Abstract
Introduction
Carcinomatous meningitis (CM) is a devastating disease characterized by the dissemination of malignant tumor cells into the subarachnoid space along the brain and spine. Systemic treatment with monoclonal antibody (mAb) trastuzumab can be effective against HER2-positive systemic breast carcinoma but like other therapies, is ineffective against CM. The goal of this study was to evaluate the therapeutic effect of α-particle emitting 211At-labeled trastuzumab following intrathecal administration in a rat model of breast carcinoma CM.
Methods
Athymic rats were injected intrathecally with MCF-7/HER2-18 breast carcinoma cells through a surgically-implanted indwelling intrathecal catheter. In Experiment 1, animals received 33 or 66 µCi 211At-labeled trastuzumab, cold trastuzumab, or saline. In Experiment 2, animals were inoculated with a lower tumor burden and received 46 or 92 µCi 211At-labeled trastuzumab, or saline. In Experiment 3, animals received 28 µCi 211At-labeled trastuzumab, 30 µCi 211At-labeled TPS3.2 control mAb or saline. Histopathological analysis of the neuroaxis was performed at the end of the study.
Results
In Experiment 1, median survival increased from 21 days for the saline and cold trastuzumab groups to 45 and 48 days for 33 and 66 µCi 211At-labeled trastuzumab, respectively. In Experiment 2, median survival increased from 23 days for saline controls to 68 and 92 days for 46 and 92 µCi 211At-labeled trastuzumab, respectively. In Experiment 3, median survival increased from 20 days to 29 and 36 days for animals treated with 211At-labeled TPS3.2 and 211At-labeled trastuzumab, respectively. Long-term survivors were observed exclusively in the 211At-trastuzumab-treated groups.
Conclusion
Intrathecal 211At-labeled trastuzumab shows promise as a treatment for patients with HER2-positive breast CM.
Keywords: astatine-211, radioimmunotherapy, trastuzumab, HER2, targeted radiotherapy
1. Introduction
Carcinomatous meningitis (CM) is a devastating disease characterized by the dissemination of malignant tumor cells within the leptomeningeal space and metastatic spread throughout the cerebrospinal fluid compartment along the brain and spine. The disease occurs in approximately 4 to 15% of patients with solid tumors and is most frequently diagnosed in patients with breast carcinoma (27 to 50%), lung carcinoma (22 to 36%), lymphoma or leukemia (5 to 15%) as well as tumors of the central nervous system (CNS), mostly notably, medulloblastoma (30%) [1,2]. Developing better treatments for CM accompanying breast cancer is particularly important not only because of its relative frequency but also due to the fact that its incidence appears to be increasing, in part because systemic disease has become more effectively managed [3]. The prognosis for patients with CM is grim with a median survival of only 2 to 6 months. This is due in large part to the fact that external beam radiotherapy and chemotherapy are generally self-limited in this setting by their cytotoxicity to normal tissues, particularly those of the CNS. An attractive approach to increasing the specificity of CM treatment is targeted radiotherapy, in which a vector such as a monoclonal antibody (mAb) is utilized to selectively deliver a radionuclide to cancer cells.
With regard to the choice of vehicle for this purpose, the epidermal growth factor receptor (EGFR) tyrosine kinase known as both ErbB2 and HER2/neu is a potentially valuable target for cancer therapeutics. The p185 trans-membrane protein HER2 oncogene product is over expressed on about 25% of breast carcinomas and other malignancies but only at low levels on normal tissues [4–6]. Trastuzumab (Herceptin, Genentech, South San Francisco, CA) is a humanized mAb that specifically binds to a cysteine rich motif within the extracellular domain of this p185 protein [7]. Systemically administered Trastuzumab is broadly utilized, primarily in combination with chemotherapy, for the treatment of patients with HER2-positive breast carcinoma with responses observed in about half of the time [8,9].
For unknown reasons, HER2 positive breast carcinoma patients have a relatively high incidence of CM [3]. The impermeability of the blood-brain barrier (BBB) hinders the delivery of systemically administered macromolecules to lesions located within the CNS, such as brain metastasis or CM, in a therapeutically-significant manner: following intravenous administration, the CSF concentration of trastuzumab remains 300-fold lower than its systemic concentration [10]. Compartmental administration (intratumoral, intrathecal) by-passes the BBB, thereby allowing for significantly higher doses available for binding to HER2-positive tumor cells. This has lead to a number of case reports investigating the therapeutic effectiveness of high-dose intrathecal trastuzumab, with 2 of 5 patients treated surviving for more than 6 months [11–15].
We hypothesize that the efficacy of intrathecal trastuzumab could be enhanced by combining the mAb with a radionuclide possessing emission characteristics that are well matched to the geometrical features of CM. Because leptomeningeal spread of malignancies present as free floating cancer cells in the CSF and sheet-like deposits on compartmental walls, radionuclides emitting short range radiation are recommended to minimize radiation dose to the spinal cord, [16]. Alpha particles such as those emitted by 211At have a range in tissue of only a few cell diameters and thus might be ideally suited to this purpose. In addition the 7.2-h half life of this radiohalogen reduces the risk of systemic toxicity after CSF protein resorbtion into the general circulation. Finally, as a consequence of the high linear energy transfer nature of α-particles, the cytotoxicity of 211At-labeled compounds is considerably higher than those labeled with β-emitters such as 131I and 90Y in routine use for clinical radioimmunotherapy [17].
In the present study, we describe a rat model for HER2-positive breast carcinoma CM. This model was utilized to evaluate the therapeutic potential of 211At-labeled trastuzumab and our results indicate that significant survival prolongation could be obtained after intrathecal administration of this targeted radiotherapeutic.
2. Materials and methods
2.1 Antibodies
Trastuzumab was obtained from the Duke University Medical Center hospital pharmacy and was dialyzed overnight into 100 mM pH 8.5 borate buffer prior to labeling. The chimeric human/murine TPS3.2 mAb, produced as described previously [18], was treated in similar fashion and served as a control. Fluorescence activated cell sorting analysis of this anti-dansyl IgG2 mAb confirmed its lack of reactivity to the HER2-expressing cell line used in these studies (data not shown).
2.2 Labeling mAbs with 211At
Astatine-211 was produced on the Duke University Medical Center CS-30 cyclotron by bombarding natural bismuth targets with 28-MeV α-particles via the 209Bi(α,2n)211At reaction. The 211At was isolated from the cyclotron target into chilled chloroform using a dry distillation procedure. Trastuzumab and TPS3.2 were labeled at a specific activity of about 5 mCi/mg with 211At using the N-succinimidyl 3-[211At]astatobenzoate reagent as described [19] and then diluted as needed for the radioimmunotherapy studies. The integrity of the labeled mAbs was evaluated by size exclusion HPLC, which indicated that greater than 98% of the 211At activity eluted with a retention time corresponding to that of intact IgG. The immunoreactive fraction of 211At-labeled trastuzumab, determined by incubation with 50, 100 and 200 ng of HER2 extracellular domain, was 80–90% for the preparations used in these studies.
2.3 Cell line and culture
The MCF-7/HER2-18 cell line [20] is the stable HER2-transfected version of the human breast carcinoma MCF-7 cell line, which was provided by Genentech. All cell culture reagents were obtained from Gibco (Grand Island, NY). Cells were grown in DMEM:Ham’s F-12 (50:50) media enriched with 10% inactivated fetal bovine serum and 400 mg/ml G418 (geneticin) to select for HER2-expressing cells. Cells were cultured at 37oC in a 5% CO2 atmosphere. Prior to intrathecal inoculation, cells were detached from culture flasks using rubber cell scrapers, washed three times, and suspended in PBS at a concentration of 1.25 × 108 cells/ml for use in Experiment 1, and 6.25 × 107 cells/ml for use in Experiment 2 and 3. The cell suspension solution was then loaded into 1000 µ1 Hamilton syringes and injectors (Hamilton Co., Reno, NV).
2.4 Athymic rat model of breast carcinomatous meningitis
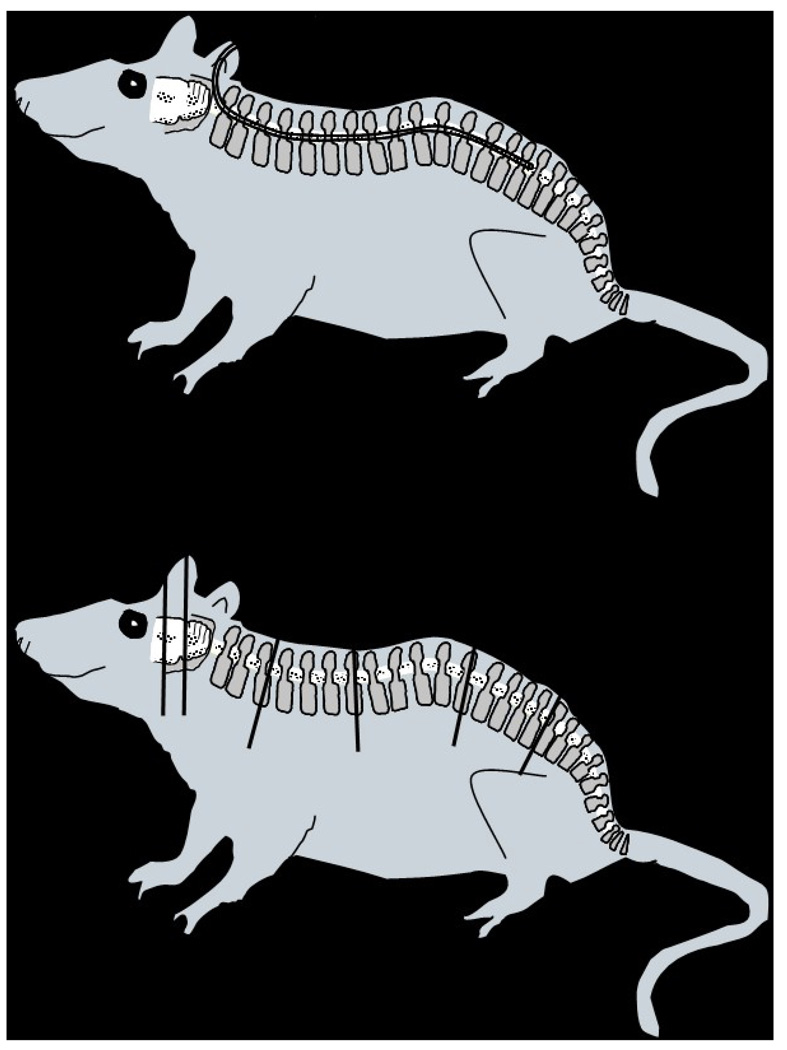
All procedures involving animals were performed in accordance with protocols approved by the Duke University Institutional Animal Care and Use Committee. Female athymic rats (Big:NIMRrnu/rnu) weighing 350–450 g and 5–6 months of age were maintained in the Duke University Cancer Center Isolation Facility. Animals were anesthetized with a mixture of ketamine (55 mg/ml) and xylazine (9 mg/ml) given intraperitoneally in a total volume of 1 ml/kg body weight. Intrathecal catheters were placed using the procedures described by Fuchs et al. [21]. Briefly, rats were placed in a Kopf stereotactic frame (David Kopf Instruments, Tujunga, CA) with the neck flexed at 90°. A midline sagittal incision was made from the inion to the arch of C1 lamina. The atlanto-occipital membrane was then exposed, and the underlying cisterna magna dura was opened. A PE-10 catheter (Intramedic; Clay Adams, Franklin Lakes, NJ) with a 5-0 stainless steel wire stylet was inserted into the subarachnoid space and passed along the posterior aspect of the spinal cord so that the tip rested in the lumbar region (8.5 cm) as illustrated in Figure 1. A loose knot was tied in the catheter and fixed with dental epoxy (Lang Dental Manufacturing Co., Chicago, IL). The catheter was passed through the skin lateral to the incision, and the wound was closed with surgical staples. The animals were allowed to recover for 10–14 days. Beginning 8–10 days before tumor inoculation, a 1.6 mg continuous release 17β-estradiol pellet (Innovative Research, Toledo, OH) was placed subcutaneously. Animals were anesthetized by inhalation of isoflurane and CM was initiated by a single bolus of tumor cells (0.5–1.25 × 107 in 80–100 µl) via the indwelling catheter using the Hamilton syringe and injector. The catheter was flushed with 20 µl of saline and the catheter was re-occluded using a small piece of 2-0 stainless steel wire.
Figure 1.
(Top) An intrathecal catheter was surgically implanted in athymic female rats to permit intrathecal tumor cell inoculation and development of carcinomatous meningitis as well as intrathecal treatment administration. The PE-10 14-cm long catheter was inserted into the subarachnoid space and passed along the posterior aspect of the spinal cord to reach the lumbar area. (Bottom) In order to perform a histopathological analysis of the neuroaxis, animals were necropsied and sections from the brain, at the level of the bregma (A) and lambda (B), and the spine at the cervical (C), thoracic (D), lumbar (E) and sacral (F) levels were obtained; tissues, previously fixed in 10% formalin, were embedded in paraffin, sectioned and stained with H&E.
2.5 Targeted radiotherapy studies
A total of three experiments were performed. In each case, treatment was initiated three days after tumor inoculation. Treatment solutions were injected in volumes of 40 µl, followed by a 20 µl flush, via the indwelling catheter using the Hamilton syringe and injector. The tumor inoculum in the first experiment was 1.25 × 107 cells and was 5 × 106 cells in the other two experiments. In Experiment 1, animals received 33 µCi/14 µg 211At-labeled trastuzumab (n=8), 66 µCi/14 µg 211At-labeled trastuzumab (n=9), 14 µg cold trastuzumab (n=10), or saline (n=9). In Experiment 2, animals were treated with 46 µCi/19 µg 211At-labeled trastuzumab (n=10), 92 µCi/19 µg 211At-labeled trastuzumab (n=11), or saline (n=10). In Experiment 3, animals received 28 µCi//20 µg 211At-labeled trastuzumab (n=10), 30 µCi/20 µg 211At-labeled TPS3.2 (n=9), or saline (n=11). Following treatment administration, animals were examined daily for neurological status and survival, and were weighed twice weekly. Median survival was determined using the Kaplan-Meier plot method. The Wilcox rank sum test was used to analyze the statistical significance of differences among groups, with a P < 0.05 considered to be significant.
2.6 Histopathological analysis
Within 24 h of death or, for survivors, at the end of the experiment, animals were necropsied in order to perform a histopathological analysis of the neuroaxis. The skull and spine were removed, fixed in 10% buffered neutral formalin for 7–10 days and treated with a decalcifying solution for 1–2 days. Sections from the brain, at the level of the bregma (A) and lambda suture (B), and the spine at the cervical (C), thoracic (D), lumbar (E) and sacral (F) levels were obtained (Figure 1). The tissues were embedded in paraffin, cut at 6-µm sections, and stained with hematoxylin and eosin-Luxol fast blue for light microscopic examination by a neuropathologist.
3. Results
3.1 Breast carcinomatous meningitis model
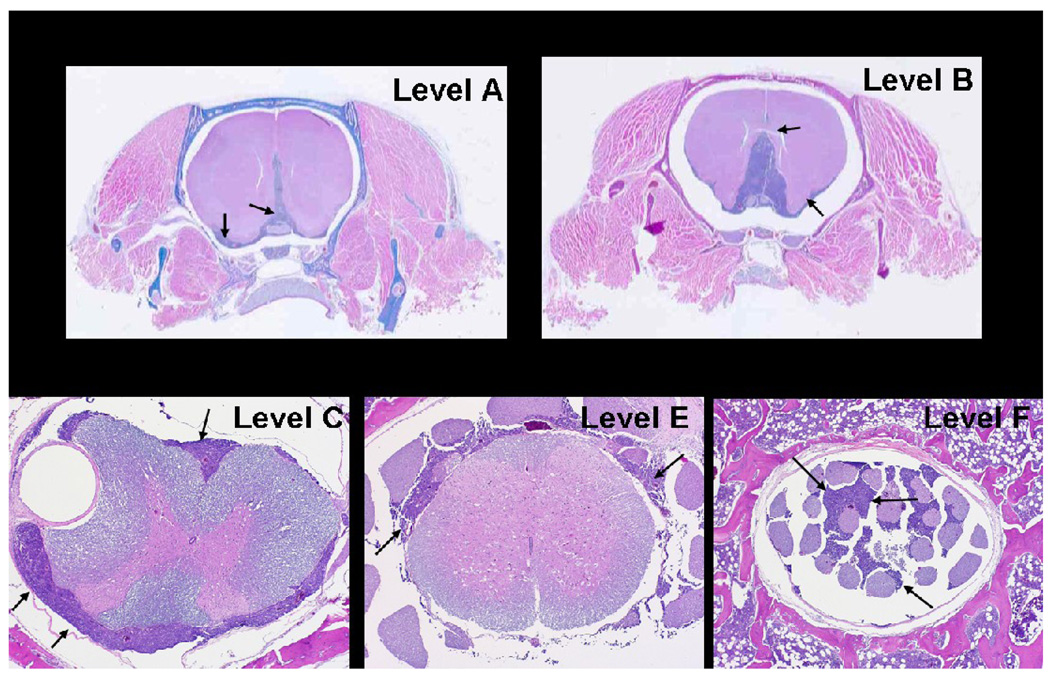
The effect of altering the number and volume of inoculated cells was evaluated in preliminary experiments where it was found that CM could be established reproducibly with volumes of 80–100 µl and 0.5–1.25 × 107 MCF-7/HER2-18 cells. As observed previously, in order to use this cell line to establish intracerebral xenografts in this rat strain [22], consistent tumor growth required use of a continuous-release 17β-estradiol pellet. At these cell doses, animals developed progressive neurological deficits and died on Days 18–24. Histological analysis revealed the presence of leptomeningeal tumor extension from the base of the brain to the cauda equina (Figure 2).
Figure 2.
Carcinomatous meningitis model in the athymic rat resulting from the intrathecal administration of MCF7/HER2-18 breast carcinoma cells. Arrows indicate the presence of tumor in histopathological sections obtained from the neuroaxis at the levels indicated in Figure 1, bottom.
3.2 Targeted radiotherapy studies
Details, including tumor inoculum, number of animals in each treatment group and the protein doses of trastuzumab, for the three radioimmunotherapy experiments are summarized in Table 1.
Table 1.
Treatment response observed in athymic rats with carcinomatous meningitis treated with single intrathecal injection of 211At-trastuzumab or controls
| Experiment 1: 1.25 × 107 cell inoculum | |||||
|---|---|---|---|---|---|
| Treatment | Dose | Rats/group | Median survival (days) |
Survival advantage (%)a |
Significance vs. saline |
| Saline | -- | 9 | 21 | -- | -- |
| Cold trastuzumab | 14 µg | 10 | 21 | 0 | Not significant |
| 211At-trastuzumab | 33 µCi/14 µg | 8 | 45 | 214 | P <0.005 |
| 211At-trastuzumab | 66 µCi/14 µg | 9 | 48 | 229 | P <0.005 |
| Experiment 2: 5.0 × 106 cell inoculum | |||||
|---|---|---|---|---|---|
| Treatment | Dose | Rats/group | Median survival (days) |
Survival advantage (%) |
Significance vs. saline |
| Saline | -- | 10 | 23 | -- | -- |
| 211At-trastuzumab | 46 µCi/19 µg | 10 | 68 | 296 | P <0.001 |
| 211At-trastuzumab | 92 µCi/19 µg | 11 | 92 | 400 | P <0.001 |
| Experiment 3: 5.0 × 106 cell inoculum | |||||
|---|---|---|---|---|---|
| Treatment | Dose | Rats/group | Median survival (days) |
Survival advantage (%) |
Significance vs. saline |
| Saline | -- | 11 | 20 | -- | -- |
| 211At-trastuzumab | 28 µCi/20 µg | 10 | 36 | 180 | P <0.005 |
| 211At-TPS3.2 | 30 µCi/16 µg | 9 | 29 | 145 | P <0.005 |
Percent survival advantage is calculated as the median survival of treated mice divided by the median survival of mice receiving saline × 100
Experiment 1
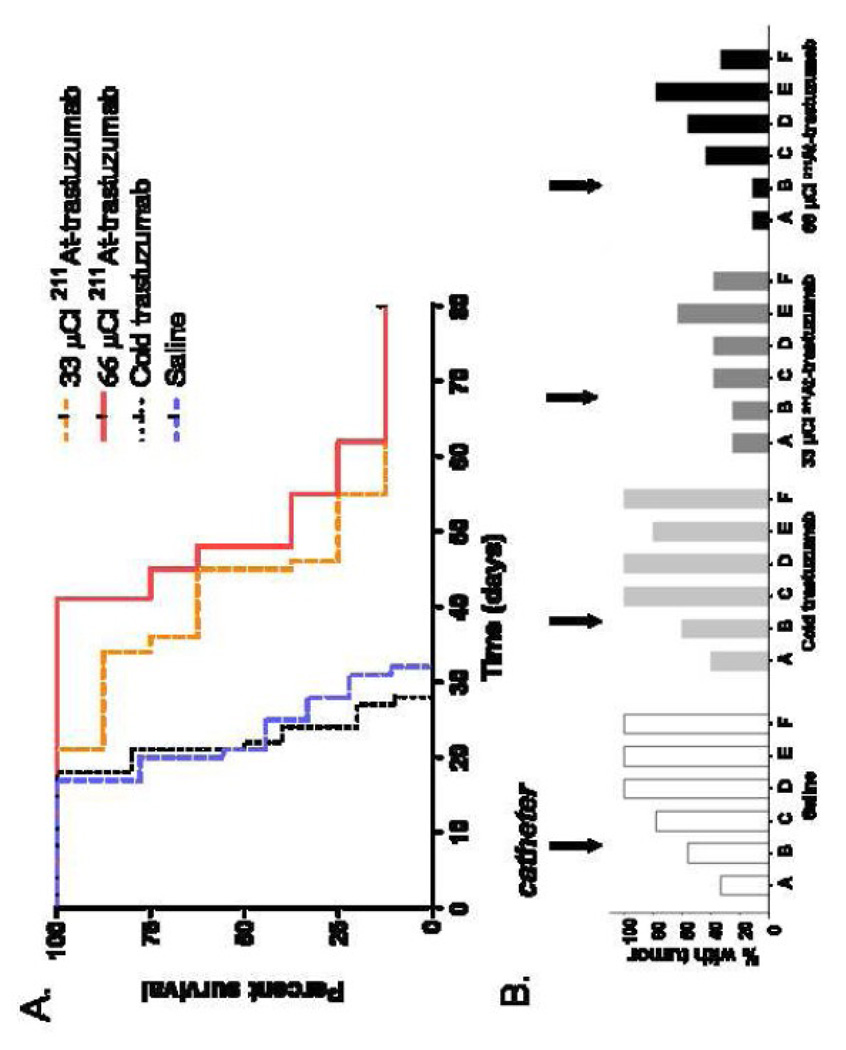
As shown in Figure 3A, animals treated with saline or cold trastuzumab had a median survival of 21 days. Median survival was more than doubled to 45 and 48 days for groups that received 33 and 66 µCi 211At-labeled trastuzumab, respectively; one long-term survivor was observed in each radiolabeled trastuzumab-treated group. Doubling the level of 211At on the mAb did not result in a significant increase in survival (P = 0.252). The incidence of tumor in histopathological sections of the brain (A, B) and spine (C-F) was significantly reduced among animals treated with 211At-labeled trastuzumab, compared to those treated with saline or cold trastuzumab (Figure 3B). Histopathological analysis reveal that 60% and 67% of the animals treated with saline or cold trastuzumab, respectively, were diagnosed with intracranial tumor growth, in comparison with 25% and 11% for those treated with the low and high dose of 211At-labeled trastuzumab, respectively. Along the spine, tumor was found in 78–100% of the control animals and in 38–63% and 33–78% of those treated with 211At-labeled trastuzumab at the low and high doses, respectively.
Figure 3.
(A) Percentage of athymic rats with MCF7/HER2-18 breast carcinoma carcinomatous meningitis surviving after intrathecal administration of 33 or 66 µCi 211At-labeled trastuzumab (14 µg), cold trastuzumab (14 µg), or saline 3 days after intrathecal injection of 1.25 × 107 tumor cells. (B) Percentage of animals having evidence of tumor in sections of the neuroaxis after treatment, as determined by histopathologial analysis. Location of sections as indicated in Figure 1, bottom.
Experiment 2
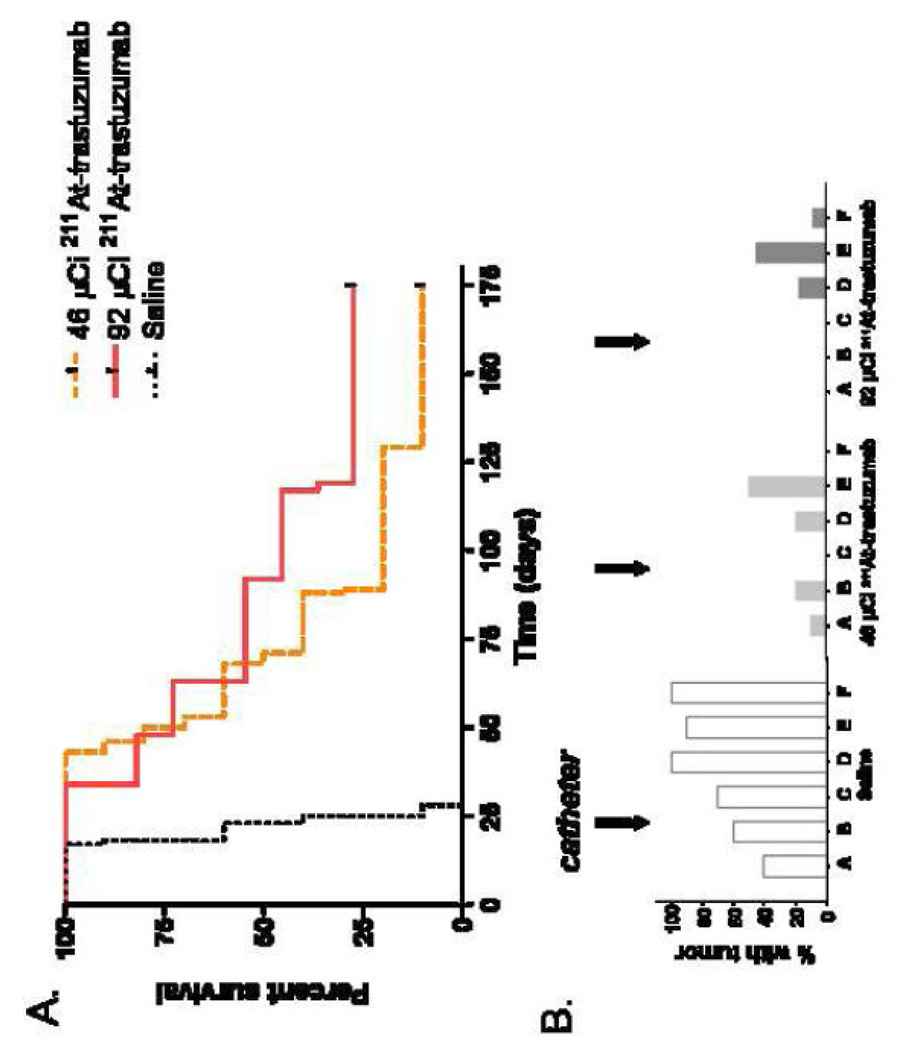
Animals in this and the following experiment were inoculated with a tumor burden of 5 × 106 cells compared with 1.25 × 107 cells used in Experiment 1. The median survival of the saline group was 23 days in this study, longer than observed in Experiment 1(21 days); however, the difference was not statistically significant. As shown in Figure 4A, treatment with 46 µCi and 92 µCi 211At-labeled trastuzumab increased median survival to 68 and 92 days, respectively, equivalent to a three and four fold survival prolongation advantage compared with saline. Nonetheless, according to the Wilcox rank sum test, the difference in median survival between the high and low dose groups was not statistically significant (P = 0.20). There were 3 long term survivors out of 11 animals in the high dose group and one long term survivor out of 10 animals in the low dose 211At-labeled trastuzumab group. For animals receiving both the low and high dose of 211At-labeled trastuzumab, histopathological analysis documented the presence of tumor in 0–20% of animals in all regions except the lumbar spine (Figure 4B). In comparison, 40–100% of animals in the saline group had evidence of tumor in these regions.
Figure 4.
(A) Percentage of athymic rats with MCF7/HER2-18 breast carcinoma carcinomatous meningitis surviving after intrathecal administration of 46 or 92 µCi 211At-labeled trastuzumab, or saline 3 days after intrathecal injection of 5 × 106 tumor cells. (B) Percentage of animals having evidence of tumor in sections of the neuroaxis after treatment, as determined by histopathologial analysis. Location of sections as indicated in Figure 1, bottom.
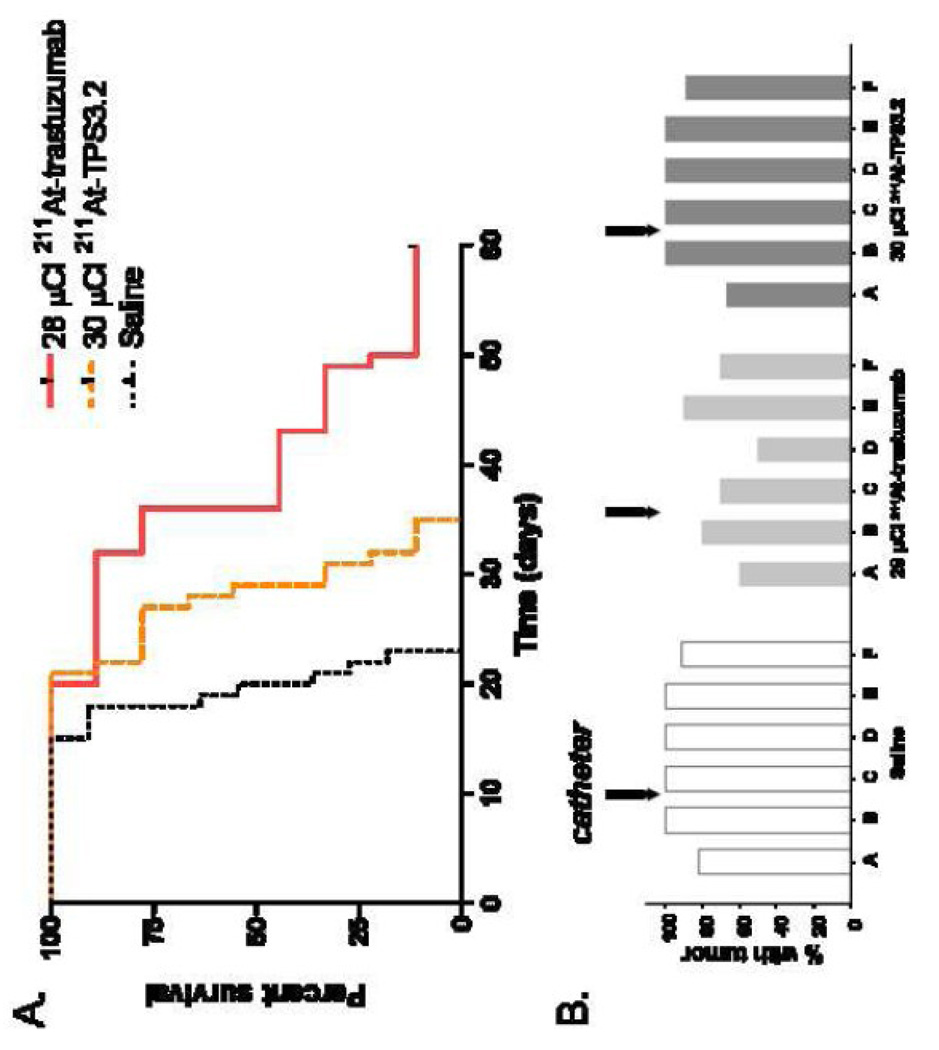
Experiment 3
The third experiment was performed to evaluate the specificity of the 211At-labeled trastuzumab therapeutic effect. Animals treated with saline had a median survival of 20 days, compared to 29 and 36 days for those treated with 211At-labeled TPS3.2 (30 µCi) and 211At-labeled trastuzumab (28 µCi), respectively (Figure 5A). The median survival for both 211At-labeled mAb groups was significantly longer than that of the saline control, and the median survival for the 211At-labeled trastuzumab group was significantly longer than that for the group treated with the HER2 nonreactive 211At-labeled TPS3.2 mAb. One long-term survivor was observed in the 211At-labeled trastuzumab-treated group. Tumor was found in 82–100% of brain sections in the saline group compared with 67–100% and 60–80% in the TPS3.2 and trastuzumab groups, respectively (Figure 5B). Likewise, tumor was found in 91–100% of spine sections in the saline group compared with 89–100% and 50–90% in the TPS3.2 and trastuzumab groups, respectively.
Figure 5.
(A) Percentage of athymic rats with MCF7/HER2-18 breast carcinoma carcinomatous meningitis surviving after intrathecal administration of 28 µCi 211At-labeled trastuzumab, 30 µCi 211At-labeled TPS3.2 control mAb or saline 3 days after intrathecal injection of 5 × 106 tumor cells. (B) Percentage of animals having evidence of tumor in sections of the neuroaxis after treatment, as determined by histopathologial analysis. Location of sections as indicated in Figure 1, bottom.
3.3 Histopathological analysis for benign pathological changes
In addition to evaluation of the presence of tumor, sections of the neuraxis were assessed for toxic effects potentially associated with 211At-labeled mAb treatment. The percentages of animals in each group in which hemorrhage, necrosis, demyelination (defined as demyelination of the long tracts of the cord found along the subarachnoid space), massive spinal edema and meningeal inflammation or fibrosis were found in at least one section of an animal are summarized in Table 2. In the first experiment performed with the higher tumor inoculum, the percentage of animals exhibiting necrosis, edema and hemorrhage in the 211At-labeled trastuzumab groups was 0–38%, comparable to those seen in the saline and cold trastuzumab groups. Demyelination was only observed in the high dose 211At-labeled trastuzumab group (44%) while fibrosis was only seen in the low dose group (38%). With the exception of the observation of demyelination in 3/10 animals in Experiment 2, the percentages of animals exhibiting pathological signs in the experiments performed with the lower initial tumor burden were lower than in those receiving the higher burden. In Experiment 2, particularly in the 92 µCi 211At-labeled trastuzumab group, the percentage of animals with necrosis, edema, fibrosis and demyelination was 36, 45, 55, and 64%, respectively. However, in the 3 long term survivors in this group, the pathological observations were limited to demyelination in 1 or 2 of 7 sections in each animal, fibrosis in the cervical spine section of one animal and the sacral spine section of one animal, and edema in the cervical spine section of one animal. The only toxicity observed in Experiment 3 was demyelination in 1–3 sections of 3/10 animals receiving the 28 µCi dose of 211At-labeled trastuzumab.
Table 2.
Percentage (%) of athymic rats per treatment group with cacinomatous meningitis presenting a specific lesion in at least one histopathological tissue section of their neuroaxis
| Experiment 1 | |||||
|---|---|---|---|---|---|
| Treatment | Necrosis | Edema | Hemorrhage | Fibrosis | Demyelination |
| Saline | 33 | 22 | 11 | 0 | 0 |
| Cold trastuzumab | 40 | 30 | 10 | 0 | 0 |
|
211At- trastuzumab 33 µCi |
38 | 25 | 13 | 38 | 0 |
|
211At- trastuzumab 66 µCi |
11 | 11 | 0 | 0 | 44 |
| Experiment 2 | |||||
|---|---|---|---|---|---|
| Treatment | Necrosis | Edema | Hemorrhage | Fibrosis | Demyelination |
| Saline | 10 | 10 | 0 | 0 | 30 |
|
211At- trastuzumab 46 µCi |
0 | 30 | 0 | 20 | 80 |
|
211At- trastuzumab 92 µCi |
36 | 45 | 0 | 55 | 64 |
| Experiment 3 | |||||
|---|---|---|---|---|---|
| Treatment | Necrosis | Edema | Hemorrhage | Fibrosis | Demyelination |
| Saline | 0 | 0 | 0 | 0 | 0 |
|
211At- trastuzumab 28 µCi |
0 | 0 | 0 | 0 | 30 |
|
211At-TPS3.2 30 µCi |
0 | 0 | 0 | 0 | 0 |
4. Discussion
Better treatments for malignancies that metastasize to the subarachnoid space are critically needed because current therapies are largely ineffective [1,2]. Although radiation has been utilized in patients with CM, external beam therapy can rarely achieve a meaningful therapeutic effect because of dose-limiting toxicity to the normal neuraxis [3]. Targeted radiotherapy is attractive in this setting because of the potential for achieving high levels of tumor cell irradiation while sparing normal tissues. The feasibility of this approach has been demonstrated in pilot clinical studies performed in CM patients with glioma, melanoma and neuroblastoma, all utilizing mAbs labeled with the β-particle emitter 131I [23–25]. Although responses were observed in some patients, in others, significant toxicities were seen [26].
A more effective strategy might be to utilize radionuclides emitting radiation with a range in tissue that is better matched to the geometry of CM, which frequently is characterized by 5 to 10 cell thick layers of tumor cells and free floating cancer cells within the CSF [27]. Dosimetry calculations suggest that using a shorter range β-particle emitter such as 199Au (P90 280 µm) would increase CSF:spinal cord dose ratios by a factor of three [16]. A further enhancement in the selectivity of CM irradiation would be reasonable to expect based on the 55–80 µm range of 211At α-particles. This feature, combined with the radiobiological advantages in utilizing high linear energy transfer radiation for targeted radiotherapy, provided motivation for the current study investigating the therapeutic efficacy of a single dose of 211At-labeled trastuzumab in an athymic rat model of CM.
A HER-2 expressing CM model was established in athymic rats and histopathological analyses documented the presence of leptomeningeal tumor extension from the base of the brain to the cauda equina. Fluorescence activated cell sorting analysis of the MCF-7/HER2-18 line in vitro indicated that receptor expression on this cell line was heterogeneous with a bimodal distribution of receptors per cell observed [28]. Heterogeneous receptor expression is one of the major hurdles to molecularly targeted cancer therapy [29] and thus a CM model with varying levels of HER-2 provides a realistic setting for the evaluation of therapeutic agents that target the p185 trans-membrane protein HER2 oncogene product. The existence of subpopulations of cancer cells with low receptor number is particularly problematic for treatments with short-range radionuclides such as α-particles because killing of non-targeted cells by physical cross fire will be limited.
The cytotoxicity of 211At-labeled trastuzumab for MCF-7/HER2-18 cells has been evaluated in vitro [28]. When survival fraction was plotted against activity concentration, a two phase relationship was observed, consistent with the bimodal HER2 receptor concentration on this line. The intrinsic cell sensitivity for 211At-labeled trastuzumab was measured to be 0.36 Gy compared with a D37 value of 3.1 Gy for external beam radiation, indicating a relative biological effectiveness of 8.6, consistent with the high linear energy transfer characteristic of α-particles. As observed with other 211At-labeled targeted radiotherapeutics, a significant reduction in tumor cell survival could be achieved with less than 10 211At decays per cell [17], making 211At-labeled trastuzumab an attractive reagent for the treatment of HER2 expressing malignancies.
Our results demonstrate that a single dose of 211At-labeled trastuzumab was effective in the treatment of MCF-7/HER2-18 breast CM in the athymic rat. In the first experiment, performed 3 days after injection of 1.25 × 107 tumor cells, intrathecal administration of 211At-labeled trastuzumab more than doubled median survival compared with untreated controls as well as a group of animals receiving unlabeled trastuzumab at the same protein dose present in the 211At-labeled mAb preparations. Thus, the tumor response observed with 211At-labeled trastuzumab resulted solely from the radiation emitted by 211At and not the mAb. Higher doses of cold trastuzumab were not evaluated in the CM model; however, in a previous study evaluating the therapeutic potential of trastuzumab administered by convection enhanced delivery to rats with intracerebral MCF-7/HER2-18 xenografts, a 7-day infusion of 16 mg/kg trastuzumab was required to increase median survival by 181% [22].
Because no dose-related differences in median survival were seen between the lower (33 µCi) and higher (66 µCi) doses of 211At-labeled trastuzumab, the animals were inoculated with a lower tumor burden, 5 × 106 cells in further experiments. Administration of 46 µCi and 92 µCi of 211At-labeled trastuzumab increased median survival to 296% and 400% of that observed in the saline group and there were 1/10 and 3/11 long term survivors, respectively. The more pronounced therapeutic response along with the emergence of a dose-response pattern suggest that the more advanced stage of disease at the time when treatment was administered in the first experiment might have interfered with uniform delivery of the labeled mAb throughout the neuraxis. Comparison of the percentage of animals with tumor detected on histopathological analysis following radiolabeled mAb treatment at similar dose suggests that this might indeed be the case.
Comparisons were made to the therapeutic effectiveness of a 211At-labeled HER2 nonreactive mAb in order to determine the specificity of the 211At-labeled trastuzumab treatment. Because the small size of the intrathecal compartment in the rat could lead to considerable irradiation of tumor from unbound labeled mAb in the CSF, it would be expected that demonstrating specificity might be difficult in this setting. The 211At-labeled control mAb did result in a 45% increase in median survival compared with saline while 211At-labeled trastuzumab resulted in an 80% increase in median survival. In addition, histopathological analysis revealed a higher incidence of tumor in sections from animals receiving labeled control mAb compared with labeled trastuzumab. Presumably, these differences reflect binding of 211At-trastuzumab to receptors on cancer cell surface coupled with the rapid clearance of IgG from the rat intrathecal space [30].
One of the motivations for performing histopathological analysis of the neuroaxis was to determine whether regional differences occurred with regard to therapeutic effect. This was indeed the case because in animals receiving 211At-labeled trastuzumab treatment, tumor foci were most effectively eliminated from the regions rostral to the injection site (brain at the level of the bregma and lambda, cervical spine). The region of the neuroaxis with the highest percentage of animals with tumor foci was the lumbar spine. This pattern of tumor after 211At-labeled mAb treatment is the opposite of that observed in a prior study in which rats with TE-671 rhabdomyosarcoma neoplastic meningitis were treated 8 days after receiving an inoculation of 5 × 105 cancer cells [31]. Although the reason for this difference is not known, differences in tumor cell inoculation number, interval before initiation of radioimmunotherapy and nature of the cell line are all possible contributing factors.
Histopathological analyses also were performed in order to get a preliminary indication of the toxicities associated with intrathecal administration of 211At-labeled mAbs. Because these were tumor bearing animals, the effects of 211At-labeled mAb therapy were considered to be those pathological alterations in the treated animals but not the saline controls. Animals receiving saline in the experiment with the higher initial tumor burden had a higher incidence of CNS necrosis, edema and hemorrhage than the saline groups in experiments with the lower initial tumor burden. In animals receiving the lower initial tumor burden, those receiving the highest dose (92 µCi) of 211At-labeled trastuzumab had the highest incidence of CNS necrosis, edema and fibrosis. However, there were 3 long term survivors in this group and in these, benign pathological changes were limited to one or two of seven sections from each animal. It should be noted that because of the small size of the intrathecal compartment in the rat, the neuroaxis likely is within range of a greater fraction of α-particles emitted by unbound 211At-labeled mAb in the CSF than in the human. For this reason, it is possible that the pathological changes observed in the rat model may over emphasize those that might occur in the human.
The survival advantage resultant from 211At-labled trastuzumab treatment compares favorably with those reported in two previous studies evaluating mAbs labeled with α-particle emitters for the treatment of leptomeningeal carcinomatosis in the athymic rat. Using the anti-disialoganglioside GD2 mAb 3F8 labeled with 10-day half life 225Ac, median survival in rats with neuroblastoma meningeal carcinomatosis could be increased twofold from 16 to 34 days [27]. And treatment of rats with TE-671 rhabdomyosarcoma neoplastic meningitis with only 18 µCi of 211At-labeled anti-tenascin mAb 81C6 increased median survival from 23.5 days to 84 days, a 357% survival prolongation [31]. Although comparison of results obtained with different animal models and mAbs must be done with caution, we note that achieving similar therapeutic benefit required at least three times higher activity with 211At-labeled trastuzumab. This may reflect differences in the pattern of radionuclide delivery along the neuroaxis as suggested by the histopathological analyses noted above, or perhaps the more rapid degradation of an internalizing mAb [28] compared with one binding to a molecular target found in the extracellular matrix.
In this study, a 400% increase in median survival was obtained after injection of 92 µCi of 211At-labeled trastuzumab in 350–450 g rats. Assuming a body weight of 58 kg for females, this would scale a dose of 13.3 mCi in humans. An alternative approach for estimating the corresponding human dose would be to scale according to the volume of the cerebrospinal fluid compartment, which are estimated to be 350 µl in the rat [32] and 125 ml in the human [33]. On this basis, a 32.9 mCi dose of 211At-labeled trastuzumab would be estimated in humans. The radiotoxicity of intrathecally administered 211At-labeled trastuzumab has not been determined. In the past, the radiotoxicity of systemically administered 211At-labeled chimeric 81C6 mAb [34] was determined over one year prior to initiating its clinical evaluation after injection into surgically created brain tumor resection cavities [35]. Systemic administration was utilized to represent the worst possible case scenario, i.e. total and immediate release of 211At from the resection cavity. The lethal dose in 10% of animals (LD10) measured for intravenously administered 211At-labeled chimeric 81C6 mAb in normal mice was 46 kBq/g in females (102 kBq/g in males), equivalent to a dose of 72 mCi in humans [34]. Thus, the projected therapeutically relevant doses in humans for intrathecal 211At-labeled trastuzumab based both on body weight and CSF volume, are less than the extrapolated LD10 determined after systemic administration of another 211At-labeled chimeric 81C6.
In summary, the data presented here have demonstrated that significant prolongation in median survival, and in some animals, cure of a HER2-expressing breast CM could be achieved following intrathecal administration of 211At-labeled trastuzumab. Future studies to define the pharmacokinetics and regional distribution of radiolabeled trastuzumab in larger animals or humans will be critical for optimizing the efficacy of this promising targeted radiotherapeutic approach. It is envisioned that more homogeneous delivery of radiation dose and enhanced therapeutic effectiveness might be possible through the use of fractionated delivery, smaller fragments of trastuzumab [36] and 211At labeling methods designed for maximizing tumor retention of label with internalizing mAbs [37].
Acknowledgments
This work was supported by grants CA42324 and NS20023 from the National Institutes of Health and Grant DOE-FG02-08ER64697 from the Department of Energy. Dr. Abraham Boskovitz was supported by the SICPA Foundation (Prilly, Switzerland), the Swiss Cancer League and the 450th Anniversary of the University of Lausanne Foundation (Lausanne, Switzerland). We thank Dr. Xiao-Guang Zhao for his assistance in preparing the 211At-labeled mAbs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by Grants CA42324 and CA42324 from the National Institutes of Health and Grant DOE-FG02-08ER64697 from the Department of Energy.
References
- 1.Prados MD. Primary neoplasms of the central nervous system in adults. In: Kufe DW, editor. Cancer Medicine. 7th Edition. Hamilton, ON: BC Decker; 2006. pp. 1037–1064. [Google Scholar]
- 2.Chamberlain MC. Neoplastic meningitis. J Clin Oncol. 2005;23:3605–3613. doi: 10.1200/JCO.2005.01.131. [DOI] [PubMed] [Google Scholar]
- 3.Jaeckle KA. Neoplastic meningitis from systemic malignancies: diagnosis, prognosis and treatment. Semin Oncol. 2006;33:312–323. doi: 10.1053/j.seminoncol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- 5.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 6.Natali PG, Nicotra MR, Bigotti A. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer. 1990;45:457–461. doi: 10.1002/ijc.2910450314. [DOI] [PubMed] [Google Scholar]
- 7.Sliwkowski MX, Lofgren JA, Lewis GD. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60–70. [PubMed] [Google Scholar]
- 8.Hortobagyi GN. Overview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer. Semin Oncol. 2001;28:43–47. [PubMed] [Google Scholar]
- 9.Baselga J. Clinical trials of Herceptin (trastuzumab) Eur J Cancer. 2001;37(Suppl 1):S18–S24. [PubMed] [Google Scholar]
- 10.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 11.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 12.Stemmler HJ, Schmitt M, Harbeck N. Application of intrathecal trastuzumab (Herceptin) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep. 2006;15:1373–1377. doi: 10.3892/or.15.5.1373. [DOI] [PubMed] [Google Scholar]
- 13.Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer. 2001;2:235. doi: 10.1016/S1526-8209(11)70419-0. [DOI] [PubMed] [Google Scholar]
- 14.Stemmler HJ, Mengele K, Schmitt M. Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER-2 overexpressing metastatic breast cancer: a case report. Anticancer Drugs. 2008;19:832–836. doi: 10.1097/CAD.0b013e32830b58b0. [DOI] [PubMed] [Google Scholar]
- 15.Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. High dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2-expressing breast cancer. Annals Oncol. 2008;19:1978–1980. doi: 10.1093/annonc/mdn654. [DOI] [PubMed] [Google Scholar]
- 16.Millar WT, Barrett A. Dosimetric model for antibody targeted radionuclide therapy of tumor cells in cerebrospinal fluid. Cancer Res. 1990;50(Suppl):1043s–1048s. [PubMed] [Google Scholar]
- 17.Vaidyanathan G, Zalutsky MR. Astatine radiopharmaceuticals: prospects and problems. Curr Radiopharm. 2008;1:177–196. doi: 10.2174/1874471010801030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalutsky MR, Archer GE, Garg PK, Batra SK, Bigner DD. Chimeric anti-tenascin antibody 81C6: increased tumor localization compared with its murine parent. Nucl Med Biol. 1996;23:449–458. doi: 10.1016/0969-8051(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 19.Zalutsky MR, Zhao XG, Alston KL, Bigner D. High-level production of alpha-particle-emitting 211At and preparation of 211At-labeled antibodies for clinical use. J Nucl Med. 2001;42:1508–1515. [PubMed] [Google Scholar]
- 20.Benz CC, Scott GK, Sarup JC. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs HE, Archer GE, Colvin OM. Activity of intrathecal 4-hydroperoxycyclo-phosphamide in a nude rat model of human neoplastic meningitis. Cancer Res. 1990;50:1954–1959. [PubMed] [Google Scholar]
- 22.Grossi PM, Ochiai H, Archer GE. Efficacy of intracerebral microinfusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Cancer Res. 2003;9:5514–5520. [PubMed] [Google Scholar]
- 23.Brown MT, Coleman E, Friedman AH. Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: Phase I trial results. Clin Cancer Res. 1996;2:963–972. [PubMed] [Google Scholar]
- 24.Cokgor I, Akabani G, Friedman HS. Long-term response in a patient with neoplastic meningitis secondary to melanoma treated with 131I-radiolabeled anti-chondroitin proteoglycan sulfate Me1-14 F(ab’)2: A case study. Cancer. 2001;91:1809–1813. [PubMed] [Google Scholar]
- 25.Kramer K, Humm JL, Souweidane MM. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25:5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 26.Moseley RP, Benjamin JC, Ashpole RD. Carcinomatous meningitis: antibody guided therapy with I-131 HMFG1. J Neurol Neurosurg Psychiatry. 1991;54:260–265. doi: 10.1136/jnnp.54.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miederer M, McDevitt MR, Borchardt P. Treatment of neuroblastoma meningeal carcinomatosis with intrathecal application of α-emitting atomic nanogenerators targeting disialo-ganglioside GD2. Clin Cancer Res. 2004;10:6985–6992. doi: 10.1158/1078-0432.CCR-04-0859. [DOI] [PubMed] [Google Scholar]
- 28.Akabani G, Carlin S, Welsh P, Zalutsky MR. In vitro cytotoxicity of 211At-labeled trastuzumab in human breast cancer cell lines: effect of specific activity and HER2 receptor heterogeneity on survival fraction. Nucl Med Biol. 2006;33:333–347. doi: 10.1016/j.nucmedbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Braun S, Hepp F, Sommer HL, Pantel K. Tumor-antigen heterogeneity of disseminated breast cancer cells: implications for immunotherapy of minimal residual disease. Int J Cancer. 1999;84:1–5. doi: 10.1002/(sici)1097-0215(19990219)84:1<1::aid-ijc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Bergman I, Burckart GJ, Pohl CR. Pharmacokinetics of IgG and IgM anti-ganglioside antibodies in rats and monkeys after intrathecal administration. J Pharmacol Exp Ther. 1998;284:111–115. [PubMed] [Google Scholar]
- 31.Zalutsky MR, McLendon RE, Garg PK, Archer GE, Schuster JM, Bigner DD. Radioimmunotherapy of neoplastic meningitis in rats using an α-particle-emitting immunoconjugate. Cancer Res. 1994;54:4719–4725. [PubMed] [Google Scholar]
- 33.van den Berg MP, Romeijn SG, Verhoef JC, Merkus FWHM. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J Neurosci Methods. 2002;116:99–107. doi: 10.1016/s0165-0270(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 34.Nopoulos P, Flaum M, Andreasen NC. Sex differences in brain morphology in schizophrenia. Am J Psychiatry. 1997;154:1648–1654. doi: 10.1176/ajp.154.12.1648. [DOI] [PubMed] [Google Scholar]
- 35.McLendon RE, Archer GE, Larsen RH, Akabani G, Bigner DD, Zalutsky MR. Radiotoxicity of systemically administered 211At-labeled human/mouse chimeric monoclonal antibody: a long-term survival study with histological analysis. Int J Radiat Oncol Biol Phys. 1999;45:491–499. doi: 10.1016/s0360-3016(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 36.Zalutsky MR, Reardon DA, Akabani G. Clinical experience with α-emitting astatine-211: treatment of recurrent brain tumor patients with 211At-labeled chimeric 81C6 anti-tenascin monoclonal antibody. J Nucl Med. 2008;49:30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olafsen T, Kenanova VE, Sundaresan G. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005;65:5907–5916. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaidyanathan G, Affleck DJ, Bigner DD, Zalutsky MR. N-succinimidyl 3-[211At]astato-4-guanidinomethylbenzoate: an acylation agent for labeling internalizing antibodies with α-particle emitting 211At. Nucl Med Biol. 2003;30:351–359. doi: 10.1016/s0969-8051(03)00005-2. [DOI] [PubMed] [Google Scholar]