Abstract
Many chronic pain syndromes including fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome, migraine headache, chronic back pain, and complex regional pain syndrome are associated with hypersensitivity to painful stimuli and with reduced endogenous pain inhibition. These findings suggest that modulation of pain-related information may be related to the onset and/or maintenance of chronic pain. Although pain sensitivity and pain inhibition are normally distributed in the general population, they are not useful as reliable predictors of future pain. The combination of heightened pain sensitivity and reduced pain-inhibition, however, appears to predispose individuals to greater risk for increased acute clinical pain (e.g., postoperative pain). It is unknown at this time whether such pain processing abnormalities may also place individuals at increased risk for chronic pain. Psychophysical methods, including heat sensory and pressure pain testing have become increasingly available and can be used for the evaluation of pain sensitivity and pain inhibition. However, long-term prospective studies in the general population are lacking which could yield insight into the role of heightened pain sensitivity and pain disinhibition for the development of chronic pain disorders like fibromyalgia.
Keywords: Modulation, Fibromyalgia, Chronic pain, DNIC, Analgesia
Introduction
Population surveys estimate the prevalence of chronic pain in the United States at more than 45% of the general population [25] with minimal recovery rates over 4-year follow-up periods [24]. Furthermore, the total cost of chronic pain is estimated at more than 100 billion dollars annually [88]. Rheumatologists are frequently involved in the treatment of chronic pain syndromes, including various neuropathic and musculoskeletal pain syndromes such as complex regional pain syndrome (CRPS), postherpetic neuralgia (PHN), headache, facial pain, back pain, chronic fatigue syndrome, and fibromyalgia (FM), as well as the painful aspects of conditions such as rheumatoid arthritis and osteoarthritis.
Although many chronic pain syndromes are defined by their anatomic location, they often share similar pathophysiological mechanisms [52,96]. Many patients with chronic pain syndromes (e.g., FM, chronic headache, low back pain, temporomandibular disorder [TMD]), report insidious onsets and their physical findings are often poor predictors of their symptoms, specifically pain. Similarly, easily apparent tissue damage (e.g., in patients with osteoarthritis, PHN, chronic postsurgical pain) often bears only a modest relationship to reported symptoms, including pain. Thus inter-individual differences in pain sensitivity seem to play an important role for clinical pain which is best illustrated by many patients with PHN, post-surgical pain, or severe osteoarthritis, who present with similar extents of tissue damage but may have either no pain at all or severe disabling pain. Such discrepancies in pain sensitivity suggest profound differences in pain processing of noxious stimulation.
Continuing nociceptive input can have many biological, psychological, and functional consequences, ranging from receptor modification and central sensitization to depressed affect, inappropriate cognition, and social disruption. Thus persistent pain states may represent a disease entity in its own right [74]. Like any disease, the extent of experienced symptoms is greatly influenced by internal and external factors, in particular the environment, i.e. genetic, psychological and social factors strongly contribute to the perception and severity of persistent pain. Therefore persistent pain states cannot solely be defined by their associated tissue damage, but are influenced by many other relevant contributors. Several characteristic features of many chronic pain patients are of special importance: First, there is substantial overlap amongst many pain conditions [2], suggesting common pathophysiological mechanisms; second, many chronic pain syndromes are characterized by hyperalgesia and abnormal endogenous pain-inhibition [64]. These pain conditions are often associated with secondary hyperalgesia at sites distant from the affected area, suggesting sensitization of the central nervous system (CNS) (e.g., diabetic neuropathy, PHN) [76].
Individual differences in endogenous pain modulation may place people at increased or reduced risk for the development of chronic pain (e.g., FM pain, headache, visceral pain); specifically individuals who are highly pain-sensitive and who show the lowest degree of endogenous pain inhibition, may be at greater risk for the onset and persistence of chronic pain.
Inter-individual differences of pain in animals and humans
Individual differences in the perception and modulation of pain have been reported in animals [54] and humans [50,59]. Most commonly pain sensitivity is evaluated by psychophysical testing, including mechanical, thermal, and electrical threshold and suprathreshold stimuli [23]. Subjects usually rate the pain intensity of the stimulus using a validated pain rating scale, like the visual analogue scale (VAS) [66] or the numerical pain scale [66]. Endogenous pain modulation can be assessed with phasic or tonic heat stimuli, spatial summation [84], or by simultaneous administering two noxious stimuli [counter-irritation or diffuse noxious inhibitory controls (DNIC)] and measuring the resultant pain inhibition (“pain inhibits pain”) [21,82]. Importantly, high pain sensitivity and low endogenous pain inhibition have been observed in many patients with chronic pain syndromes like FM [48]. Whereas pain thresholds and endogenous analgesia show only minor correlations [22], the combination of high pain sensitivity with low endogenous pain inhibition seem to confer cumulative risk. Overall, individual differences in endogenous pain inhibition are better predictors of future chronic pain than increased pain sensitivity [36].
What are the mechanisms for individual differences of pain sensitivity?
The measures described above are important indexes of central nervous system (CNS) pain processing. As pain is actively modulated by the nervous system at multiple levels of the pain pathways, testing individual differences in the endogenous modulation of pain is crucial for understanding the variability of pain responses [17]. Well defined psychophysical methods, including heat and pressure stimuli have been increasingly used to characterize inter-individual differences in pain sensitivity [59].
Individual differences in pain responses are normally distributed in the general population [34,97] and reflect a combination of genetic and environmental factors related to CNS pain processing. Human twin studies found heritability estimates for pain sensitivity ranging from 22% to 55% for a number of different pain stimuli [60]. Of specific interest were genetic effects on pain sensitivity that explained 60% of the variance in cold-pressor pain and 26% of the variance in contact-heat pain. Importantly, there were distinct genetic and environmental factors influencing these two pain modalities. Only 6% of the variance in cold-pressor pain and 3% of the variance in heat pain was attributable to genetic factors that were common to both pain modalities. Similarly, only 5% of the variance in cold-pressor pain and 8% of the variance in heat pain was attributable by environmental factors that were common to both pain modalities.
Furthermore, genetic studies in human subjects show that single-nucleotide polymorphisms (SNP) of several pain related genes significantly contribute to basal pain sensitivity, including polymorphisms of the mu and delta-opioid receptor genes [30,44], the catechol-O-methyltransferase (COMT) gene [15], as well as the GTP cyclohydrolase-1 gene [87]. These findings are supported by rodent studies [56] which also emphasized the important effects of pain genes on sensitivity to noxious stimuli [56] and on analgesia [55]. Environmental influences can strongly influence pain sensitivity in animals as well as in humans [51]. In particular, exposure to strong and prolonged pain [86] seems to alter individuals subsequent pain sensitivity and increases their risk for more severe acute [31] and chronic pain [1,32,45,69].
In addition to genetic and environmental factors, pain sensitivity is influenced by cognitive factors such as catastrophizing. Specifically, high levels of catastrophizing seem to be associated with lower pain thresholds [85] and enhanced pain related brain activation [33]. Therefore, individual differences in pain responsiveness may not only reflect the influence of genetic and environmental factors, but also may impact an individual's risk for developing a variety of persistent pain conditions, including FM.
Acute pain intensity as predictor of chronic pain
Several studies provide evidence that high pre-operative pain sensitivity can be used to predict post-operative pain, in particular pain related to cesarean section [36], hysterectomy [8], limb amputation [61], and cholecystectomy [7]. Experimental pain responses prior to surgery significantly predicted the magnitude of postoperative pain for several weeks after surgery. Specifically, patients who were highly sensitive to noxious heat or cold stimuli reported more severe pain following cesarean section [36]. These patients' preoperative ratings of noxious heat stimuli predicted more than 50% of the variance in their postoperative pain. This study also showed that suprathreshold heat stimuli seem to have clinical relevance as predictors of post-surgical pain [23]. Severe acute pain has been consistently demonstrated as a risk factor for the development of chronic pain after injury. For example, more than 50% of patients after spinal cord injury (SCI) or herpes zoster infection will develop chronic pain syndromes [63,68]. High pain ratings following surgery, like mastectomy, cholecystectomy, amputations, thoracotomy, herniorraphy, prostatectomy, and total knee arthroplasty also seem to increase the risk for developing persistent pain syndromes [37,63,77,93]. Some of the possible mechanisms contributing to future chronic pain may involve sensitization of the CNS [63] and inadequate pain modulation. Specifically, high pain sensitivity seems to predispose individuals to higher levels of acute pain after tissue traumas, resulting in sensitization of the CNS and subsequent chronic pain.
Several studies of healthy adults who were free of chronic pain demonstrated a significant relationship between increased pain sensitivity and frequent pain complaints like headaches, backaches, muscle aches, etc. and impaired functional status [19] [20] [28]. These highly sensitive individuals not only rated noxious thermal stimuli as more painful [19], but also showed the lowest levels of pain tolerance [28], the greatest temporal summation of pain (central sensitization) [20],http://www.neurology.org/cgi/content/full/65/3/437?cookietest=yes - R27-20 and the lowest levels of endogenous pain inhibition [22]. Similarly, high sensitivity to experimental pain also predicted more clinical pain and lower levels of physical functioning in chronic pain patients [11,18].
Variability of analgesic responses
In animals and humans, the effect of genetic factors on pain sensitivity and analgesia seems to be moderate [46,47,54,59]. However, there appears to be a strong relationship between high pain sensitivity and low analgesic responsiveness to opioid analgesics in animals [26,56,57] and humans, particularly in men [29]. These findings suggest concordance between high pain sensitivity and reduced analgesic responsiveness both of which may predispose individuals to chronic pain while making them insensitive to opioids therapy.
Predictor for new-onset chronic musculoskeletal pain
Most research of risk factors for new-onset chronic musculoskeletal pain has been done in patients with low back pain (LBP) [38] or knee pain [42]. Mechanical factors, including lifting and pulling heavy weights seem to be important predictors of new-onset LBP and knee pain. Psychosocial and physical environment factors also appear to significantly predict future pain in both conditions, including monotonous work and poor working conditions. However, work-related psychosocial and environmental factors seem to be most relevant in predicting new symptom onset.
Few studies have addressed the interaction of genetic and/or environmental factors with future FM pain [3,53]. One case-controlled study of previously healthy individuals found that 22% of patients with neck injury, and 2% of patients with leg injury, developed FM one year after a motor vehicle accident [9], suggesting that neck trauma increases the risk for FM more than 10-fold in predisposed patients. Using individual differences in pain sensitivity as a predictor of new-onset TMD a recent prospective study followed a large number of pain-free female participants over three years [15], detecting new-onset TMD in 7.43 % of study participants who were not only highly pain sensitive at study entry but also shared mutations of the COMT gene (high or average pain sensitivity haplotypes). Because of considerable overlap between TMD and FM, the results of this study suggest that similar genetic mutations may also contribute to the risk of predisposed individuals for future FM. Thus, multiple lines of evidence indicate that increased pain sensitivity may increase individuals' risk for the development of future chronic pain, including FM.
Abnormal pain modulation of FM patients
Several studies have provided psychophysical evidence that pain processing is abnormal in FM patients [67,78-80,83,89], showing that perceived pain from experimental stimuli (mechanical, heat, cold, or electricity) was greater for FM patients compared to normal controls (NC), as was the amount of temporal summation of pain or wind-up (WU) within a series of heat stimuli (Figure 1). WU was used as a non-invasive method of assessing C-fiber dependent central sensitization in human subjects. Following multiple stimuli, WU after-sensations were greater in magnitude, lasted longer and were more frequently painful in FM subjects. These results indicate both augmentation and prolonged decay of nociceptive input in FM patients and provide convincing evidence for central sensitization in this syndrome. Several points related to central sensitization appear relevant for understanding FM pain. A) When central sensitization has occurred in chronic pain patients, including FM patients, little additional nociceptive input is required to maintain the sensitized state. Thus, seemingly innocuous daily activities may contribute to the maintenance of the chronic pain state. B) The decay of painful sensations is very prolonged in FM and therefore patients may not experience robust changes of their pain levels during brief therapeutic interventions. Many frequently used analgesic medications do not improve central sensitization, and some medications, including opioids have been shown to maintain or even worsen this CNS phenomenon [10,16]. However, there is evidence that the anti-epileptic pregabalin which was recently approved for the treatment of FM, can reduce central sensitization [41].
Figure 1.
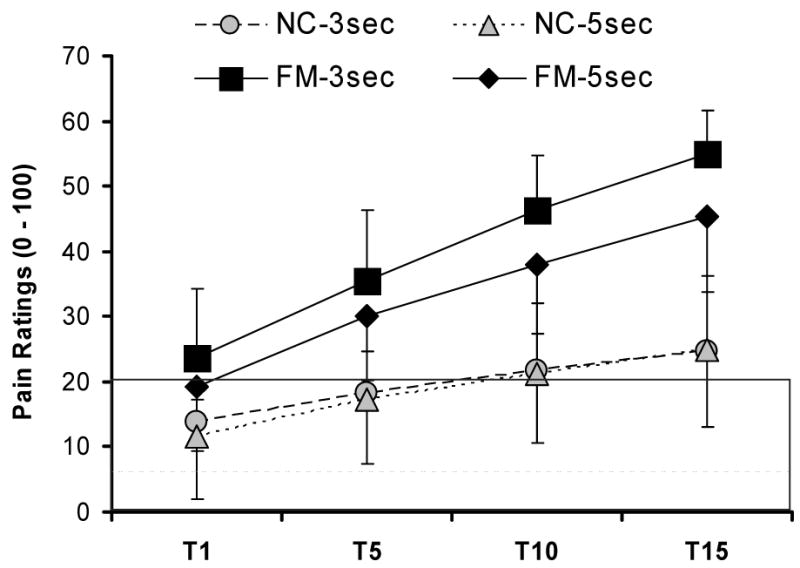
WU pain ratings of NC and FM patients. All subjects received 15 mechanical stimuli to the adductor pollicis muscles of the hands at interstimulatory intervals (ISI) of 3 sec and 5 sec. FM patients showed mechanical hyperalgesia during the first tap and greater temporal summation than NC at both ISIs. A numerical pain scale was used (0 – 100). The shaded area represents pain threshold. FM, fibromyalgia syndrome; ISI, interstimulatory interval; NC, normal control; T, mechanical tap
WU Measures as Predictors of FM Pain Intensity
The important role of central pain mechanisms for clinical pain is also supported by their usefulness as predictors of clinical pain intensity of FM patients. Heat WU ratings correlate well with clinical pain intensity (Peason's r = 0.53), thus emphasizing the important role of this pain mechanism for FM. In addition, hierarchical regression models that include tender point count, pain related negative affect, and WU ratings have been shown to account for 50% of the variance in FM clinical pain intensity [81].
Mechanisms Underlying Abnormal Pain Sensitivity in FM
The mechanisms underlying central sensitization that occurs in patients with FM relies on hyperexcitability of spinal dorsal horn neurons that transmit nociceptive input to the brain. As a consequence, low intensity stimuli delivered to the skin or deep muscle tissue generate high levels of nociceptive input to the brain as well as the perception of pain. Specifically, intense or prolonged impulse input from A-δ and C afferents sufficiently depolarizes the dorsal horn neurons and results in the removal of the Mg2+ block of NMDA-gated ion channels. This is followed by the influx of extracellular Ca2+ and production of nitric oxide which diffuses out of the dorsal horn neurons [12]. Nitric oxide, in turn, promotes the exaggerated release of excitatory amino acids and substance P from presynaptic afferent terminals and causes the dorsal horn neurons to become hyperexcitable [58]. Subsequently, low intensity stimuli evoked by minor physical activity may be amplified in the spinal cord resulting in painful sensations [35].
Role of Glia in Central Sensitization
Accumulating evidence suggests that dorsal horn glia cells might have an important role in producing and maintaining abnormal pain sensitivity [92,94]. Synapses within the CNS are encapsulated by glia that do not normally respond to nociceptive input from local sites. Following the initiation of central sensitization, however, spinal glia cells are activated by a wide array of factors that contribute to hyperalgesia, such as immune activation within the spinal cord, substance P, excitatory amino acids, nitric oxide, and prostaglandins. Precipitating events known to induce glial activation include viral infections, including HIV, Hepatitis C, and influenza [40]. Once activated, glia cells release proinflammatory cytokines, including tumor necrosis factor, IL-6 and IL-1, substance P, nitric oxide, prostaglandins, excitatory amino acids, ATP, and fractalkine [95] that, in turn, further increase the discharge of excitatory amino acids and substance P from the A-δ and C afferents that synapse in the dorsal horn and also enhance the hyper-excitability of the dorsal horn neurons [91,92]. Recent evidence also points towards a possible role of NMDA receptors in glial activation and pain [72]
Possible Causes of Central Sensitization in FM
As a normal response to tissue trauma, injury is followed by repair and healing. Inflammation occurs, which results in a cascade of electrophysiological and chemical events that resolve over time and the patient becomes pain free. In persistent pain, however, the local, spinal, and even supraspinal responses are considerably different from those that occur during acute pain. While defining the relationship between tissue events and pain is necessary for understanding the clinical context of these pathologies, defining the relationship between injury and specific and relevant nociceptive responses is crucial for understanding the central mechanisms of persistent pain in FM. It must be emphasized however, that specific abnormalities in persons with FM have not been identified that might produce the prolonged impulse input that is necessary to initiate the events underlying the development of central sensitization and/or spinal glia cell activation. After central sensitization has occurred, low threshold A-β afferents, which normally do not serve to transmit a pain response, are recruited to transmit spontaneous and movement-induced pain. This central hyperexcitability is characterized by a “windup” response of repetitive C fiber stimulation, expanding receptive field areas, and spinal neurons taking on properties of wide dynamic range neurons [13]. Ultimately, A-β fibers stimulate postsynaptic neurons to transmit pain, where these A-β fibers previously had no role in pain transmission, all leading to central sensitization. Nociceptive information is transmitted from the spinal cord to supraspinal sites, such as the thalamus and cerebral cortex by ascending pathways.
Muscle Tissue as a Source of Nociceptive Input
A potential source of nociceptive input that might account for FM pain is muscle tissue [39]. Several types of muscle abnormalities have been reported in FM patients including the appearance of ragged red fibers, inflammatory infiltrates, and moth-eaten fibers [4,5,65]. Possible mechanisms for such muscle changes might include repetitive muscle microtrauma, which could contribute to the postexertional pain and other painful symptoms experienced by these patients. In addition, prolonged muscle tension and ischemia was found in muscles of FM patients [6,27,49]. Changes in muscle pH related to ischemia [14] might provide a powerful mechanisms for the sensitization of spinal and supraspinal pain pathways [75]. Investigations using 31P nuclear magnetic resonance (NMR) spectroscopy have shown that FM patients display significantly lower phosphorylation potential and total oxidative capacity in the quadriceps muscle during rest and exercise [62]. FM patients also exhibit significantly lower levels of muscle phosphocreatine and ATP, as well as a lower phosphocreatine/inorganic phosphate ratio [4,5]. Furthermore, NMR testing of muscles in FM patients showed an increased prevalence of phosphodiester peaks which have been associated with sarcolemmal membrane damage [43,62].
Focal muscle abnormalities, including trigger points (TrP) are frequently detectable in FM patients and may play an important role as pain generators. Using sensitive microdialysis techniques, concentrations of protons, bradykinin, calcitonin gene-related peptide, substance P, TNF-α, IL-1b, serotonin, and norepinephrine have been found to be significantly higher in TrP of myofascial pain patients than normal muscle tissue [70,73]. Recent studies have shown that advanced glycation end products (AGE) may also be relevant for FM pain. AGE can trigger the synthesis of cytokines particularly IL-1b and TNF-α and elevated AGE levels have been detected in interstitial connective tissue of muscles and in serum of FM patients [71]. All these biochemical mediators can sensitize muscle nociceptors and thus indirectly contribute to central sensitization and chronic pain. Because nociceptive input from muscles is very powerful in inducing and maintaining central sensitization [90] FM muscle abnormalities may strongly contribute to pain through important mechanism of pain amplification.
Conclusions
Many different factors seem to contribute to current pain in chronic pain patients and may affect previously healthy individuals' risk for future musculoskeletal pain. These factors include individual variability of pain-facilitatory and analgesic mechanisms, like temporal summation and analgesia from counter-irritation (DNIC). Such individual differences in pain sensitivity/modulation can be readily assessed in the laboratory and could be used as sensitive biomarkers of current pain sensitivity as well as risk factors for future chronic pain. What specific abnormalities, however, are critical for maintenance of the chronic pain state is unclear at this time. They most likely include a host of psychological and physical stressors. There is accumulating evidence for genetic risk factors for chronic pain, specifically COMT and GTP cyclohydrolase-1 polymorphisms, which may interfere with pain modulation in chronic pain patients, including FM sufferers. Because the effectiveness of treatments for chronic musculoskeletal pain is limited at this time, emphasis should be placed on prevention and/or modification of risk factors that may result in worsening of current pain as well as occurrence of future chronic pain disorders. Specifically, highly pain-sensitive individuals should be identified, evaluated, and counseled about risk modifications for future chronic pain. Psychophysical test methods, including WU and DNIC play an increasingly important role in the assessment of chronic pain patients and may become useful biomarkers for individual pain sensitivity and ability to modulate pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aaron LA, Bradley LA, Alarcon GS, et al. Perceived physical and emotional trauma as precipitating events in fibromyalgia. Associations with health care seeking and disability status but not pain severity. Arthritis Rheum. 1997;40(3):453–460. doi: 10.1002/art.1780400311. [DOI] [PubMed] [Google Scholar]
- 2.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160(2):221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Al Allaf AW, Sanders PA, Ogston SA, et al. A case-control study examining the role of physical trauma in the onset of rheumatoid arthritis. Rheumatology. 2001;40(3):262–266. doi: 10.1093/rheumatology/40.3.262. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson A, Henriksson KG, Jorfeldt L, et al. Primary fibromyalgia. A clinical and laboratory study of 55 patients. Scand J Rheumatol. 1986;15(3):340–347. doi: 10.3109/03009748609092601. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson A, Henriksson KG, Larsson J. Muscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findings. Scand J Rheumatol. 1986;15(1):1–6. doi: 10.3109/03009748609092661. [DOI] [PubMed] [Google Scholar]
- 6.Bennett RM, Clark SR, Goldberg L, et al. Aerobic fitness in patients with fibrositis. A controlled study of respiratory gas exchange and 133xenon clearance from exercising muscle. Arthritis Rheum. 1989;32(4):454–460. doi: 10.1002/anr.1780320415. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg. 2001;167(2):84–96. doi: 10.1080/110241501750070510. [DOI] [PubMed] [Google Scholar]
- 8.Brandsborg B, Nikolajsen L, Hansen CT, et al. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007;106(5):1003–1012. doi: 10.1097/01.anes.0000265161.39932.e8. [DOI] [PubMed] [Google Scholar]
- 9.Buskila D, Neumann L, Vaisberg G, et al. Increased rates of fibromyalgia following cervical spine injury. A controlled study of 161 cases of traumatic injury. Arthritis Rheum. 1997;40(3):446–452. doi: 10.1002/art.1780400310. [DOI] [PubMed] [Google Scholar]
- 10.Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92(2):465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Clauw DJ, Williams D, Lauerman W, et al. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine. 1999;24(19):2035–2041. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 12.Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12(9):3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook AJ, Woolf CJ, Wall PD, et al. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325(7000):151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 14.de Kerviler E, Leroy-Willig A, Jehenson P, et al. Exercise-induced muscle modifications: study of healthy subjects and patients with metabolic myopathies with MR imaging and P-31 spectroscopy. Radiology. 1991;181(1):259–264. doi: 10.1148/radiology.181.1.1887044. [DOI] [PubMed] [Google Scholar]
- 15.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar SA, Pulai IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther. 1998;284(2):678–686. [PubMed] [Google Scholar]
- 17.Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65(3):437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Doleys DM, Fillingim RB, et al. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61(3):346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: Clinical relevance in healthy older and younger adults. J Pain. 2001;2(6):307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(12):155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Ness TJ, Weigent DA, et al. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106(3):427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Edwards RR, Sarlani E, Wesselmann U, et al. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114(3):315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Elliott AM, Smith BH, Hannaford PC, et al. The course of chronic pain in the community: results of a 4-year follow-up study. Pain. 2002;99(12):299–307. doi: 10.1016/s0304-3959(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 25.Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–1252. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 26.Elmer GI, Pieper JO, Negus SS, et al. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75(1):129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 27.Elvin A, Siosteen AK, Nilsson A, et al. Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: A contrast media enhanced colour doppler study. Eur J Pain. 2006;10(2):137–144. doi: 10.1016/j.ejpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83(3):419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 29.Fillingim RB, Hastie BA, Ness TJ, et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69(1):97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Fillingim RB, Wilkinson CS, Powell T. Self-reported abuse history and pain complaints among young adults. Clin J Pain. 1999;15(2):85–91. doi: 10.1097/00002508-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RT, Goldstein R. A comparison of chronic pain patients and controls on traumatic events in childhood. Disabil Rehabil. 2000;22(17):756–763. doi: 10.1080/09638280050200250. %20. [DOI] [PubMed] [Google Scholar]
- 33.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 34.Gracely RH, Grant MAB, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17(4):593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 35.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51(2):175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 36.Granot M, Lowenstein L, Yarnitsky D, et al. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98(6):1422–1426. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Harden RN, Bruehl S, Stanos S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106(3):393–400. doi: 10.1016/j.pain.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Harkness EF, Macfarlane GJ, Nahit ES, et al. Risk factors for new-onset low back pain amongst cohorts of newly employed workers. Rheumatology (Oxford) 2003;42(8):959–968. doi: 10.1093/rheumatology/keg265. [DOI] [PubMed] [Google Scholar]
- 39.Henriksson KG. Is fibromyalgia a distinct clinical entity? Pain mechanisms in fibromyalgia syndrome. A myologist's view. Best Pract Res Clin Rheumatol. 1999;13(3):455–461. doi: 10.1053/berh.1999.0035. [DOI] [PubMed] [Google Scholar]
- 40.Holguin A, O'Connor KA, Biedenkapp J, et al. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-1 (nNOS) Pain. 2004;110(3):517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Iannetti GD, Zambreanu L, Wise RG, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proceedings of the National Academy of Sciences. 2005;102(50):18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones GT, Harkness EF, Nahit ES, et al. Predicting the onset of knee pain: results from a two year prospective study of new workers. Ann Rheum Dis. 2006 doi: 10.1136/ard.2006.057570. doi:10.1136/ard.2006.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jubrias SA, Bennett RM, Klug GA. Increased incidence of a resonance in the phosphodiester region of 31P nuclear magnetic resonance spectra in the skeletal muscle of fibromyalgia patients. Arthritis Rheum. 1994;37(6):801–807. doi: 10.1002/art.1780370604. [DOI] [PubMed] [Google Scholar]
- 44.Kim HS, Neubert JK, Miguel AS, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109(3):488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Lampe A, Doering S, Rumpold G, et al. Chronic pain syndromes and their relation to childhood abuse and stressful life events. J Psychosom Res. 2003;54(4):361–367. doi: 10.1016/s0022-3999(02)00399-9. [DOI] [PubMed] [Google Scholar]
- 46.Lariviere WR, Chesler EJ, Mogil JS. Transgenic studies of pain and analgesia: mutation or background genotype? J Pharmacol Exp Ther. 2001;297(2):467–473. [PubMed] [Google Scholar]
- 47.Lariviere WR, Wilson SG, Laughlin TM, et al. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97(12):75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 48.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13(3):189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Lund N, Bengtsson A, Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. Scan J Rheumatol. 1986;15(2):165–173. doi: 10.3109/03009748609102084. [DOI] [PubMed] [Google Scholar]
- 50.MacGregor AJ. The heritability of pain in humans. In: Mogil JS, editor. The genetics of pain (Progress in Pain Research and Management, vol. 28) Seattle: IASP Press; 2004. pp. 154–70. [Google Scholar]
- 51.MacGregor AJ, Griffiths GO, Baker J, et al. Determinants of pressure pain threshold in adult twins: evidence that shared environmental influences predominate. Pain. 1997;73(2):253–257. doi: 10.1016/S0304-3959(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 52.Max MB. Is mechanism-based pain treatment attainable? Clinical trial issues. J Pain. 2000;1(3 Suppl):2–9. doi: 10.1054/jpai.2000.9819. [DOI] [PubMed] [Google Scholar]
- 53.McLean SA, Clauw DJ, Abelson JL, et al. The development of persistent pain and psychological morbidity after motor vehicle collision: Integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67(5):783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 54.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. PNAS. 1999;96(14):7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mogil JS, Sternberg WF, Marek P, et al. The genetics of pain and pain inhibition. PNAS. 1996;93(7):3048–3055. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mogil JS, Wilson SG, Bon K, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(12):67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 57.Mogil JS, Wilson SG, Bon K, et al. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999;80(12):83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 58.Neugebauer V, Schaible HG, Weiretter F, et al. The involvement of substance P and neurokinin-1 receptors in the responses of rat dorsal horn neurons to noxious but not to innocuous mechanical stimuli applied to the knee joint. Brain Res. 1994;666(2):207–215. doi: 10.1016/0006-8993(94)90774-9. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: measurement, causation, and consequences. J Pain. 2009;10(3):231–237. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen CS, Stubhaug A, Price DD, et al. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136(12):21–29. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Nikolajsen L, Ilkjaer S, Jensen TS. Relationship between mechanical sensitivity and postamputation pain: a prospective study. Eur J Pain. 2000;4(4):327–334. doi: 10.1053/eujp.2000.0194. [DOI] [PubMed] [Google Scholar]
- 62.Park JH, Phothimat P, Oates CT, et al. Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum. 1998;41(3):406–413. doi: 10.1002/1529-0131(199803)41:3<406::AID-ART5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 63.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 64.Peters ML, Schmidt AJ, van den Hout MA. Chronic low back pain and the reaction to repeated acute pain stimulation. Pain. 1989;39(1):69–76. doi: 10.1016/0304-3959(89)90176-0. [DOI] [PubMed] [Google Scholar]
- 65.Pongratz DE, Spath M. Morphologic aspects of fibromyalgia. Z Rheumatol. 1998;57:47–51. doi: 10.1007/s003930050234. [DOI] [PubMed] [Google Scholar]
- 66.Price DD, Patel R, Robinson ME, et al. Characteristics of electronic visual analogue and numeric scales for ratings of experimental pain in healthy subjects and fibromyalgia patients. Pain. 2008;140:158–166. doi: 10.1016/j.pain.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 67.Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 68.Putzke JD, Richards JS, Hicken BL, et al. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain. 2002;100(3):231–242. doi: 10.1016/S0304-3959(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 69.Ren TH, Wu J, Yew D, et al. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G849–G856. doi: 10.1152/ajpgi.00400.2006. [DOI] [PubMed] [Google Scholar]
- 70.Rosendal L, Kristiansen J, Gerdle B, et al. Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain. 2005;119(13):201–209. doi: 10.1016/j.pain.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Ruster M, Franke S, Spath M, et al. Detection of elevated N-epsilon-carboxymethyllysine levels in muscular tissue and in serum of patients with fibromyalgia. Scand J Rheumatol. 2005;34(6):460–463. doi: 10.1080/03009740510026715. [DOI] [PubMed] [Google Scholar]
- 72.Salter MW. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Current Topics in Medicinal Chemistry. 2005;5(6):557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- 73.Shah JP, Phillips TM, Danoff JV, et al. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99(5):1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 74.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: Implications for clinical management. Anesth Analg. 2004;99(2):510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 75.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24(1):37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 76.Sommer C. Painful neuropathies. Curr Opin Neurol. 2003;16(5):623–628. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- 77.Stammberger U, Steinacher C, Hillinger S, et al. Early and long-term complaints following video-assisted thoracoscopic surgery: evaluation in 173 patients. Eur J Cardiothorac Surg. 2000;18(1):7–11. doi: 10.1016/s1010-7940(00)00426-7. [DOI] [PubMed] [Google Scholar]
- 78.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4(4):299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 79.Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Med. 2001;2(3):208–215. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- 80.Staud R, Domingo M. New Insights into the pathogenesis of fibromyalgia syndrome. Med Aspect Human Sexual. 2001:151–57. [Google Scholar]
- 81.Staud R, Robinson ME, Vierck CJ, et al. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105(12):215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 82.Staud R, Robinson ME, Vierck CJ, et al. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 83.Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(12):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 84.Staud R, Vierck CJ, Robinson ME, et al. Spatial summation of heat pain within and across dermatomes in fibromyalgia patients and pain-free subjects. Pain. 2004;111(3):342–350. doi: 10.1016/j.pain.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs. 2005;7(4):245–257. doi: 10.2165/00148581-200507040-00004. [DOI] [PubMed] [Google Scholar]
- 87.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12(11):1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 88.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18(6):355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Vierck CJ, Staud R, Price DD, et al. The effect of maximal exercise on temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. J Pain. 2001;2(6):334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 90.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol Lond. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watkins LR, Maier SF. When good pain turns bad. Current Directions in Psychological Science. 2003;12(6):232–236. [Google Scholar]
- 92.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 93.Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Relationships among clinical characteristics of chronic pain after spinal cord injury. Arch Phys Med Rehabil. 2001;82(9):1191–1197. doi: 10.1053/apmr.2001.25077. [DOI] [PubMed] [Google Scholar]
- 94.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45(23):389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 96.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology. 2001;95(1):241–249. doi: 10.1097/00000542-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 97.Yarnitsky D, Sprecher E, Zaslansky R, et al. Heat pain thresholds: normative data and repeatability. Pain. 1995;60(3):329–332. doi: 10.1016/0304-3959(94)00132-x. [DOI] [PubMed] [Google Scholar]


