Abstract
Islet transplantation is a method of restoring endogenous insulin secretion in individuals with type 1 diabetes by transplanting insulin-secreting islet cells from cadaveric donor pancreases into eligible recipients. Since 2000, the one-year insulin independence rate observed in islet transplant recipients has risen from less than 10% to approximately 80%. However, the continued requirement for at least two donor pancreases for each islet transplant recipient, the occurrence of suboptimal islet engraftment, the need for chronic immunosuppressive therapy and a decline in islet function over time continue to make this procedure unsuitable for the majority of patients with type 1 diabetes. Despite these challenges, the recent progress in islet transplantation has reinforced the potential of beta cell replacement for the treatment of type 1 diabetes.
Keywords: Hypoglycemia, Islet transplantation, Type 1 diabetes
Abstract
La transplantation d’îlots de Langerhans est une méthode pour restaurer la sécrétion d’insuline endogène chez les personnes atteintes de diabète insulinodépendant, grâce à la transplantation d’îlots de Langerhans sécrétant de l’insuline prélevés sur le pancréas de donneurs cadavériques à des receveurs admissibles. Depuis 2000, le taux d’indépendance à l’insuline observé après un an chez les receveurs de transplantation est passé de moins de 10 % à environ 80 %. Cependant, le besoin continu d’au moins deux pancréas de donneurs par receveur d’îlots de Langerhans, l’occurrence de greffes sous-optimales d’îlots, le besoin chronique d’immunosuppresseurs et une diminution de la fonction des îlots au fil du temps rendent cette intervention inapplicable pour la plupart des patients atteints de diabète insulinodépendant. Malgré ces défis, les progrès récents dans la transplantation d’îlots de Langerhans ont renforcé le potentiel de remplacement de cellules bêta pour le traitement du diabète insulinodépendant.
Islet transplantation is a method of restoring endogenous insulin secretion in individuals with type 1 diabetes by transplanting insulin-secreting islet cells from cadaveric donor pancreases into eligible recipients. Increased interest in this procedure was generated in 2000 after the publication of a series of seven type 1 diabetes patients who became insulin-independent after receiving islet transplants (1). Since the introduction of steroid-free immunosuppressive regimens in the late 1990s, the one-year insulin independence rate observed in islet transplant recipients has risen from less than 10% to approximately 80% (2). These results represent a marked improvement in the clinical outcome of islet transplantation and have encouraged further study of beta cell replacement as a viable therapy for selected individuals with type 1 diabetes.
The Edmonton Protocol
Introduced in 1999, the Edmonton Protocol (1) combined steroid-free immunosuppression with at least two separate islet infusions to deliver a sufficient total islet mass to the liver to achieve insulin independence. Islets are isolated by enzymatic digestion from donor pancreases and infused through a percutaneous transhepatic catheter under fluoroscopic guidance into the portal circulation to lodge in hepatic sinusoids. Immunosuppression usually consists of daclizumab induction with sirolimus and tacrolimus maintenance therapy.
Clinical and metabolic outcomes
At the University of Alberta (Edmonton, Alberta), the median age of islet recipients is 42 years (range 29 to 59 years) and the median duration of diabetes is 26 years (range 5 to 50 years). The mean cumulative number of transplanted islets in individuals receiving at least two islet infusions is 874,742±28,053. In most cases, islets are obtained from two separate donors. After the first islet infusion, daily insulin requirements are reduced by a mean of 52% (range 12% to 100%) and glycemic stability is usually greatly improved. However, recipients do not generally achieve insulin independence before receiving a second islet infusion, for a total transplanted islet mass of at least 9000 islets/kg to 12,000 islets/kg (3).
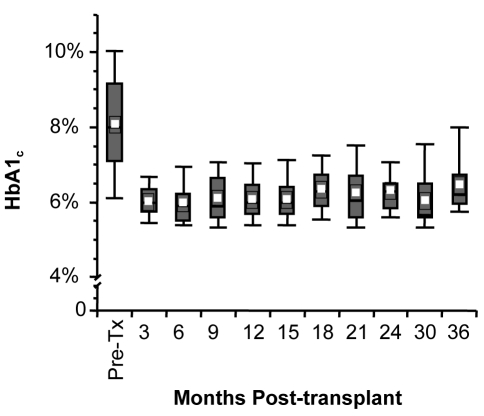
The most immediate benefit of islet transplantation is the achievement of ‘physiological’ insulin secretion and stabilization of blood glucose. The one-year insulin independence rate for all recipients who have received at least two islet infusions is 80% using Kaplan-Meier survival analysis. Mean glycated hemoglobin is normalized in insulin-independent recipients (Figure 1) and near-normal in noninsulin-independent islet transplant recipients. However, there appears to be a gradual loss of islet function over time, with a two-year insulin independence rate of less than 60%. A complete loss of all C-peptide secretion after transplantation has occurred in 11% of recipients. The reason for the decline or complete loss of islet function is not clear, but may involve direct immunosuppressive toxicity, allo- or autoimmune rejection, or islet cell apoptosis (potentially reflecting the natural life cycle of islets) (4).
Figure 1.
Hemoglobin A1c (HbA1c) at three-month intervals after islet transplantation in insulin-independent subjects. Values are means ± SEM. Pre-Tx Pretransplant
Complications of islet transplantation
The most common procedure-related adverse events are abdominal pain and nausea (Table 1). Intraperitoneal hemorrhage has occurred in approximately 10% to 15% of recipients (2). Portal hypertension can occur acutely during islet infusion; however, portal pressures tend to normalize after the acute phase of transplantation (5). Portal vein thromboses have occurred in 4% of recipients but have generally been limited to branch veins and have resolved with appropriate anticoagulation. Post-transplant elevation of liver enzymes (54%) and catheter-related puncture of the gallbladder (3%) have also occurred, although these tend to be self-limited (3).
TABLE 1.
Adverse events associated with clinical islet transplantation*
| Immunosuppressive-related (%) | Procedure-related (%) |
|---|---|
| Oral ulcers (96) | Elevated liver enzymes (54) |
| Anemia (60) | Abdominal pain (50) |
| Diarrhea (50) | Nausea/vomiting (50) |
| Weight loss (50) | Fatty liver (long term) (20) |
| Fatigue (50) | Peritoneal hemorrhage (15) |
| LDL elevation (50) | Portal vein thrombosis (4) |
| Hypertension (50) | Gall bladder puncture (3) |
| Decline in GFR (50) | |
| Proteinuria (50) | |
| Peripheral edema (30) | |
| Acne (10) | |
| Neutropenia (<10) | |
| Tremor (<10) | |
| Miscellaneous – arthralgia, pneumonitis, hematuria, infection, PTLD (rare) |
Approximate per cent incidence. GFR Glomerular filtration rate; LDL Low density lipoprotein cholesterol; PTLD Post-transplant lymphoproliferative disorder
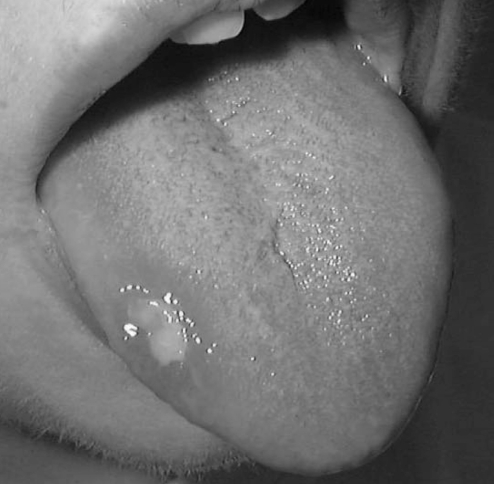
The most common adverse effect of immunosuppressive therapy is mucosal ulceration involving the tongue or buccal mucosa (Figure 2), which occurs in the vast majority (96%) of recipients. This is presumed to be a consequence of sirolimus therapy and tends to be dose-dependent. The ulcers are generally superficial, but more severe ulceration has occurred (rarely) which can compromise oral intake. Increased use of lipid-lowering or antihypertensive agents has occurred in approximately one-half of all patients. Post-transplant anemia is common (greater than 50%), and a reduction in total white blood cell count is also frequently seen, but severe neutropenia is uncommon. Post-transplant weight loss is also frequently seen. The appearance of intra-hepatic periportal steatosis in 20% of recipients on magnetic resonance imaging has raised cautious concern (6,7). This finding is thought to be benign due to the local effects of insulin on surrounding liver parenchyma. These changes also appear to be reversible because complete resolution has been seen in one patient whose graft failed completely.
Figure 2.
Oral ulcers are a common complication of immunosuppressive therapy in islet transplant patients. Most ulcers are small and self-limited
The impact of islet transplantation on diabetic microvascular complications remains uncertain. Immunosuppressive agents can be nephrotoxic and may be partially responsible for an elevation of serum creatinine and a reduction in the glomerular filtration rate observed after transplantation (8). Retinal bleeds have also occurred, but because of the limited duration of follow-up, no firm conclusions can be drawn regarding the long-term effects of islet transplantation on microvascular complications. The risk of post-transplant infection and malignancy are also of concern, although thus far, no malignancy has been observed in islet transplant recipients at the University of Alberta.
Who should get an islet transplant?
In many respects, the current selection criteria are similar to those for solitary pancreas transplantation (9,10). Currently, the basic criteria used for the selection of islet transplant candidates are:
frequent episodes of severe, undetected hypoglycemia;
severe glycemic lability; or
progressive diabetic complications despite optimization of insulin injection therapy.
The percentage of recipients meeting at least one of these criteria is listed in Table 2. Because the greatest impact of islet transplantation is the stabilization of blood glucose and no long-term data are yet available regarding the impact of islet transplantation on diabetic complications, the ‘progressive complications’ indication rarely justifies transplantation without another indication also being present.
TABLE 2.
Selection criteria for islet transplant candidates at the University of Alberta
| Selection criteria | % transplanted |
|---|---|
| Frequent undetected hypoglycemia | 94 |
| Glycemic lability | 57 |
| Progressive diabetic complications | 4 |
CONCLUSIONS
Islet transplantation can provide insulin independence and marked stabilization of blood glucose in selected patients with type 1 diabetes if an adequate transplant islet mass is infused, toxic immunosuppressive drugs such as glucocorticoids are avoided, and allo- and autoimmune destruction of the transplanted islets are minimized. However, suboptimal islet engraftment, a decline in islet function over time, the continued requirement of at least two donor pancreases for each islet transplant recipient and the need for chronic immunosuppressive therapy continue to make this procedure unsuitable for the majority of patients with type 1 diabetes.
For islet transplantation to become more widely used in the treatment of diabetes, the current severe lack of transplantable insulin-secreting tissue must be overcome. Furthermore, the efficacy and side effect profiles of immunosuppressive agents must be further improved to enable consistent islet engraftment with fewer adverse effects. Despite these challenges, the recent progress in islet transplantation has reinforced the potential of beta cell replacement for the treatment of diabetes and given hope to many diabetic patients that they may one day be free from the risk of secondary complications and the daily burden of insulin injections.
ACKNOWLEDGEMENTS
The author gratefully acknowledges the valuable contributions of Drs Edmond Ryan, Peter Senior, Jose Oberholzer, Jonathan Lakey and James Shapiro.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro A, Ryan EA, Paty B, et al. Three-year follow-up in clinical islet alone transplant with the Edmonton Protocol, and preliminary impact of infliximab and campath-1H. Am J Transplantation. 2003;3 [Google Scholar]
- 3.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: Continued insulin reserve provides long-term control. Diabetes. 2002;51:2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S. Beta-cell turnover: Its assessment and implications. Diabetes. 2001;50(Suppl 1):S20–4. doi: 10.2337/diabetes.50.2007.s20. [DOI] [PubMed] [Google Scholar]
- 5.Casey JJ, Lakey JR, Ryan EA, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74:913–5. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Markmann JF, Rosen M, Siegelman ES, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: A functional footprint of islet graft survival? Diabetes. 2003;52:1591–4. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311–7. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 8.Senior P, Zeman M, Paty B, Shapiro A, Ryan E. Renal outcomes after clinical islet allotransplantation at the University of Alberta 4-year follow-up. Diabetes. 2004;53 [Google Scholar]
- 9.Clinical practice recommendations. Pancreas transplantation for patients with diabetes. Diabetes Care. 2002;25(Suppl 1):S111. [Google Scholar]
- 10.Canadian Diabetes Association, Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(Suppl 2):S55–56. [Google Scholar]