Abstract
Introduction
A 105 kDa double mutant single-chain Fv-Fc fragment (scFv-Fc DM) derived from the anti-p185HER2 hu4D5v8 antibody (trastuzumab; Herceptin™) has been described recently. The goal of this study was to investigate whether improved tumor targeting could be achieved with this fragment through the use of residualizing radioiodination methods.
Methods
The scFv-Fc DM fragment was radioiodinated using N-succinimidyl 4-guanidinomethyl 3-[131I]iodobenzoate ([131I]SGMIB) and N-(3-[131I]iodobenzoyl)-Lys5-N- maleimido-Gly 1-GEEEK ([131I]IB-Mal-D-GEEEK), two residualizing radioiodination agents that have been used successfully with intact antibodies. Paired-label internalization assays of the labeled fragments were performed in vitro using MCF7 human breast cancer cells transfected to express HER2 (MCF7-HER2); comparisons were made to scFv-Fc DM directly radioiodinated using Iodogen. The tissue distribution of the scFv-Fc DM labeled with [125I]IB-Mal-D-GEEEK and [131I]SGMIB was compared in athymic mice bearing MCF7-HER2 xenografts.
Results
The scFv-Fc DM fragment was labeled with [131I]SGMIB and [131I]IB-Mal-D-GEEEK in conjugation yields of 53% and 25%, respectively, with preservation of immunoreactivity for HER2. Internalization assays indicated that labeling via SGMIB resulted in a 1.6- to 3.5-fold higher (p < 0.05) retention of radioactivity, compared to that from the directly labeled fragment, in HER2-expressing cells during a 24 h observation period. Likewise, the amount of radioactivity retained in cells from the IB-Mal-D-GEEEK-labeled fragment was 1.4- to 3.3-fold higher (p < 0.05). Tumor uptake of radioiodine activity in athymic mice bearing MCF7-HER2 xenografts in vivo was significantly higher for the [125I]IB-Mal-D-GEEEK-labeled scFv-Fc DM fragment compared with that of the [131I]SGMIB-labeled fragment, particularly at later time points. The uptake of 125I was 3-fold (3.6 ± 1.1%ID/g versus 1.2 ± 0.4%ID/g) and 4-fold (3.1 ± 1.7%ID/g versus 0.8 ± 0.4%ID/g) higher than that for 131I at 24 and 48 h, respectively. However, the [125I]IB-Mal-D-GEEEK-labeled scFv-Fc DM fragment also exhibited considerably higher levels of radioiodine activity in liver, spleen and kidney.
Conclusions
The overall results further demonstrate the potential utility of these two prosthetic groups for the radiohalogenation of internalizing monoclonal antibodies and their fragments. Specifically, the trastuzumab-derived double mutant fragment in combination with these residualizing agents warrants further evaluation for imaging and possibly treatment of HER2 expressing malignancies.
Keywords: HER2, radioiodination, monoclonal antibody, internalization
1. Introduction
Approximately 20–30% of human primary breast cancers over express the human epidermal growth factor receptor 2 (HER2, c-erbB-2, p185HER2) and the over expression of this tyrosine kinase receptor is associated with reduced disease-free and overall survival [15, 31]. A significant improvement in the survival rates of patients with early stage HER2 over expressing breast cancer has been achieved with the addition of trastuzumab (Herceptin™) to adjuvant chemotherapy [32]. This immunotherapeutic, hu4D5v8, is version 8 of the humanized 4D5 monoclonal antibody (mAb) [8] that contains two antigen recognition sites that bind to the juxta-membrane portion of the extracellular domain of the HER2 receptor [20]. The fraction of breast cancer patients that respond to trastuzumab treatment is low and hence there has been a concerted effort to develop second generation approaches, including radiolabeled molecules, for the diagnosis and treatment of p185HER2-expressing breast cancers.
Because trastuzumab treatment is only effective for patients with breast cancers expressing HER2 and because of the high cost and deleterious side effects associated with this modality, prior determination of the HER2 status is a sine qua non for individualizing trastuzumab therapy. Noninvasive molecular imaging offers significant advantages for the quantification of various receptors [37]; however, the pharmacokinetic properties of intact mAbs like trastuzumab are not ideal for imaging applications. With radiolabeled mAbs, their slow clearance from the circulation results in high background radioactivity levels that can hinder tumor detection and interfere with quantification. To overcome this, several smaller mAb fragments such as single chain Fv (scFv; ~25 kDa) and diabody (scFv dimer; 55 kDa) have been engineered [10, 11]. While scFv and diabody fragments clear faster from the circulation, their tumor uptake is considerably lower than that of intact mAbs. Furthermore, since their molecular weight is below the cutoff for renal filtration (<60 kDa), their use is associated with very high background in the kidneys which is problematic from both an imaging and radiation dosimetry perspective. A slightly larger engineered antibody fragment is the minibody that consists of scFv-CH3 dimers (80 kDa) [19]. Lower renal accumulation, in addition to higher tumor uptake was seen when carcinoembryonic antigen (CEA) was targeted with a radiometal labeled minibody compared to that with diabody [46]. However, radiometal labeled anti-p185HER2 minibodies demonstrated unexpectedly high kidney uptake and a lower than anticipated levels of tumor accumulation [34]. A recombinant mAb fragment of 105 kDa, a size intermediate between that of an intact IgG and a minibody, consisting of a scFv-CH2-CH3 dimer, was then developed [22, 34]. The Fc domain was retained in these fragments to augment tumor uptake and retention; however, two histidine residues—310 in CH2 domain and 435 in CH3 domain—were mutated in order to minimize Fc receptor mediated accumulation in normal tissues. The persistence of intact IgG in the circulation is largely due to the binding of their Fc domain to neonatal Fc receptors (FcRn; Brambell receptor) essentially diverting them from the lysosomal degradation pathway [6, 21], and the two mutated histidines are involved in this interaction [24].
An important consideration for determining the optimal radionuclide and labeling approach for use with molecules that bind to receptors, is the degree to which receptor-mediated internalization occurs. There are conflicting reports concerning the internalization of trastuzumab into breast cancer cells after binding to p185HER2. Austin et al. [2] have shown that, in SKBr3 human breast carcinoma cells, HER2-bound trastuzumab was predominantly surface-localized undergoing endocytosis at a rate of 1–2% per min, followed by efficient recycling to the cell surface; the half-life of internalization was ~19 h. On the other hand, trastuzumab-induced internalization of HER2 receptor in a dose- and time-dependent manner has been demonstrated [30]. Other research groups including our own also have obtained evidence for the internalization of radiolabeled trastuzumab [1, 5, 25]. Recently, the internalization of trastuzumab—naked and decorated with peptides containing nuclear localization sequence (NLS)—labeled with 111In also has been demonstrated [9].
Consistent with most of the evidence that trastuzumab is internalized after receptor binding, the tumor uptake of a minibody, derived from an internalizing anti-p185HER2 10H8 mAb and radioiodinated by a direct electrophilic method, was relatively low compared to that of the anti-CEA minibody [33]. This observation was attributed to rapid loss of the label after internalization and degradation. Several investigators including us have developed methods for the radiohalogenation of internalizing mAbs that have provided considerable advantages of tumor delivery of radioactivity compared with direct iodination procedures such as those using Iodogen [18, 36, 38, 39, 41]. The purpose of the current study was to evaluate the potential utility of two promising residualizing labels—SGMIB [42] and IB-Mal-D-GEEEK [41]—for labeling the 105 kDa double mutant scFv-Fc fragment (scFv-CH2-CH3 dimer) derived from trastuzumab; hereafter referred to as scFv-Fc DM [34]. These prosthetic groups were chosen not only because of the excellent results obtained with them for the radioiodination of internalizing anti-EGFRvIII mAbs but also because they are amenable to use with the α-particles emitting halogen radionuclide 211At, which is of great interest for targeted radiotherapy. Herein, the scFv-Fc DM fragment radioiodinated using SGMIB and IB-Mal-D-GEEEK templates was evaluated for immunoreactivity, cell processing in vitro by HER2 expressing breast cancer cells, and tissue distribution in athymic mice bearing human breast carcinoma xenografts.
2. Materials and Methods
2.1. General
All reagents were purchased from Sigma Aldrich except where noted. Sodium [131I]iodide (1200 Ci/mmol) and sodium [125I]iodide (2200 Ci/mmol) in 0.1 N NaOH were supplied by Perkin-Elmer Life and Analytical Sciences (Boston, MA). HPLC was performed using a Beckman Gold HPLC system equipped with a Model 126 programmable solvent module, a Model 168 diode array detector, and Beckman System Gold remote interface module SS420X, using 32 Karat® software. Radioactivity was detected using an IN/US -RAM radioactivity detector and Laura Lite® software (IN/US Systems, Tampa, FL); this system has the capability to detect two isotopes simultaneously. Size exclusion HPLC was performed using a TSK-GEL G3000SW (7.5 × 600 mm; 10 :m) column (TOSOH Bioscience LLC, Montgomeryville, PA) eluted with PBS pH 7.14 at a flow rate of 1 ml/min.
2.2. Trastuzumab scFv-Fc DM and HER2-ECD
The generation of anti-HER2 scFv-Fc DM has been described [34]. For this study, the protein was purified by affinity chromatography using protein L agarose beads (Pierce now called Thermo Scientific, Rockford, IL). Briefly, terminal cell culture supernatants were passed over the Protein L column. Captured protein was eluted with 0.1 M glycine, pH 2.5 and immediately neutralized by 2M Tris-HCl, pH 8 (10% v/v). The purified protein was dialyzed against PBS and concentrated with a Vivascience Vivaspin 20 (mwco: 30,000). Final protein concentrations were determined by measuring UV absorbance at 280 nm. Purified proteins were analyzed by SDS-PAGE under non-reducing and reducing conditions as described [34]. Binding was evaluated as described [33] by flow cytometry using MCF7-HER2 cells and anti-human PE-conjugated antibody [Fc specific] (Jackson ImmunoResearch Labs, West Grove, PA) for detection.
Soluble extracellular domain of HER2 (HER2-ECD), fused to human IgG1 or mouse IgG2a Fc (~96 kDa), was expressed in NS0 murine myeloma cells as described for other constructs [34] and purified by Protein A affinity chromatography (Poros20 A; Applied Biosystems, Foster City, CA).
2.3. Cells and culture conditions
MCF7 human breast cancer cells, transfected to express HER2 (MCF7-HER2–18) [4], were used in this study. Cells were cultured in DMEM/F12 (1:1) containing 10% FBS and 0.5 mg/ml G418 (geneticin) to select for HER2-expressing cells. The cell culture medium was supplemented with penicillin/streptomycin (100 U/ml) and fungizone (2 mg/ml). Cells were cultured at 37°C in a 5% CO2 atmosphere. All cell culture reagents were obtained from GIBCO (Grand Island, NY).
2.4. Direct radioiodination of scFv-Fc DM
A solution of scFv-Fc DM (150 :g; 4.5 mg/ml) in phosphate buffer, pH 7.4, was added to a 2-dram vial coated with Iodogen (10 :g) and containing a solution of sodium [131I]- or [125I]-iodide (~0.5 mCi; 1–2 :l). The mixture was incubated at room temperature for 10 min with occasional shaking, and the labeled protein was isolated by gel filtration on a PD-10 column (GE Healthcare, Piscataway, NJ) using PBS as running buffer.
2.5. Radioiodination of scFv-Fc DM using [131I]SGMIB and [125I]IB-Mal-D-GEEEK
The radioiodinated prosthetic groups were synthesized from their corresponding trialkylstannyl precursors and purified as reported before [40, 41]. For labeling with [131I]SGMIB, scFv-Fc DM in borate buffer, pH 8.5 (50 :l of 6 mg/ml; 2.86 nmol) was incubated with 0.4 – 1.6 mCi (0.33 – 1.33 nmol) of the tracer at room temperature for 15–20 min and the labeled fragment was purified by gel filtration as above. For labeling with [125I]IB-Mal-D-GEEEK, the protein was first treated with 2-iminothiolane (Thermo Scientific) to generate free sulfhydryl groups. For this, a solution of the scFv-Fc DM in PBS, pH 7.4, containing 1 mM EDTA (100 :l of 2.6 mg/ml; 2.48 nmol) was incubated at room temperature with a solution of 2-iminothiolane hydrochloride in 1 mM EDTA in PBS (2.0 :l of 4 mg/ml; 58 nmol) for 90 min. The thiol-derivatized fragment was isolated using a Microspin G-25 column (GE Healthcare, Piscataway, NJ) and then added to [125I]IB-Mal-D-GEEEK (1– 2 mCi; 0.45 – 0.91 nmol). The mixture was incubated at room temperature for 70 min with occasional mixing. Iodoacetamide (10 :l; 100 mg/ml PBS/EDTA) was added to quench the reaction. After a further incubation for 10 min, the labeled scFv-Fc DM was purified by passing over a PD-10 column as described above.
2.6. Determination of protein-associated radioactivity and immunoreactive fraction of labeled fragments
The protein-associated radioactivity of labeled fragments was determined in paired-label format by TCA precipitation. About 5 ng of each radiolabeled fragment in 25 :l PBS was mixed with 0.8 ml of 2% (v/v) human serum albumin in PBS, 0.1 ml of 20% (w/v) TCA, vortexed and incubated at room temperature for 45 min. The precipitated protein was pelleted, and the pellets and the supernatants were counted for 125I and 131I activity using an LKB 1282 dual-channel automated gamma counter with cross over correction for 131I in the 125I window. The percentage of the total radioactivity that was protein-associated was calculated from this. Immunoreactive fractions were determined in a paired-label format by incubating the scFv-Fc DM fragment labeled with 125I using Iodogen and that labeled with 131I using either [131I]SGMIB or [131I]IB-Mal-D-GEEEK for 30 min at 37°C with ~20-fold excess of HER2 extra cellular domain (ECD) in PBS containing 1% (v/v) HSA. The fraction of radioactivity bound to HER2 ECD was determined by injecting this mixture onto a size exclusion HPLC column [46].
2.7. Paired-label internalization of scFv-Fc DM labeled with [131I]SGMIB versus 125I-Iodogen and [125I]IB-Mal-D-GEEEK versus 131I-Iodogen by MCF7-HER2 cells
Cells at a density of 5 × 105 cells per well in 6-well plates were incubated with the tracer pair (0.5 – 1.0 :g each) at 4°C for 1h. Unbound radioactivity-containing medium was removed, and the cells were washed and supplemented with fresh medium and brought to 37°C. After 1, 2, 4, 8, 16, and 24 h, cell culture supernatants from triplicate wells were removed for analysis. Cells in these wells were then washed with culture medium (pH 2.0) to isolate surface-bound radioactivity, and then solubilized in 0.5 ml of 0.5 N NaOH. The cell culture supernatants, acid washes and the solubilized cells were counted in a gamma counter for 125I and 131I activity. The percent of initially bound radioactivity that was internalized and that present in cell culture supernatants and acid washes were calculated.
2.8. Paired-label biodistribution of labeled scFv-Fc DM in athymic mice bearing MCF7-HER2 subcutaneous xenografts
Animal experiments were performed under the guidelines established by the Duke University Institutional Animal Care and Use Committee. Eight to twelve week old athymic female mice (Balb c/nu/nu) weighing about 20 g were used in these studies. To augment tumor growth, 17-β-estradiol receptors were replenished by the administration of β-estradiol (Innovative Research of America, Sarasota, FL, USA) [7]. For this, mice were anesthetized (0.3 ml 1:10 ketamine/xylazine) and a 2 mm incision was made in the upper torso of each animal. A 0.72 mg, 60-d release β-estradiol pellet was inserted and the incision was closed with a staple. Three to five days later, 1 × 107 MCF7-HER2 cells in logarithmic growth phase in 0.1 ml of PBS were injected subcutaneously in the flank. Biodistribution experiments were performed two weeks later when the tumors were 100–150 mm3 in size.
Groups of five mice were injected with 1.2 :Ci of [125I]IB-Mal-D-GEEEK-labeled scFv-Fc DM and 2.0 :Ci of scFV-Fc DM labeled with [131I]SGMIB via the tail vein. At 2, 4, 12, 24, and 48 h after the tracer administration of the radioiodinated fragment pairs, mice were killed by an overdose of isofluorane. Organs of interest were harvested, blot-dried, weighed, and counted in an automated gamma counter for 131I and 125I activity along with injection standards. The results are expressed as percent injected dose per gram of tissue (%ID/g) except for the thyroid. The statistical significance of the uptake difference between the two tracers was calculated by a paired Student t test using the Microsoft Excel program statistical function. The differences were considered to be significant if the p values were less than 0.05.
3. Results
3.1. Radiolabeling of scFv-Fc DM
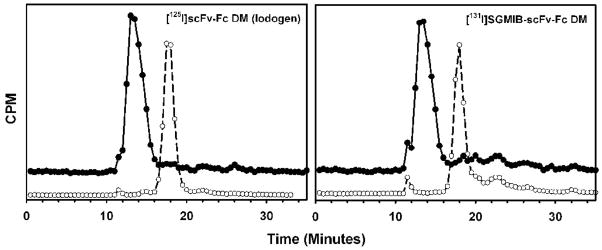
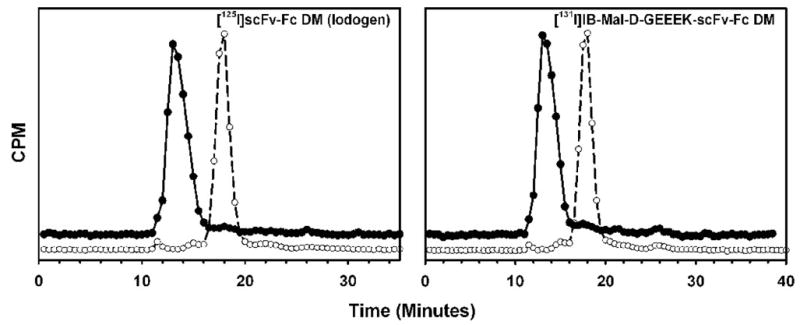
The fragment was radioiodinated by a direct method using Iodogen with an average radiochemical yield of 85%. The efficiency for the labeling of scFv-Fc DM with SGMIB and IB-Mal-D-GEEEK reagents was 53% and 25%, respectively. The specific activity of the labeled fragments was in the range of 0.4 – 2.8 :Ci/:g with the variation depending more on the initial radioiodine activity level than the method utilized. Because the labeling agents were prepared at a no-carrier-added level and thus used in the conjugation reactions in sub-stoichiometric amounts, the average number of residualizing residues per scFv-Fc DM molecule would be less than one. The highest specific activity obtained for the [131I]SGMIB-labeled constructs was 1.1 mCi/mg, which corresponds to about 1 SGMIB residue per 10 protein molecules. Likewise, the highest specific activities for the [131I]IB-Mal-GEEEK and [125I]IB-Mal-GEEEK conjugates were 0.36 mCi/mg and 0.76 mCi/mg, respectively. These correspond to about 1 substitution per 33 and 25 protein molecules, respectively. Protein-associated radioactivity as determined by TCA precipitation was 97.3 ± 0.7%, 97.6 ± 1.5%, and 99.4 ± 0.9% for the scFv-Fc DM labeled using Iodogen, SGMIB and IB-Mal-D-GEEEK, respectively. When analyzed by size-exclusion HPLC, fragments labeled by all 3 methods showed a single major peak with a tR of 17.5 min consistent with a molecular weight of 105 kDa (Figures 1 and 2). When the labeled fragments were incubated with excess of HER2-ECD, almost 100% of the peak shifted to a retention time of 13 min, corresponding to a molecular weight of about 215 kDa, in all three cases. These results suggest that the immunoreactivity of the scFv-Fc DM fragment was not compromised when it was radioiodinated using any of the three methods.
Figure 1.
Size exclusion chromatography of scFv-Fc DM fragment following radioiodination (open circles) and after incubation with 10- to 20-fold excess of ECD at 37°C for 30 min (filled circles). Left: 125I-scFv-Fc DM (Iodogen method); Right = [131I]SGMIB-scFv-Fc DM
Figure 2.
Size exclusion chromatography of scFv-Fc DM fragment following radioiodination (open circles) and after incubation with 10- to 20-fold excess of ECD at 37°C for 30 min (filled circles). Left: 125I-scFv-Fc DM (Iodogen method); Right = [131I]IB-Mal-D-GEEEK-scFv-Fc DM
3.2. Paired-label internalization of labeled fragments by MCF7-HER2 cells
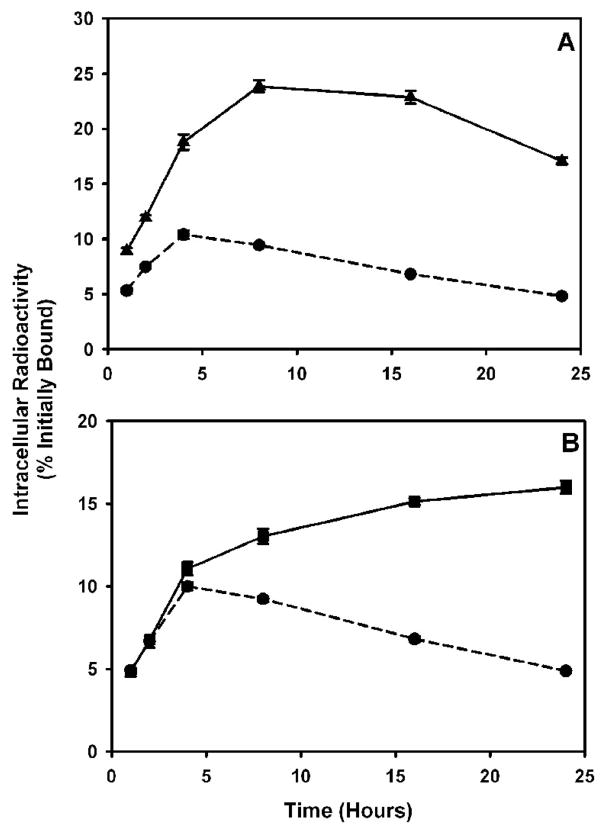
The ability to enhance the retention of intracellular radioactivity from the radioiodinated fragments through the use of the two residualizing labeling methods was determined by paired-label internalization assays using MCF7-HER2 cells. As seen in Figure 3, the intracellular radioactivity from scFv-Fc DM labeled using [131I]IB-Mal-D-GEEEK increased steadily with time. When the fragment was labeled with [131I]SGMIB, an increase in intracellular radioactivity up to 8–16 h was seen that dropped to some degree at 24 h. On the other hand, the radioactivity retained by the cells from the directly radioiodinated scFv-Fc DM fragment decreased over time, after an initial increase. Between 1–24 h, 1.6- to 3.5-fold higher (p < 0.05) radioactivity was retained from the SGMIB-labeled scFv-Fc DM fragment compared to that of the directly labeled fragment. The fraction of initially bound radioactivity that was in the intracellular compartment was 23.9 ± 0.5%, 22.9 ± 0.6%, and 17.1 ± 0.3% for scFv-Fc DM labeled with [131I]SGMIB and 9.4 ± 0.0%, 6.8 ± 0.1%, and 4.8 ± 0.1% for the directly labeled fragment at 8, 16, and 24 h, respectively. Similarly, the amount of radioactivity retained from the [131I]IB-Mal-D-GEEEK-labeled scFv-Fc DM fragment was 1.4- to 3.3-fold higher, compared to the directly labeled scFv-Fc DM fragment, during the 4–24 h period. Internalized counts accounted for 13.0 ± 0.5%, 15.1 ± 0.2%, and 16.0 ± 0.4% of the initially bound radioactivity for the scFv-Fc DM fragment labeled with [131I]IB-Mal-D-GEEEK and 9.2 ± 0.2%, 6.8 ± 0.2%, and 4.9 ±0.1% for scFv-DM directly labeled with 125I. The radioactivity pattern seen in the cell culture supernatants was complementary to that seen in the intracellular compartment; i.e. the radioactivity levels from the fragment labeled using the residualizing labeling methods were generally correspondingly less than those from scFv-Fc DM labeled directly (data not shown). A third paired-label assay was performed to directly compare the intracellular trapping of the scFv-Fc DM fragment labeled using [131I]SGMIB with that of [125I]IB-Mal-D-GEEEK labeled scFv-Fc DM fragment (Figure 4). The pattern of intracellularly-retained radioactivity with time was similar for both labeling methods; however, between 8 and 24 h, [125I]IB-Mal-D-GEEEK-labeled scFv-Fc DM fragment was retained to a significantly higher degree according to the paired t-test (p < 0.05).
Figure 3.
Paired-label internalization of scFv-Fc DM fragment radiolabeled with 125I using Iodogen (circles) and with 131I either by [131I]SGMIB (triangles; panel A) or by [131I]IB-Mal-D-GEEEK (squares; panel B) in MCF7-HER2 cells. Cells were incubated with the tracer pair at 4°C for 1 h, and after removal of the unbound radioactivity, brought to 37°C and processed at various time periods. Data shown are the percent of initially bound radioactivity that was trapped intracellularly.
Figure 4.
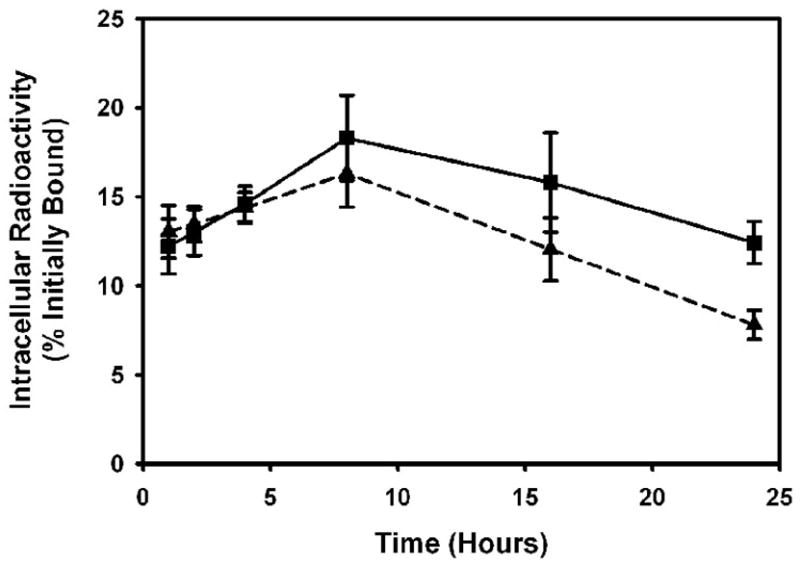
Paired-label internalization of scFv-Fc DM fragment radiolabeled with [125I]IB-Mal-D-GEEEK (squares) and [131I]SGMIB (triangles) in MCF7-HER2 cells. Cells were incubated with the tracer pair at 4°C for 1 h, and after removal of the unbound radioactivity, brought to 37°C and processed at various time periods. Data shown are the percent of initially bound radioactivity that was trapped intracellularly.
3.3. Tissue distribution of scFv-Fc DM: SGMIB versus IB-Mal-D-GEEEK
The uptake of [125I]IB-Mal-D-GEEEK-scFv-Fc DM and [131I]SGMIB-scFv-Fc DM in tumor and other tissues in athymic mice bearing MCF7-HER2 xenografts are shown in Table 1. At all time points studied, the uptake of 131I was significantly (p < 0.05) lower than that for 125I. At 24 and 48 h, the uptake of 125I was 3-fold (3.6 ± 1.1% versus 1.2 ± 0.4%) and 4-fold (3.1 ± 1.7% versus 0.8 ± 0.4%) higher than that for 131I. The thyroid uptake of 131I was 1.2- to 2.4-fold lower than that of 125I suggesting that the use of the SGMIB reagent resulted in a lower level of in vivo deiodination.
Table 1.
Paired-label biodistribution of [125I]IB-Mal-D-GEEEK scFv-Fc DM and [131I]SGMIB-scFv-Fc DM in athymic mice bearing MCF7/HER2 xenograftsa
| Organ | 2 h | 4 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|
| [125I]IB-Mal-D-GEEEK scFv-Fc DM | |||||
| Liver | 10.75±1.36b | 13.75±1.27 | 13.52±3.55 | 6.39±1.59 | 3.09±0.88 |
| Spleen | 4.27±0.73 | 10.27±0.39b | 12.65±12.97b | 3.17±1.56 | 2.69±1.17 |
| Lungs | 6.93±1.11 | 5.07±0.96 | 2.94±0.59 | 1.40±0.37 | 0.47±0.22 |
| Heart | 4.38±0.70 | 3.22±0.99 | 2.13±0.16 | 0.85±0.14 | 0.26±0.11 |
| Kidney | 7.82±1.16 | 7.53±1.09 | 7.57±0.61 | 5.68±1.33 | 3.04±1.14 |
| Stomach | 1.18±0.49b | 0.68±0.32b | 0.63±0.19 | 0.54±0.15 | 0.18±0.06 |
| Sm. intestine | 3.28±0.38b | 3.82±2.27 | 2.20±1.68 | 1.87±1.04 | 0.50±0.43 |
| Lg. intestine | 1.35±0.26 | 3.45±0.62b | 2.16±0.43 | 1.69±0.26 | 0.55±0.15 |
| Thyroid | 0.58±0.25 | 0.39±0.11 | 0.14±0.28b | 0.06±0.02b | 0.08±0.03 |
| Muscle | 1.16±0.33 | 0.95±0.31 | 1.28±1.11b | 0.61±0.09 | 0.18±0.13 |
| Blood | 19.96±2.67 | 9.77±4.21 | 4.10±2.33 | 1.19±0.24 | 0.33±0.18 |
| Bone | 1.47±0.19 | 2.87±1.36 | 3.54±3.30b | 1.14±0.78 | 0.41±0.29 |
| Brain | 0.52±0.07 | 0.30±0.12 | 0.15±0.09 | 0.06±0.01 | 0.02±0.01 |
| Tumor | 3.37±0.49 | 3.84±0.83 | 5.46±3.05 | 3.62±1.14 | 3.09±1.67 |
| [131I]SGMIB-scFv-Fc DM | |||||
| Liver | 11.67±0.88 | 10.90±3.67 | 6.83±3.63 | 1.54±0.51 | 0.53±0.12 |
| Spleen | 4.88±1.14 | 8.51±5.69 | 7.78±7.87 | 1.16±0.63 | 0.58±0.35 |
| Lungs | 5.63±0.86 | 3.88±0.72 | 1.93±0.41 | 0.87±0.29 | 0.21±0.12 |
| Heart | 3.65±0.52 | 2.55±0.73 | 1.54±0.15 | 0.58±0.14 | 0.15±0.06 |
| Kidney | 6.49±0.63 | 4.61±0.68 | 2.44±0.77 | 0.85±0.27 | 0.25±0.11 |
| Stomach | 1.07±0.40 | 0.57±0.24 | 0.46±0.13 | 0.34±0.14 | 0.09±0.06 |
| Sm. intestine | 3.23±0.31 | 3.20±1.87 | 1.47±1.18 | 0.90±0.57 | 0.16±0.15 |
| Lg. intestine | 2.16±0.43 | 3.94±0.46 | 1.38±0.15 | 0.89±0.26 | 0.22±0.12 |
| Thyroid | 0.48±0.21 | 0.31±0.08 | 0.10±0.19 | 0.04±0.01 | 0.03±0.01 |
| Muscle | 1.63±0.40 | 0.74±0.22 | 0.91±0.81 | 0.22±0.02 | 0.11±0.09 |
| Blood | 15.32±1.88 | 7.13±2.91 | 3.41±2.13 | 0.72±0.05 | 0.18±0.11 |
| Bone | 1.29±0.22 | 2.15±1.20 | 2.45±2.36 | 0.76±0.49 | 0.15±0.12 |
| Brain | 0.41±0.06 | 0.22±0.07 | 0.10±0.05 | 0.04±0.00 | 0.01±0.00 |
| Tumor | 2.84±0.44 | 3.02±0.77 | 3.41±2.13 | 1.23±0.28 | 0.79±0.37 |
Values are mean % ID/g ± S.D. (n = 5) except for thyroid for which % ID/organ is used
No significant difference between the two tracers (P > 0.05)
At 2 h after injection, the uptake of both radionuclides was similar in most other tissues; however, at later time points, the scFv-Fc DM fragment labeled using [125I]IB-Mal-D-GEEEK exhibited higher sequestration in a number of tissues. For example, in the liver the uptake of 125I was 2-, 4-, and 6-fold higher than that of 131I at 12 h, 24 h and 48 h, respectively; these ratios were 3, 6, and 12 in the case of kidneys. Other tissues including lungs, heart, spleen, and blood had 1.5- to 4-fold higher uptake of 125I at these time points. Notwithstanding the higher 125I uptake in some tissues, the tumor-to-tissue ratios were generally higher for 125I (Figure 5), suggesting an advantage for the [125I]IB-Mal-D-GEEEK-scFv-Fc DM conjugate. Liver and kidneys are the two notable tissues for which the tumor-to-tissue ratios were higher at later time points with [131I]SGMIB-scFv-Fc DM. The tumor-to-liver ratios for 131I at 12 h, 24 h and 48 h (0.7 ± 0.6, 0.9 ± 0.4, and 1.5 ± 0.8, respectively) were about 1.5-fold higher than those calculated for 125I; however the differences were statistically significant (p < 0.05) only at 24 h and 48 h time points. The tumor-to-kidney ratios at 4 h, 12 h, 24 h, and 48 h for 131I were 0.7 ± 0.2, 1.7 ± 1.2, 1.6 ± 0.8, and 3.7 ± 2.5, respectively compared to 0.5 ± 0.2, 0.7 ± 0.4, 0.7 ± 0.3, and 1.1 ± 0.6 for 125I; the differences were statistically significant except at 12 h.
Figure 5.
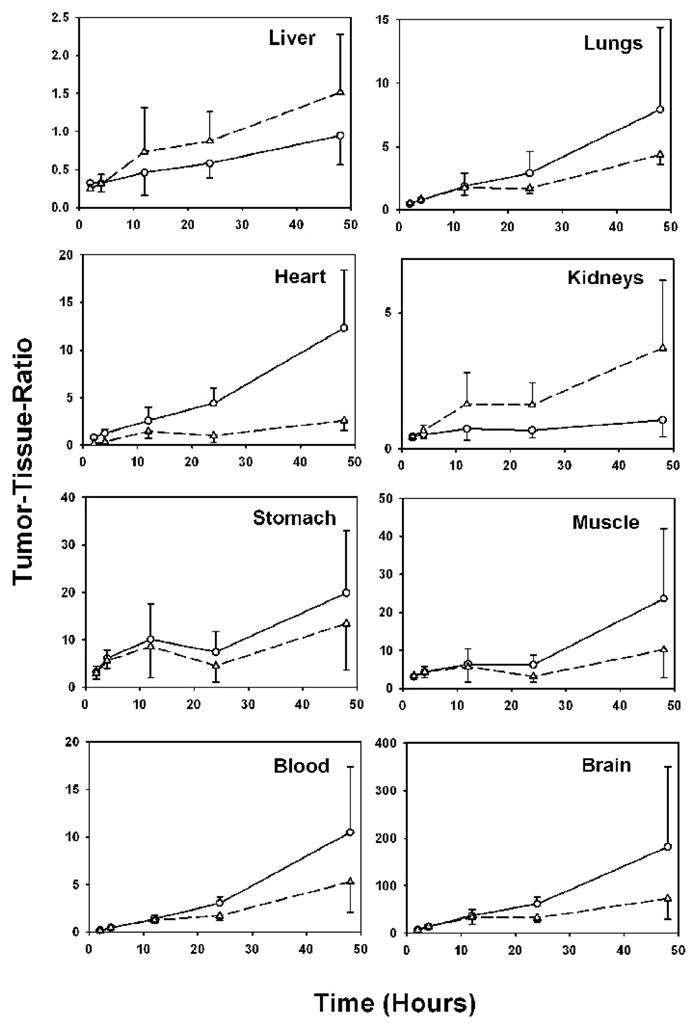
Tumor-to-tissue ratios observed in athymic mice bearing MCF7-HER2 xenografts and injected with [131I]SGMIB-scFv-Fc DM (triangles) and [125I]IB-Mal-D-GEEEK-scFv-Fc DM (circles).
4. Discussion
Previously, we have reported on the usefulness of the residualizing labels SGMIB and IB-Mal-D-GEEEK for the efficient tumor delivery of halogen radionuclides attached to mAbs that undergo internalization [38, 39, 41]. Indeed, an up to fivefold advantage in tumor delivery, both in vitro and in vivo, was achieved when an intact mAb reactive to a mutant form of the epidermal growth factor receptor (EGFRvIII) was radiohalogenated using these prosthetic groups. The goal of this study was to investigate whether improved tumor targeting of scFv-Fc DM, an engineered fragment derived from the anti-HER2 trastuzumab (Herceptin™) mAb, could be achieved by radioiodinating the fragment with these residualizing labeling methods.
Here, we demonstrated that the trastuzumab scFv-Fc DM fragment could be radioiodinated with both [131I]SGMIB and [125I]IB-Mal-D-GEEEK in reasonable conjugation yields. Both [131I]SGMIB and [125I]IB-Mal-D-GEEEK-labeled scFv-Fc DM fragments were immunoreactive as demonstrated by size-exclusion HPLC following incubation of the fragment and the p185HER2 extracellular domain (antigen).
The scFv-Fc DM fragment radioiodinated with either the positively charged SGMIB or the negatively charged IB-Mal-D-GEEEK reagents demonstrated enhanced intracellular retention of radioactivity in vitro compared to the directly radioiodinated scFv-Fc DM fragment. Thus it has to be concluded that the trastuzumab scFv-Fc DM fragment does indeed undergo internalization on receptor binding and that these residualizing labels enhance the cellular retention of radioactivity following receptor-mediated internalization. This result corroborates that of other researchers who have demonstrated the internalization of radiolabeled intact trastuzumab [5, 9, 25]. When the internalization assay was performed independently, the scFv-Fc DM fragment labeled with [131I]SGMIB accumulated greater amounts of radioactivity than the [131I]IB-Mal-D-GEEEK-labeled fragment within the intracellular compartments, whereas in direct comparison of the two, the negatively charged [131I]IB-Mal-D-GEEEK-scFv-Fc DM showed a slight advantage. The latter results are congruent with the data obtained with L8A4, an anti-EGFRvIII mAb; intracellular retention of radioactivity from L8A4 that was radioiodinated with the glutamate-containing peptide was higher than that seen for the same mAb labeled with SGMIB prosthetic group [40, 41].
The tissue distribution of directly radioiodinated scFv-Fc DM fragment has not been evaluated extensively. A microPET imaging study performed 20 h after injection of scFv-Fc DM labeled with 124I using the Iodogen method in mice with MCF7-HER2 xenografts indicated a tumor accumulation of only 1.52 0.28%ID/g (unpublished results). Likewise, preliminary necropsy experiments with scFv-Fc DM labeled with 125I using Iodogen also showed poor tumor uptake and very high thyroid uptake (6–8% ID/organ) for the directly labeled fragment (unpublished results). In concert with the in vitro data, the [125I]IB-Mal-D-GEEEK-scFv-Fc DM demonstrated higher tumor uptake than that seen with the [131I]SGMIB-scFv-Fc DM. The absolute tumor uptake values were considerably lower (2- to 3-fold) than that reported for the same fragment labeled with 64Cu [34]. While the 64Cu-labeled scFv-Fc DM demonstrated higher tumor uptake, there was a concomitant higher uptake in the liver and the kidneys with tumor-to-liver and tumor-to-kidney ratios 0.6 ± 0.1 and 1.1 ± 0.2, respectively at 21 h. Compared with this, at 24 h the [131I]SGMIB-scFv-Fc DM fragment exhibited slightly improved tumor-to-tissue ratios that were 1.3- and 1.5-fold higher in liver and kidney, respectively. In contrast, the [125I]IB-Mal-D-GEEEK-scFv-Fc DM fragment yielded lower tumor-to-tissue ratios for these two organs. Here we show that the scFv-Fc DM fragment radioiodinated with SGMIB and IB-Mal-D-GEEEK prosthetic groups demonstrated remarkable inertness to in vivo deiodination. The low degree of in vivo deiodination of the IB-Mal-D-GEEEK template presumably contributed to the significantly higher tumor accumulation and retention of its radioiodinated scFv-Fc DM conjugate.
It would be ideal if the positive features of the IB-Mal-GEEEK reagent for labeling this scFv-Fc DM construct could be exploited without its negative feature, i.e., increased accumulation and retention of activity in the liver and spleen at time points beyond 2 h after injection. The question arises whether the increased reticuloendothelial uptake is a result from treating the protein with 2-iminothiolane and/or some property of the IB-Mal-GEEEK reagent itself. The generation of free sulfhydryl groups on proteins via 2-iminothiolane treatment is a widely used technique that offers advantages compared with reduction of disulfide bonds because of the possibility of less disruption of the tertiary structure of the protein. With regard to the effect on the biodistribution of radiolabeled proteins that have been treated with 2-iminothiolane, there are some reports indicating a higher liver uptake of mAbs labeled via this route compared to other methods in the literature [12]. However, in most cases, treatment of mAbs with 2-iminothiolane has not resulted in increased reticuloendothelial uptake [13, 26, 27]. We have previously used the IB-Mal-GEEEK reagent to radiolabel the intact L8A4 mAb with radioiodine and did not observe excessive liver and spleen uptake when compared to the Iodogen or the SGMIB labeled L8A4 [38, 41]. Thus, the increased liver and spleen accumulation observed with the IB-Mal-GEEEK scFv-Fc DM conjugate probably does not reflect a general property of this acylation agent or 2-iminothiolane treatment but an alteration that is specific to this construct. Such an alteration could be the presence of multimeric species derived by the formation of disulfide bonds between different scFv-Fc DM molecules. However, size exclusion HPLC did not indicate presence of any significant amounts of multimers (Figure 2). Another possible explanation is that the increased activity in the liver and spleen may reflect the formation of a disulfide linked species between the scFv-Fc DM and an endogenous molecule with a free sulfhydryl group. This is consistent with the fact that peak spleen and liver activity is not observed until 4 to 12 h after injection. Finally, it is worth noting that the liver accumulation observed with the [125I]IB-Mal-GEEEK-scFv-Fc DM fragment actually was 2 to 3 times lower than that observed when the same molecule was labeled with 64Cu-DOTA [34].
IB-Mal-D-GEEEK was designed to offset the high renal uptake seen with another residualizing template containing several cationic amino acids [16]. The retention in kidneys of low molecular weight peptides and proteins is attributed to the binding of their positively charged amino acid residues to the negatively charged renal proximal tubules [28]. It was envisaged that exchanging the cationic lysine and arginines in the D-KRYRR-containing residualizing label [16] with the negatively charged glutamic acids might yield a prosthetic group, which would help augment the tumor uptake of radioiodinated internalizing mAbs or their fragments and at the same time would not result in significant uptake in the kidneys. This tactic was used to effectively reduce kidney uptake of radiolabeled peptides by other investigators as well [29]. On the other hand, a number of investigators have reported a high kidney uptake of molecules containing glutamic acid residues [14, 44, 45] and efforts have been made to reduce the renal uptake by administering polyglutamic acid [3, 17]. The existence of a mechanism for the reuptake of negatively charged amino acid-containing peptides in kidneys has been proposed. It is known that several amino acid transport systems in mammals contribute to renal proximal tubular reabsorption of amino acids and EAAT3 is the major transporter of L-glutamic acid [35, 43]; however, the D-glutamic acid is not expected to be a substrate for this transporter.
The scFv-Fc DM fragment is anticipated to clear rapidly from the blood and thereby to yield very high tumor-to-blood ratios due to the mutations in the Fc region that interferes with the binding to the antibody recycling receptor, known as FcRn or Brambell receptor [22]. Indeed, rapid blood clearance was seen with both [131I]SGMIB- and [125I]IB-Mal-GEEEK-scFv-Fc DM fragments and the rate was similar to that reported for another scFv-Fc DM fragment targeting CEA [23].
5. Conclusion
The engineered trastuzumab scFv-Fc DM could be radioiodinated in reasonable yields using two residualizing labels with the preservation of immunoreactivity. Compared to the directly labeled fragment, the fragments labeled with the residualizing labels demonstrated higher retention in tumor cells in vitro. Although [125I]IB-Mal-D-GEEEK-scFv-Fc DM yielded higher tumor targeting and generally higher tumor-to-tissue ratios, uptake in liver and kidney was greater compared with the [131I]SGMIB-scFv-Fc DM. Easier chemistry and higher inertness to in vivo deiodination are added advantages of the SGMIB method. With regard to potentially using the scFv-Fc DM fragment for imaging HER2 activity, it will be important to determine the best balance between maximizing tumor uptake and obtaining optimal contrast with normal organs. Usefulness of scFv-Fc DM labeled with the -emitter 211At using the SGMIB analogue [211At]SAGMB [39] and the Mal-D-GEEEK template is being explored.
Acknowledgments
The authors want to thank Donna Affleck, Philip Welsh, and Xiao Zhao for their excellent technical assistance. This work was supported by Grants CA42324, CA14236, CA43904 and CA86306 from the National Institutes of Health. AMW is a member of the UCLA Johnson Comprehensive Cancer center (CA16042).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akabani G, Carlin S, Welsh P, Zalutsky MR. In vitro cytotoxicity of 211At-labeled trastuzumab in human breast cancer cell lines: effect of specific activity and HER2 receptor heterogeneity on survival fraction. Nucl Med Biol. 2006;33:333–47. doi: 10.1016/j.nucmedbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci U S A. 2005;102:17987–92. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behe M, Kluge G, Becker W, Gotthardt M, Behr TM. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J Nucl Med. 2005;46:1012–5. [PubMed] [Google Scholar]
- 4.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 5.Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003;63:5084–90. [PubMed] [Google Scholar]
- 6.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087–93. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- 7.Brooks SC, Hansen ER, Saunders DE, Battelli MG, Shafie SM. Effect of growth on the estrogen receptor levels in MCF-7 cells. Cancer Res. 1984;44:3724–9. [PubMed] [Google Scholar]
- 8.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-Labeled Trastuzumab (Herceptin) Modified with Nuclear Localization Sequences (NLS): An Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357–68. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 10.da Silva FA, Corte-Real S, Goncalves J. Recombinant antibodies as therapeutic agents - Pathways for modeling new biodrugs. Biodrugs. 2008;22:301–14. doi: 10.2165/00063030-200822050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Demarest SJ, Glaser SM. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr Opin Drug Discov Dev. 2008;11:675–87. [PubMed] [Google Scholar]
- 12.DeNardo GL, DeNardo SJ, Peterson JJ, Miers LA, Lam KS, Hartmann-Siantar C, et al. Preclinical evaluation of cathepsin-degradable peptide linkers for radioimmunoconjugates. Clin Cancer Res. 2003;9:3865S–72S. [PubMed] [Google Scholar]
- 13.DeNardo GL, Kukis DL, Shen S, DeNardo DA, Meares CF, DeNardo SJ. 67Cu-versus 131I-labeled Lym-1 antibody: comparative pharmacokinetics and dosimetry in patients with non-Hodgkin’s lymphoma. Clin Cancer Res. 1999;5:533–41. [PubMed] [Google Scholar]
- 14.Dijkgraaf I, Liu S, Kruijtzer JA, Soede AC, Oyen WJ, Liskamp RM, et al. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F. HER2/neu role in breast cancer: from a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62. doi: 10.1097/GCO.0b013e328012980a. [DOI] [PubMed] [Google Scholar]
- 16.Foulon CF, Reist CJ, Bigner DD, Zalutsky MR. Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 2000;60:4453–60. [PubMed] [Google Scholar]
- 17.Gotthardt M, van Eerd-Vismale J, Oyen WJ, de Jong M, Zhang H, Rolleman E, et al. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med. 2007;48:596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 18.Govindan SV, Griffiths GL, Stein R, Andrews P, Sharkey RM, Hansen HJ, et al. Clinical-scale radiolabeling of a humanized anticarcinoembryonic antigen monoclonal antibody, hMN-14, with residualizing 131I for use in radioimmunotherapy. J Nucl Med. 2005;46:153–9. [PubMed] [Google Scholar]
- 19.Hu S, Shively L, Raubitschek A, Sherman M, Williams LE, Wong JY, et al. Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 1996;56:3055–61. [PubMed] [Google Scholar]
- 20.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 21.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the 2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–6. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenanova V, Olafsen T, Crow DM, Sundaresan G, Subbarayan M, Carter NH, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res. 2005;65:622–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Kenanova V, Olafsen T, Williams LE, Ruel NH, Longmate J, Yazaki PJ, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res. 2007;67:718–26. doi: 10.1158/0008-5472.CAN-06-0454. [DOI] [PubMed] [Google Scholar]
- 24.Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29:2819–25. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Shirakawa K, Kawamoto S, Saga T, Sato N, Hiraga A, et al. Rapid accumulation and internalization of radiolabeled herceptin in an inflammatory breast cancer xenograft with vasculogenic mimicry predicted by the contrast-enhanced dynamic MRI with the macromolecular contrast agent G6-(1B4M-Gd)(256) Cancer Res. 2002;62:860–6. [PubMed] [Google Scholar]
- 26.Kukis DL, DeNardo GL, DeNardo SJ, Mirick GR, Miers LA, Greiner DP, et al. Effect of the extent of chelate substitution on the immunoreactivity and biodistribution of 2IT-BAT-Lym-1 immunoconjugates. Cancer Res. 1995;55:878–84. [PubMed] [Google Scholar]
- 27.Lewis MR, Boswell CA, Laforest R, Buettner TL, Ye D, Connett JM, et al. Conjugation of monoclonal antibodies with TETA using activated esters: biological comparison of 64Cu-TETA-1A3 with 64Cu-BAT-2IT-1A3. Cancer Biother Radiopharm. 2001;16:483–94. doi: 10.1089/10849780152752083. [DOI] [PubMed] [Google Scholar]
- 28.Maack T, Park CH, Camargo MJ. Filtration, transport, and metabolism of proteins. In: Seldin DW aGG, editor. Physiology and Pathophysiology. Raven Press; New York.: 1992. pp. 3005–3038. [Google Scholar]
- 29.Miao Y, Fisher DR, Quinn TP. Reducing renal uptake of 90Y- and 177Lu-labeled -melanocyte stimulating hormone peptide analogues. Nucl Med Biol. 2006;33:723–33. doi: 10.1016/j.nucmedbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–98. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Morris SR, Carey LA. Trastuzumab and beyond: New possibilities for the treatment of HER2-positive breast cancer. Oncology (Williston Park) 2006;20:1763–71. [PubMed] [Google Scholar]
- 32.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–43. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 33.Olafsen T, Cheung CW, Yazaki PJ, Li L, Sundaresan G, Gambhir SS, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21–7. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olafsen T, Kenanova VE, Sundaresan G, Anderson AL, Crow D, Yazaki PJ, et al. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005;65:5907–16. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rexhepaj R, Grahammer F, Volkl H, Remy C, Wagner CA, Sandulache D, et al. Reduced intestinal and renal amino acid transport in PDK1 hypomorphic mice. Faseb J. 2006;20:2214–22. doi: 10.1096/fj.05-5676com. [DOI] [PubMed] [Google Scholar]
- 36.Stein R, Govindan SV, Hayes M, Griffiths GL, Hansen HJ, Horak ID, et al. Advantage of a residualizing iodine radiolabel in the therapy of a colon cancer xenograft targeted with an anticarcinoembryonic antigen monoclonal antibody. Clin Cancer Res. 2005;11:2727–34. doi: 10.1158/1078-0432.CCR-04-2100. [DOI] [PubMed] [Google Scholar]
- 37.Torigian DA, Huang SS, Houseni M, Alavi A. Functional imaging of cancer with emphasis on molecular techniques. CA Cancer J Clin. 2007;57:206–24. doi: 10.3322/canjclin.57.4.206. [DOI] [PubMed] [Google Scholar]
- 38.Vaidyanathan G, Affleck DJ, Bigner DD, Zalutsky MR. Improved xenograft targeting of tumor-specific anti-epidermal growth factor receptor variant III antibody labeled using N-succinimidyl 4-guanidinomethyl-3-iodobenzoate. Nucl Med Biol. 2002;29:1–11. doi: 10.1016/s0969-8051(01)00277-3. [DOI] [PubMed] [Google Scholar]
- 39.Vaidyanathan G, Affleck DJ, Bigner DD, Zalutsky MR. N-succinimidyl 3-[211At]astato-4-guanidinomethylbenzoate: an acylation agent for labeling internalizing antibodies with -particle emitting 211At. Nucl Med Biol. 2003;30:351–9. doi: 10.1016/s0969-8051(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 40.Vaidyanathan G, Affleck DJ, Li J, Welsh P, Zalutsky MR. A polar substituent-containing acylation agent for the radioiodination of internalizing monoclonal antibodies: N-succinimidyl 4-guanidinomethyl-3-[131I]iodobenzoate ([131I]SGMIB) Bioconjug Chem. 2001;12:428–38. doi: 10.1021/bc0001490. [DOI] [PubMed] [Google Scholar]
- 41.Vaidyanathan G, Alston KL, Bigner DD, Zalutsky MR. N-(3-[*I]Iodobenzoyl)-Lys5-N-maleimido-Gly1-GEEEK ([*I]IB-Mal-D-GEEEK): a radioiodinated prosthetic group containing negatively charged D-glutamates for labeling internalizing monoclonal antibodies. Bioconjug Chem. 2006;17:1085–92. doi: 10.1021/bc0600766. [DOI] [PubMed] [Google Scholar]
- 42.Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate: a radio-iodination agent for labeling internalizing proteins and peptides. Nat Protoc. 2007;2:282–6. doi: 10.1038/nprot.2007.20. [DOI] [PubMed] [Google Scholar]
- 43.Verrey F, Ristic Z, Romeo E, Ramadan T, Makrides V, Dave MH, et al. Novel renal amino acid transporters. Annu Rev Physiol. 2005;67:557–72. doi: 10.1146/annurev.physiol.67.031103.153949. [DOI] [PubMed] [Google Scholar]
- 44.von Guggenberg E, Behe M, Behr TM, Saurer M, Seppi T, Decristoforo C. 99mTc-labeling and in vitro and in vivo evaluation of HYNIC- and (N-His)acetic acid-modified [D-Glu1]-minigastrin. Bioconjug Chem. 2004;15:864–71. doi: 10.1021/bc0300807. [DOI] [PubMed] [Google Scholar]
- 45.Wen X, Jackson EF, Price RE, Kim EE, Wu Q, Wallace S, et al. Synthesis and characterization of poly(L-glutamic acid) gadolinium chelate: a new biodegradable MRI contrast agent. Bioconjug Chem. 2004;15:1408–15. doi: 10.1021/bc049910m. [DOI] [PubMed] [Google Scholar]
- 46.Yazaki PJ, Wu AM, Tsai SW, Williams LE, Ikler DN, Wong JY, et al. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12:220–8. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]