minimal testosterone was aromatized to estrogen, consistent with prior investigations of transdermal testosterone use at this dose 11, 12. Contrary to the findings of a prior study with the same testosterone dose 6, transdermal testosterone use in our study had no significant effect on lipid levels, including HDL, and the number of women receiving lipid lowering medications did not differ between treatment groups.
Study related adverse events were similar between groups, without significant differences in hirsutism score, hair pattern, acne or skin irritation, or changes in menstrual pattern. The frequency of adverse events was consistent with that seen in other studies utilizing transdermal testosterone at similar or lower doses in HIV-infected women 1, 11, and none of the subjects withdrew related to such events, except for one women receiving placebo who withdrew for a change in menstrual pattern. Immune function remained stable.
Testosterone use resulted in a significant increase in lean mass in this long-term study. Prior testosterone treatment studies of a lower dose and/or shorter duration did not show an effect on lean mass among HIV-infected women 1, 3, 6. A significant effect of testosterone given at the same dose as used in this study was shown in a longer-term study of HIV-negative women, over a 12 month period 11. We selected patients who were at normal to low weight, and therefore, more likely to have lost lean body mass at baseline. Moreover, we chose patients based on low androgen levels. Indeed, 91% of HIV-infected women screened demonstrated a testosterone level below the cutoff chosen, with mean baseline levels very close to the lower end of the normal range. Women chosen for this study, with relatively low testosterone, may have been more likely to benefit than HIV-infected women with higher androgen levels.
Weight also increased within the testosterone group over 18 months. The women were of stable, relatively low weight at baseline, and the increase in BMI may be related to the increase in lean mass. In addition, this increase in BMI did not adversely affect insulin levels, potentially because subjects were at relatively low weight to begin the study. Indeed, those randomized to testosterone actually demonstrated a significant reduction in fasting glucose compared to placebo over 18 months, suggesting an improvement in overall glucose homeostasis. This improvement may relate to increased muscle mass for glucose disposal, but further studies are needed to verify this effect and determine its mechanism. Caloric intake did not change significantly between the groups over the course of the study.
Our study demonstrates beneficial effects of testosterone on bone density among HIV-infected women. Prior investigations have reported reduced bone density among women with HIV and androgen deficiency 13, 14, although no study to date has been long enough to examine the effects of testosterone treatment on bone density in this population. Bone density was reduced at all sites among the women in this study at baseline, consistent with prior data demonstrating that HIV-infected women with low androgen levels are at greater risk for bone loss. We demonstrate that testosterone use increased BMD at the hip and tronchanter, though no effect was seen for the lumbar spine. Similar results were seen among non HIV-infected women with hypopituitarism treated with the same testosterone dose for 12 months 11. These data suggest that cortical, rather than trabecular bone may be more responsive to testosterone administration. Estradiol levels did not increase in response to testosterone, arguing against increased estradiol levels as a mechanism for increased bone density. The effects on bone density may be directly related to the anabolic effects of testosterone on bone 15, 16 or via indirect effects, related to increased lean body mass and weight seen in response to testosterone 13, 14, 17.
A significant effect on mood and problems effecting sexual function was seen among those in the testosterone treatment group. At baseline, mean scores on the BDI were consistent with “mild to moderate” depression. A significant improvement was seen in response to testosterone with mean scores suggesting “no or minimal” depression in the testosterone group after 18 months, though individual responses varied. A beneficial effect of testosterone on depression and mood scores was not seen in prior studies of testosterone administration among women with HIV 1, although a lower dose of testosterone was used for only 6 months. In contrast, a beneficial effect of testosterone administration on mood was reported among androgen deficient women with hypopituitarism over 12 months 11. In our study, testosterone use resulted in a significant decrease in the number of problems affecting sexual function, though did not have an effect on or other domains present in the questionnaire. Testosterone use in postmenopausal women has also been associated with improvement in some aspects of sexual function, though studies are limited 18. Other studies of testosterone use (300 mcg) have showed improvement in libido and sexual function among women with bilateral oophorectomy 12, and also among women with hypopituitarism 11.
This study has a number of limitations. Although testosterone was well tolerated over 18 months, without signs of virilization, and patients with low levels were specifically chosen, testosterone levels did rise above the normal range in a number of the subjects. The levels achieved were similar to those reported in the investigator brochure and in the recent study of Davis et al. among non HIV-infected women 19. Transdermal testosterone administration is not approved for use in the United States. A transdermal testosterone product identical to the one used in this study is approved for the treatment of sexual dysfunction in oophorectomized women in Europe, though is not approved for other indications or for use in HIV infected women. Though these data from a relatively small, but long-term pilot study are very encouraging, the long-term safety of such a strategy needs to be investigated and confirmed in larger studies before it can be recommended clinically. Future studies will need to assess effects of long-term testosterone on risk for breast cancer, endometrial cancer, hirsutism and menstrual pattern. We assessed pregnancy tests frequently in the current study, as testosterone use is potentially harmful to pregnant women.
Women with HIV are known to have reduced bone density, lean body mass, and reduced quality of life. Low androgen levels are common in this population, and may contribute to such changes, yet no treatment strategies exist for women and research investigating gender specific treatment strategies in HIV-infected women has been very limited. In contrast, among male HIV-infected men, treatment of hypogondism is routine and improves body composition, bone and depression 20, 21. Data from this study suggest that testosterone (300 mcg twice weekly) is well-tolerated over 18 months, and results in significant effects on body composition, BMD, and quality of life indices. Further studies of long-term testosterone are necessary in women with HIV, as this treatment strategy may ultimately prove useful for the large numbers of such women with low androgen levels, bone loss and reduced quality of life.
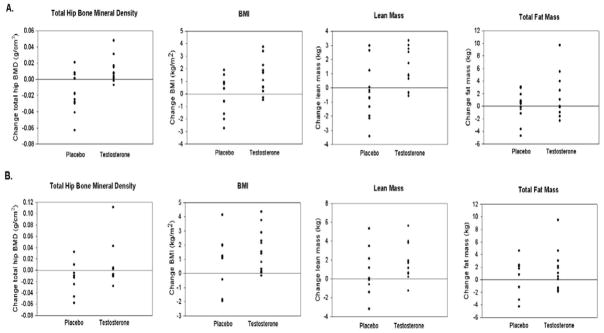
Figure 2.
Individual responses for variables from baseline to 9 months and baseline to 18 months. Individual responses for key variables represent change after 9 months (Panel A) and after 18 months (Panel B). Treatment responses above the 0 line indicate positive responders. The overall P values by longitudinal linear effects modeling include: Total hip (P = 0.02); BMI (P = 0.03); Lean mass (P = 0.04); and Fat mass (P = 0.16).
Acknowledgments
We would like to thank the subjects who participated in the study and the bionutrition, nursing, and support staff at the MGH GCRC, and the bionutrition staff at the MIT GCRC, for their dedicated patient care. Viral load testing was performed by the Emory Center for AIDS Research, Emory University, Atlanta, GA.
Funding
This study was funded by NIH DK-R01 054167 (Dr. Steven Grinspoon), M01-RR-01066, 1 UL1 RR025758-01, and the Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Procter and Gamble provided transdermal testosterone and blinded placebo patches, but did not participate in study design, analysis or manuscript preparation.
Funding Sources: NIH DK-R01 054167 (SG), M01-RR-01066, 1 UL1 RR025758-01, and the Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
Footnotes
Contributions
Dr. Looby contributed to the study recruitment, research subject management, data analysis and interpretation, and manuscript preparation. Ms. Collins contributed to data management, the coordination of study appointments/subjects, and manuscript preparation. Dr. Lee contributed to data analysis and interpretation, and manuscript preparation. Dr. Grinspoon contributed to the study conception, design, data analysis and interpretation, and manuscript preparation.
References
- 1.Dolan S, Wilkie S, Aliabadi N, et al. Effects of Testosterone Administration in Human Immunodeficiency Virus-Infected Women with Low Weight: A Randomized, Placebo-Controlled Study. Arch Intern Med. 2004;164:897–904. doi: 10.1001/archinte.164.8.897. [DOI] [PubMed] [Google Scholar]
- 2.Grinspoon S, Corcoran C, Stanley T, Rabe J, Wilkie S. Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86(9):4120–4126. doi: 10.1210/jcem.86.9.7843. [DOI] [PubMed] [Google Scholar]
- 3.Miller K, Corcoran C, Armstrong C, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab. 1998;83(8):2717–2725. doi: 10.1210/jcem.83.8.5051. [DOI] [PubMed] [Google Scholar]
- 4.Grinspoon S, Corcoran C, Miller K, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting [published erratum appears in J Clin Endocrinol Metab 1997 Oct;82(10):3360] J Clin Endocrinol Metab. 1997;82(5):1332–1337. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 5.Dolan SE, Carpenter S, Grinspoon S. Effects of weight, body composition, and testosterone on bone mineral density in HIV-infected women. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):161–167. doi: 10.1097/QAI.0b013e31804a7f4d. [DOI] [PubMed] [Google Scholar]
- 6.Choi HH, Gray PB, Storer TW, et al. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab Mar. 2005;90(3):1531–1541. doi: 10.1210/jc.2004-1677. [DOI] [PubMed] [Google Scholar]
- 7.Roche AF, Sievogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J Clin Nutr Dec. 1981;34(12):2831–2838. doi: 10.1093/ajcn/34.12.2831. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Arch Gen Psy. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Mazer NA, Leiblum SR, Rosen RC. The brief index of sexual functioning for women (BISF-W): a new scoring algorithm and comparison of normative and surgically menopausal populations. Menopause. 2000;7(5):350–363. doi: 10.1097/00042192-200007050-00009. [DOI] [PubMed] [Google Scholar]
- 10.Miller KK, Biller BM, Schaub A, et al. Effects of Testosterone Therapy on Cardiovascular Risk Markers in Androgen-Deficient Women with Hypopituitarism. J Clin Endocrinol Metab. 2007 Apr 10; doi: 10.1210/jc.2007-0195. [DOI] [PubMed] [Google Scholar]
- 11.Miller KK, Biller BM, Beauregard C, et al. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab May. 2006;91(5):1683–1690. doi: 10.1210/jc.2005-2596. [DOI] [PubMed] [Google Scholar]
- 12.Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000 Sep 7;343(10):682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 13.Huang JS, Wilkie SJ, Sullivan MP, Grinspoon S. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metab. 2001;86(8):3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 14.Dolan S, Carpenter C, Grinspoon S. Effects of Weight, Body Composition, and Testosterone on Bone Mineral Density in HIV-infected Women. J Acq Immun Def Syndr. 2007 doi: 10.1097/QAI.0b013e31804a7f4d. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82(10):3498–3505. doi: 10.1210/jcem.82.10.4306. [DOI] [PubMed] [Google Scholar]
- 16.Raisz LG, Wiita B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81(1):37–43. doi: 10.1210/jcem.81.1.8550780. [DOI] [PubMed] [Google Scholar]
- 17.Miller KK, Biller BM, Hier J, Arena E, Klibanski A. Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab Jun. 2002;87(6):2770–2776. doi: 10.1210/jcem.87.6.8557. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007 Feb 17;369(9561):597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 19.Davis SR, Moreau M, Kroll R, et al. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008 Nov 6;359(19):2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- 20.Grinspoon S, Corcoran C, Askari H, et al. Effects of androgen administration in men with the AIDS wasting syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129(1):18–26. doi: 10.7326/0003-4819-129-1-199807010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Grinspoon S, Corcoran C, Stanley T, Katznelson L, Klibanski A. Effects of androgen administration on the growth hormone-insulin-like growth factor I axis in men with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1998;83(12):4251–4256. doi: 10.1210/jcem.83.12.5305. [DOI] [PubMed] [Google Scholar]