Abstract
Background
Aging is associated with a decline in exercise capacity that may be attributable to maladaptations in both skeletal muscle perfusion and metabolism; yet very little is known regarding the real-time, within-muscle interplay between these parameters during physical activity. Therefore, we utilized an unique nuclear magnetic resonance sequence to concomitantly examine changes in lower leg skeletal muscle perfusion and metabolism.
Methods
In young (26 ± 5 years, n = 6) and older (70 ± 5 years, n = 6) healthy volunteers, arterial spin labeling measurements of muscle perfusion were combined with 31 Phosphorous (31P) nuclear magnetic resonance spectroscopy to monitor high-energy phosphate metabolites during and after 5 minutes of moderate-intensity (≈5W) plantar flexion exercise.
Results
Compared with young, end-exercise perfusion was diminished in older participants (43 ± 10 mL/100 g/minute, old; 60 ± 7 mL/100 g·minute, young), accompanied by greater phosphocreatine (PCr) depletion (–28% ± 12%, old; –19% ± 7%, young) and elevated inorganic phosphate/PCr (0.41 ± 0.2, old; 0.24 ± 0.09, young); yet the time constant for PCr recovery (τ, an index of muscle oxidative capacity) was similar between groups (51 ± 17 seconds, old; 48 ± 7 seconds, young).
Conclusions
Together, these preliminary data provide evidence of an age-related decline in tissue perfusion and increased “metabolic stress” during exercise but demonstrate that overall oxidative capacity in the elderly does not appear negatively affected by this relatively hypoperfused state.
Keywords: 31P spectroscopy, Oxidative capacity, Exercise, Aging
THERE is accumulating experimental evidence that leg blood flow is attenuated in the elderly (1–7), a condition made worse during physical activity, when metabolic demand is increased and must be met by an appropriate rise in perfusion to the exercising tissue. Despite this well-known deficit, to our knowledge no studies have extended these whole-limb observations to the level of skeletal muscle microcirculation. This is an important distinction, as it is perfusion (ie, the movement of blood through muscle tissue), not bulk blood flow through large conduit vessels, which is the most relevant measure of O2 delivery to the muscle tissue itself.
Using nuclear magnetic resonance (NMR) spectroscopy, previous work has found evidence both for (8–10) and against (11–13) a dysfunction in exercising skeletal muscle energetics with age. However, none of these prior studies evaluated skeletal muscle perfusion, leaving uncertainty as to whether altered metabolism, if present, could be related to vascular dysfunction in the elderly cohorts studied. The dynamic relationship between microcirculatory blood flow and oxidative metabolism during exercise thus remains virtually unknown.
The recent approach of utilizing multiparametric NMR techniques may address this issue, as it offers the unique capability for near-simultaneous measurements of both muscle perfusion and metabolism in vivo (14–18). The interleaving of these imaging and spectroscopic modules provides the opportunity to determine skeletal muscle perfusion and metabolism kinetics during and following the stress of physical exercise. This NMR-based paradigm thus offers the promise of improving our understanding of the causal relationship between skeletal muscle hemodynamics and energetics in the elderly.
Accordingly, we evaluated muscle perfusion (arterial spin labeling [ASL]) and metabolism (phosphocreatine [PCr] and inorganic phosphate [Pi]) during and following moderate-intensity (5W) dynamic plantar flexion exercise in younger and older adults. We hypothesized (1) that muscle perfusion would be attenuated during exercise in older participants compared with their younger counterparts (2), that metabolic demand would be similar between groups during exercise, and (3) that following the cessation of exercise, hyperemic decay would be accelerated and PCr recovery extended in older participants compared with young.
METHODS
Participants
Six young (26 ± 2 years) and six older (70 ± 2 years) volunteers were recruited for the present study. Health history and physical activity were assessed using a modified International Physical Activity Questionnaire (IPAQ). All participants were normotensive (<140/90 mmHg), normally active, and free of overt cardiovascular disease. No participants were taking prescription medication. Protocol approval and written informed consent were obtained according to Pitié Salpêtrière University Hospital guidelines, in accordance with the Declaration of Helsinki.
Experimental Protocol
Detailed accounts of the NMR setup and acquisition have been published previously (14–18) and may be found in Appendix 1. Briefly, studies were performed in a 4-T superconducting magnet (Magnex 4/60), with participants laying supine and the calf of the participant's dominant leg placed inside a volume coil for imaging and on a surface coil tuned for 31 Phosphorous (31P) spectroscopy. After a 5-minute period of baseline measurements, participants performed plantar flexion exercise (0.33 Hz, ≈5W) for 5 minutes on a custom-built ergometer (17), followed by 10 minutes of postexercise measurements.
Data Analysis
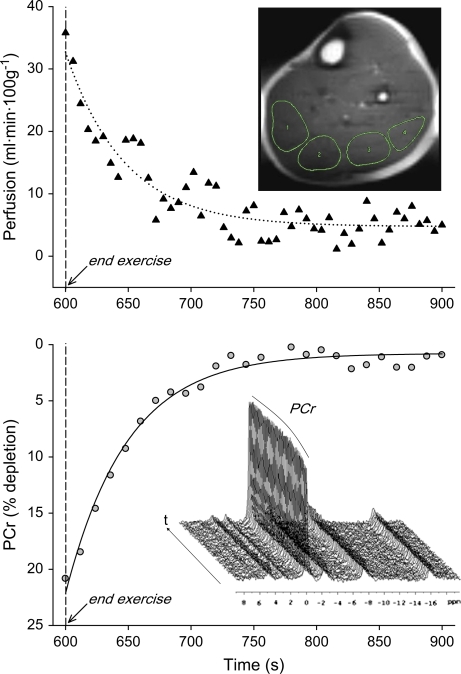
A perfusion map was generated, and four regions of interest (ROIs) of 2–3 cm2 were traced within the gastrocnemius and soleus muscles, carefully excluding voxels containing lipids or large blood vessels (Figure 1). Perfusion data are reported as the average from all ROIs (9–10 cm2). End-exercise perfusion was determined using averaged values from 10 seconds immediately preceding the cessation of exercise. Postexercise hyperemic decay was determined using cumulative perfusion area under the curve (AUC, trapezoidal rule) for 5 minutes following cessation of exercise. Relative PCr depletion/repletion and the ratio of Pi to PCr were directly measured to provide indices of muscle metabolic demand and efficiency during and immediately following exercise (11,19–21). Muscle intracellular pH was calculated from the chemical shift (δ) between the Pi and PCr peaks (22). PCr recovery was fitted monoexponentially (Systat, San Jose, CA) to determine the PCr time constant (τ).
Figure 1.
Example of arterial spin–labeled perfusion (top panel) and 31 Phosphorous (31P) phosphocreatine (PCr) recovery (bottom panel) in the lower leg upon cessation of plantar flexion exercise in an elderly volunteer. Top panel inlay is a 1H image of the lower leg (ultrafast spin echo imaging, 6 × 6 mm, field of view 22 × 11 cm, acquisition matrix 128 × 36). Four regions of interest have been highlighted from medial (left side of image) to lateral (right side of image). Bottom panel inlay illustrates a stack plot of 31P spectra (–7 to 20 ppm) with visible changes in the PCr peak during recovery.
Statistical Analyses
Statistics were performed with the use of commercially available software (SigmaStat 3.10, Systat Software Inc., Point Richmond, CA). Student t tests were used to identify between-group differences in end-exercise perfusion, PCr depletion, perfusion AUC, and PCr τ. Repeated measure analysis of variance was used to identify between-group differences in Pi/PCr, with the Bonferroni test used for post hoc analysis when a significant main effect was found. All group data are expressed as mean ± standard deviation. Significance was established at p < .05.
RESULTS
Participant characteristics are presented in Table 1.
Table 1.
Participant Characteristics
| Young | Old | |
| Age (y) | 26 ± 5 | 70 ± 5* |
| Height (cm) | 170 ± 5 | 166 ± 5 |
| Weight (kg) | 69 ± 7 | 66 ± 7 |
| Gastrocnemius/soleus muscle cross-sectional area (cm2) | 42 ± 5 | 43 ± 7 |
| Plantar flexion work rate (W) | 4.7 ± 0.1 | 4.8 ± 0.1 |
Note: *Significant difference between young and old, p < .05.
ASL Perfusion
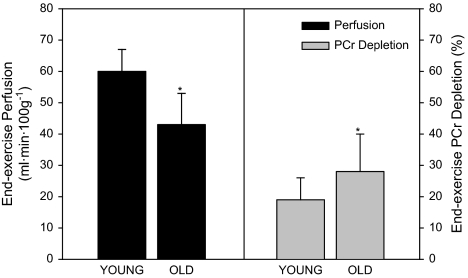
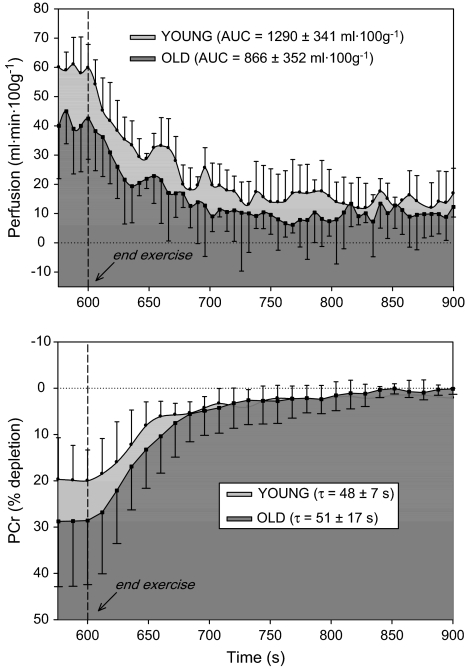
Using ASL perfusion images of the lower leg taken such as that shown in Figure 1, we observed a decrease in gastrocnemius/soleus perfusion in older participants (43 ± 10 mL/100 g·minute) compared with young (60 ± 7 mL/100 g·minute) at the end of plantar flexion exercise (Figure 2). Postexercise hyperemia (AUC) was lower in the elderly (866 ± 352 mL/100 g, old; 1290 ± 341 mL/100 g, young; Figure 3). One young participant was excluded from postexercise perfusion analysis due to poor signal quality caused by excessive movement within the volume coil.
Figure 2.
Lower leg perfusion (black bars) and phosphocreatine (PCr) depletion (gray bars) at the end of 5-minute plantar flexion exercise in young and older participants. Values are mean ± standard deviation. Asterisk indicates a significant difference between young and old, p < .05.
Figure 3.
Lower leg perfusion kinetics (top panel) and phosphocreatine (PCr) resynthesis (bottom panel) at the end of 5-minute plantar flexion exercise in young (light gray shade) and older (dark gray shade) participants. Dashed line indicates the cessation of exercise. Values are mean ± standard deviation. AUC, area under the curve; τ, time constant.
Phosphorous Depletion and Recovery
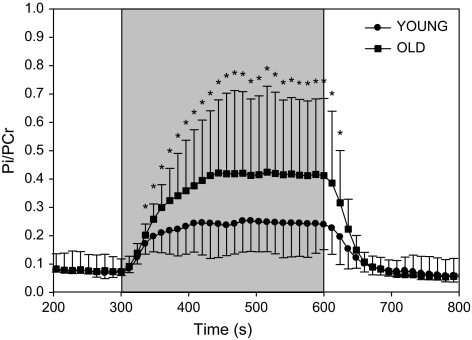
PCr depletion at the end of exercise was significantly greater in older participants (–28% ± 12%) compared with young (–19% ± 7%, young), Figure 2. Likewise, during plantar flexion exercise, Pi/PCr was significantly greater (≈40%) in older participants compared with young (Figure 4). Following cessation of exercise, the time constant (τ) of PCr recovery was not different between groups (51 ± 17 seconds, old; 48 ± 7 seconds, young; p = 0.7). In both groups, end-exercise pH (7.04 ± 0.02, old; 7.03 ± 0.07, young) did not differ from resting values (7.00 ± 0.02, old; 7.04 ± 0.02, young), and thus no correction for pH was applied to correct for the effect of acidosis on the τ of PCr recovery (23).
Figure 4.
Changes in muscle metabolism (Pi/PCr ratio) as a consequence of plantar flexion exercise in young (circles) and older (squares) participants. Shaded box indicates plantar flexion exercise. Values are mean ± standard deviation. Pi, inorganic phosphate, PCr, phosphocreatine. Asterisk indicates a significant difference between young and old, p<.05.
DISCUSSION
The present study sought to examine age-related changes in muscle hemodynamics and metabolism during and following exercise utilizing a noninvasive, clinically relevant methodology. To our knowledge, this is the first study of its kind simultaneously examining perfusion and metabolism in a healthy but aging cohort using NMR measurements of microcirculatory blood flow (ASL) and energetics (31P spectroscopy). The primary finding was that despite a ≈30% reduction in both skeletal muscle perfusion and metabolic stress (PCr depletion) during exercise in the elderly, overall muscle oxidative capacity (PCr recovery time constant, τ) was not negatively affected by this hypoperfused condition. These preliminary data provide new evidence that the documented age-related decline in skeletal muscle perfusion during physical activity minimally affect muscle metabolism, providing an indication that skeletal muscle function is preserved with healthy aging.
NMR Approach for Simultaneous Perfusion and Metabolism Measurements
In the present study, an unique NMR pulse sequence recently developed by members of our group (15) was employed to acquire interleaved perfusion and metabolic data every 3 seconds, resulting in a set of measurements with high temporal resolution. For perfusion, the arrival of the tagged arterial blood induces a modulation in tissue magnetization proportional to perfusion, and this change is recorded by NMR imaging, creating a perfusion map (Figure 1). Within the same NMR sequence, skeletal muscle energetics were simultaneously assessed through 31P NMR spectroscopy. As PCr depletion is directly proportional to adenosine triphosphate (ATP) hydrolysis (24), relative depletion of PCr and recovery can be used as an index of muscle oxidative capacity (11,19,20). When viewed together, these ASL and PCr measurements provide a unique opportunity to probe the dynamic relationship between perfusion and metabolism in skeletal muscle as a result of exercise (Figure 3) with advancing age.
Consequences of Age on Skeletal Muscle Perfusion and Metabolism
Until now, the well-documented decline in limb blood flow with age (1–7) has not been evaluated within the skeletal muscle microcirculation, an approach that offers direct examination of hemodynamics within the metabolically active region of the exercising muscle. With this new approach, we have identified a ≈30% decline in lower leg perfusion of older participants during plantar flexion exercise (Figures 2 and 3). This blunted vasodilation may result from several age-related adaptations, including changes in microvascular structure, local vascular control mechanisms, peripheral muscle sympathetic activation, and circulating vasoactive substances. Although involvement of each of these factors to the observed responses is beyond the scope of the present study, this NMR-based approach offers the promise of a spatially and temporally resolved technique to further define how each of these adaptations may influence skeletal muscle perfusion and metabolism with advancing age.
Several previous studies have applied NMR spectroscopy to examine the effect of age on skeletal muscle energetics following exercise, with evidence for (2,10,25) and against (11–13,23,26) an age-related decrement in muscle metabolism. The discrepancy between these studies may be attributed to differences in exercise modality and work rate, volunteer fitness and age, and technical considerations such as field strength and data acquisition time. In the present study, we sought to limit these confounding variables by normalizing for work rate, the recruitment of volunteers who did not participate in regular exercise, and use of a high field strength (4-T) magnet with a rapid sampling rate. Consideration of these factors thus adds credence to the current finding that PCr repletion following moderate-intensity exercise is not compromised in the elderly compared with their younger counterparts (Figures 2 and 3), supporting the existing evidence against a decline in metabolic capacity in skeletal muscle with healthy aging (11–13,23,26).
Relationship Between Perfusion and Metabolism
The localized, near-simultaneous assessment of perfusion and metabolism in the active skeletal muscle offers the unique opportunity to explore the concept of “matching” between these variables in the elderly, where the perfusion–metabolism relationship is known to be altered as a consequence of age. Although it is difficult to determine cause and effect with these measurements, it is tempting to speculate that the greater PCr depletion is a functional consequence of the relative hypoperfusion in the elderly group (Figure 3), such that older participants experience some degree of “mismatch” between perfusion and metabolism that may ultimately lead to skeletal muscle dysfunction during exercise. This concept is supported by clinical investigations in patients with peripheral artery disease, where reduced perfusion has an unfavorable effect on PCr kinetics (26–28). However, further studies with multiple work rates and refined spatial mapping of 31P are needed to fully examine the matching of perfusion and metabolism in this cohort.
Perspectives
Together, the metabolic and hemodynamic data presented herein suggest that when older individuals perform a given level of exercise, it imposes a greater metabolic challenge than in their younger counterparts, which may be attributed to the proposed decline in skeletal muscle mitochondrial function with advancing age (29). Indeed, increased Pi/PCr during exercise reflects an accumulation of adenosine diphosphate (ADP), which is thought to indicate an “uncoupling” of mitochondrial ATP production and the stimulus (ADP) for metabolism (21). Thus, in the present study it appears that the elderly were operating under a greater “metabolic stress” and a subsequent need for greater ADP levels to drive muscle metabolism during exercise (ie, elevated Pi/PCr, Figure 4); yet they successfully completed a similar amount of muscular work. These findings extend previous work from our group (2) identifying a reduced skeletal muscle perfusion but similar O2 consumption in the exercising leg of the elderly, suggesting that the elderly are capable of adapting to the condition of reduced perfusion in a way that is of minimal consequence to overall muscle function. In agreement with this concept, PCr recovery did not differ between young and old despite a decrement in perfusion in the elderly group during recovery (Figure 3). Together, these previous and present findings during exercise in older participants suggest some degree of dysregulation in both perfusion and metabolism during acute exercise but without a clear decrement in functionality. We propose that these data may be interpreted as evidence that some age-related changes, which initially appear detrimental, may in fact represent an adaptive process that ultimately preserves “normal” muscle function with advancing age.
CONCLUSION
We have identified age-specific adaptations in skeletal muscle perfusion (ASL) and metabolism (31P kinetics) during and following plantar flexion exercise using a novel, interleaved NMR sequence. End-exercise perfusion was reduced and metabolic stress (PCr depletion and Pi/PCr levels) was greater in the elderly; yet the time constant (τ) of PCr recovery did not differ between young and old. These data extend previous findings of reduced skeletal muscle blood flow in the leg with age but suggest that this hemodynamic difference does not adversely affect overall skeletal muscle function.
FUNDING
Tobacco-Related Disease Research Program (15RT-0100, R.S.R); National Institute of Health (P01 HL091830, R.S.R.); Association Française contre les Myopathies (R.S.R.); Francis Family Foundation (D.W.W.); and American Heart Association (0835209N, D.W.W.).
APPENDIX 1: METHODS
Participants
Six young (26 ± 2 years) and six older (70 ± 2 years) volunteers were recruited for the present study. Health history and physical activity were assessed using a modified IPAQ questionnaire. All participants were normotensive (<140/90 mmHg), normally active (ie, no regular exercise routine), and free of overt cardiovascular disease. No participants were taking prescription medication. Protocol approval and written informed consent were obtained according to Pitié Salpêtrière University Hospital guidelines, in accordance with the Declaration of Helsinki.
Experimental Protocol and Setup
Studies were carried out in a 4-T, 46-cm internal bore, superconducting magnet (Magnex 4/60) interfaced to a Bruker Biospec NMR spectrometer. Before the experiments, all participants were familiarized with the experimental setup and were accustomed to lying supine in the magnet. The calf of the participant's dominant leg was placed inside a 17-cm inner diameter transversal electromagnetic 1H transmit-and-receive volume coil. Affixed to the volume coil was a circular, 8-cm-diameter custom-built 31P surface coil. This volume/surface coil unit was positioned underneath the gastocnemius muscle, centered at the widest portion of the muscle as verified by sequential reference images. A reference image was also utilized for determination of cross-sectional area of the gastrocnemius/soleus muscle group, which was subsequently used for normalization of work rate.
After a 5-minute period of baseline measurements, participants performed plantar flexion exercise (0.33 Hz, ≈5W) for 5 minutes on a custom-built, nonferrous hydraulic ergometer (22), followed by 10 minutes of postexercise measurements. Participants were encouraged to perform muscle contraction as quickly as possible (0.5–1 second) in order to maximize relaxation time between contractions. To maintain optimal signal-to-noise ratio and minimize motion artifact, all images were collected during this 2-second relaxation phase.
Calf muscle perfusion and energy metabolism were studied by rapidly (3 seconds) interleaved acquisitions of saturation inversion recovery ASL perfusion imaging and 31P spectroscopy of the high-energy phosphate metabolites, as described previously (22). This interleaved acquisition scheme was initially proposed and implemented by our group (20) and was here driven by the multiscan control (MSC) tool developed and made commercially available by Bruker. A complete dataset was generated every 3 seconds. The MSC tool automatically distributed the raw interleaved data in distinct imaging, 1H, and 31P spectroscopy files, which were immediately ready for processing with standard ParaVision and XWIN NMR Bruker software.
Satir Perfusion Images
A temporary perfusion map was extracted by summing the differences between pairs of images (tagged and untagged) acquired during and after plantar flexion exercise. Four ROIs of 2–3 cm2 were traced inside sections of the gastrocnemius and soleus muscles, carefully excluding voxels containing lipids or vessels. Similar ROIs were selected in all the images of the series, and perfusion (f) was calculated according to
where M stands for the image intensity in muscle ROI after slice-selective (SS) and nonselective (NS) inversion, T is the ASL time (0.82 seconds), λ is the tissue/blood partition coefficient, and r1 is the tissue spin-lattice relaxation rate. In muscle, we assume r1 = 0.66/second and λ = 0.9.
Perfusion data are presented as the average from all ROIs (9–10 cm2). End-exercise perfusion was determined using averaged values from 10 seconds immediately preceding the cessation of exercise. Postexercise hyperemic decay was determined using cumulative perfusion AUC (trapezoidal rule) for 5 minutes following cessation of exercise, according to the equation
.
31P Spectra of High-Energy Phosphates
The 31P free induction decays were summed four by four and were processed with 8-Hz line-broadening exponential multiplication. Pi and PCr integrals were calculated with integration limits set to 5.6/3.5 ppm and 1.5/–1.5 ppm, respectively. No zero filling was utilized. Muscle intracellular pH was calculated from the chemical shift (δ) between the Pi and PCr peaks (24).
.PCr recovery was fitted monoexponentially (Systat) to determine the PCr time constant (τ), according to the equation: y = y0 + a(1 – e–bx), where y0 = the PCr baseline value and a = the difference between the baseline and recovery value.
Statistical Analyses
Statistics were performed with the use of commercially available software (SigmaStat 3.10, Systat Software Inc.). Student t tests were used to identify between-group differences in end-exercise perfusion, PCr depletion, perfusion AUC, and PCr τ. Repeated measure analysis of variance was used to identify between-group differences in Pi/PCr, with the Bonferroni test used for post hoc analysis when a significant main effect was found. All group data are expressed as mean ± standard deviation. Significance was established at p <.05.
References
- 1.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95(5):1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 3.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582(Pt 1):63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33(1):79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- 5.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100(10):1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 6.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284(4):H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 7.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290(1):H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 8.McCully KK, Forciea MA, Hack LM, et al. Muscle metabolism in older subjects using 31P magnetic resonance spectroscopy. Can J Physiol Pharmacol. 1991;69(5):576–580. doi: 10.1139/y91-084. [DOI] [PubMed] [Google Scholar]
- 9.McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75(2):813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- 10.Coggan AR, Abduljalil AM, Swanson SC, et al. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol. 1993;75(5):2125–2133. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- 11.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Tohmpson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol. 1998;83:107–117. doi: 10.1113/expphysiol.1998.sp004087. [DOI] [PubMed] [Google Scholar]
- 12.Kutsuzawa T, Shioya S, Kurita D, Haida M, Yamabayashi H. Effects of age on muscle energy metabolism and oxygenation in the forearm muscles. Med Sci Sports Exerc. 2001;33(6):901–906. doi: 10.1097/00005768-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DJ, Crowe M, Bore PJ, Styles P, Arnold DL, Radda GK. Examination of the energetics of aging skeletal muscle using nuclear magnetic resonance. Gerontology. 1984;30(1):2–7. doi: 10.1159/000212599. [DOI] [PubMed] [Google Scholar]
- 14.Brillault-Salvat C, Giacomini E, Jouvensal L, Wary C, Bloch G, Carlier PG. Simultaneous determination of muscle perfusion and oxygenation by interleaved NMR plethysmography and deoxymyoglobin spectroscopy. NMR Biomed. 1997;10(7):315–323. doi: 10.1002/(sici)1099-1492(199710)10:7<315::aid-nbm489>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Carlier PG, Brillault-Salvat C, Giacomini E, Wary C, Bloch G. How to investigate oxygen supply, uptake, and utilization simultaneously by interleaved NMR imaging and spectroscopy of the skeletal muscle. Magn Reson Med. 2005;54(4):1010–1013. doi: 10.1002/mrm.20649. [DOI] [PubMed] [Google Scholar]
- 16.Richardson RS, Noyszewski EA, Haseler LJ, Bluml S, Frank LR. Evolving techniques for the investigation of muscle bioenergetics and oxygenation. Biochem Soc Trans. 2002;30(2):232–237. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 17.Duteil S, Bourrilhon C, Raynaud JS, et al. Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1441–R1449. doi: 10.1152/ajpregu.00242.2004. [DOI] [PubMed] [Google Scholar]
- 18.Brillault-Salvat C, Giacomini E, Wary C, et al. An interleaved heteronuclear NMRI-NMRS approach to non-invasive investigation of exercising human skeletal muscle. Cell Mol Biol (Noisy-le-grand) 1997;43(5):751–762. [PubMed] [Google Scholar]
- 19.Haseler LJ, Hogan MC, Richardson RS. Oxygen uptake and 31P response to square wave and ramp exercise: implications for oxygen defecit, debt, and metabolic control. Soc Magn Reson Med Proc. 1998;6:1790. [Google Scholar]
- 20.McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunnigham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- 21.Chance B, Leigh JS, Jr, Clark BJ, et al. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda G. Bioenergetics of intact human muscle: a 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- 23.Iotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B. In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed. 1993;6(4):248–253. doi: 10.1002/nbm.1940060404. [DOI] [PubMed] [Google Scholar]
- 24.Meyer RA. A linear model of muscle respiration explains monoexponetial phosphocreatine changes. Am J Physiol. 1988:254, C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- 25.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. (Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schunk K, Romaneehsen B, Rieker O, et al. Dynamic phosphorus-31 magnetic resonance spectroscopy in arterial occlusive disease: effects of vascular therapy on spectroscopic results. Invest Radiol. 1998;33(6):329–335. doi: 10.1097/00004424-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Carlier PG, Bertoldi D. In vivo functional NMR imaging of resistance artery control. Am J Physiol Heart Circ Physiol. 2005;288(3):H1028–H1036. doi: 10.1152/ajpheart.00780.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kramer CM. Peripheral arterial disease assessment: wall, perfusion, and spectroscopy. Top Magn Reson Imaging. 2007;18(5):357–369. doi: 10.1097/rmr.0b013e31815d064c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care. 2007;10(6):688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]