Abstract
The metabolic syndrome is a constellation of metabolic abnormalities that result in an increased risk for type 2 diabetes mellitus and cardiovascular disease in adults. It emerges when a person’s predisposition for insulin resistance is worsened by increasing central obesity and is largely confined to the overweight population. The United States National Cholesterol Education Program’s Adult Treatment Panel III report proposed a set of criteria for the clinical diagnosis of metabolic syndrome in the adult population. A uniform definition for the paediatric population is lacking. Despite this, several studies have demonstrated that features of the syndrome develop in childhood and that the syndrome is present in up to 30% of obese children (body mass index at or above the 95th percentile). Ninety per cent of obese children meet at least one of the five criteria. The degree of abnormality is related to the body mass index, waist circumference and fasting insulin levels. There appears to be a genetic predisposition to the development of the syndrome and certain ethnic groups are at increased risk. The intrauterine environment also appears to play a role. Insulin resistance should be targeted for treatment through exercise and dietary intervention. The role of pharmacotherapeutic agents remains unclear. A uniform definition of the metabolic syndrome for paediatric patients needs to be created. Early intervention should be instituted because many of the features of the syndrome track from childhood into adulthood.
Keywords: Cardiovascular disease, Insulin resistance, Metabolic syndrome, Obesity, Type 2 diabetes
Abstract
Le syndrome métabolique est une constellation d’anomalies métaboliques qui entraînent une augmentation du risque de diabète non insulinodépendant et de maladies cardiovasculaires chez les adultes. Il émerge lorsque la prédisposition d’un individu à l’insulinorésistance est aggravée par une obésité centrale croissante, et il est largement confiné à la population qui fait de l’embonpoint. Le rapport du groupe III de traitement aux adultes du National Cholesterol Education Program des États-Unis a proposé une série de critères pour parvenir au diagnostic clinique du syndrome métabolique dans la population adulte. Il n’existe pas de définition uniforme pour la population pédiatrique. Malgré tout, plusieurs études ont démontré que des caractéristiques du syndrome se déclarent pendant l’enfance et que jusqu’à 30 % des enfants obèses (dont l’indice de masse corporelle est égal ou supérieur au 95e percentile) souffrent du syndrome. Quatre-vingt-dix pour cent des enfants obèses respectent au moins l’un des cinq critères. Le degré d’anomalie est relié à l’indice de masse corporelle, à la circonférence de la taille et aux taux d’insuline à jeun. Il semble exister une prédisposition génétique à l’apparition du syndrome, et certains groupes ethniques y sont plus vulnérables. L’environnement intra-utérin semble également avoir un rôle à jouer. L’insulinorésistance devrait être ciblée pour un traitement par l’exercice et une intervention diététique. Le rôle de la pharmacothérapie demeure incertain. Une définition uniforme du syndrome métabolique s’impose pour la population pédiatrique. Une intervention précoce devrait être appliquée, car bon nombre des caractéristiques du syndrome sont maintenues de l’enfance à l’âge adulte.
The metabolic syndrome is a constellation of metabolic abnormalities that result in an increased risk for type 2 in the adult population (1–7). The syndrome emerges when a person’s predisposition for insulin resistance is worsened by increasing central adiposity, and is associated with dyslipidemia, hypertension and hyperglycemia (1–7). It is largely confined to the overweight population (1–7). Adults with the metabolic syndrome have at least a twofold higher risk for atherosclerotic CVD and a fivefold increased risk for the development of T2DM (1–7). Many studies (8–12) have shown that features of the syndrome develop in childhood and are highly prevalent among overweight children and adolescents. Evidence of CVD in childhood has been demonstrated in postmortem studies, showing the presence of fatty streaks and fibrous plaques in the aorta and coronary vessels of children and adolescents (13). Body mass index (BMI), blood pressure, total cholesterol, triglycerides, low density lipoprotein-cholesterol and high density lipoprotein-cholesterol (HDL-C) were strongly associated with the extent of the lesions seen (13). Cardiovascular risk factors at 12 to 18 years of age are predictive of adult common carotid intimal thickness, suggesting that exposure to cardiovascular risk factors early in life may induce changes in arteries that contribute to the development of atherosclerosis (11). Impaired glucose tolerance (IGT) and T2DM are being diagnosed more frequently in children and adolescents, coinciding with rising rates of obesity (14,15). No long-term outcome data on the risk for T2DM and CVD in children with features of the metabolic syndrome are available.
DEFINITION
The key elements of the metabolic syndrome are central obesity, high blood pressure, dyslipidemia and hyperglycemia (7,16–19). In 2001, The United States National Cholesterol Education Program’s Adult Treatment Panel III report (7) proposed a set of criteria for the clinical diagnosis of metabolic syndrome in adults that includes: increased waist circumference, high blood pressure, high fasting blood glucose and triglycerides, and low HDL-C. The diagnosis requires that three of the five criteria be met (7). For the paediatric population, there is a lack of an uniform definition (Table 1).
TABLE 1.
Various diagnostic criteria for the metabolic syndrome in children and adolescents
| Criterion | Adult, NCEP ATP III (7), 3 of 5 criteria | Cook et al (8), NHANES III, 3 of 5 criteria | Chen et al (10), Bogalusa Heart Study, 4 criteria, 75th percentile for age and sex | Cruz et al (12), overweight Hispanic youth, 3 of 5 criteria | Raitakari et al (11), cardiovascular risk in young Finns, 3 criteria, ≥75th percentile for age and sex | Weiss et al (9), obesity and metabolic syndrome in children and adolescents, 3 of 5 criteria |
|---|---|---|---|---|---|---|
| High triglycerides | ≥1.69 mmol/L | ≥1.24 mmol/L | Triglyceride to HDL ratio | ≥90th percentile | ≥75th percentile | ≥95th percentile |
| Low HDL cholesterol | Triglyceride to HDL ratio | ≤10th percentile | ≤25th percentile | ≤5th percentile | ||
| Male | <1.03 mmol/L | <1.03 mmol/L | ||||
| Female | <1.29 mmol/L | <1.03 mmol/L | ||||
| Abdominal obesity, waist circumference* | BMI ≥75th percentile | N/A | BMI > +2 SDs | |||
| Male | >102 cm | ≥90th percentile | ≥90th percentile | |||
| Female | >88 cm | ≥90th percentile | ≥90th percentile | |||
| High fasting glucose† | ≥6.1 mmol/L | ≥6.1 mmol/L | Insulin resistance index | Impaired glucose tolerance, 2 h BG ≥7.8 mmol/L | N/A | Impaired glucose tolerance, 2 h BG >7.8 mmol/L |
| High blood pressure | ≥130/85 mmHg | ≥90th percentile | Mean arterial pressure | ≥90th percentile | ≥75th percentile | ≥95th percentile |
Accepted adult criteria are indicated by bold font. In some studies, *body mass index (BMI) percentiles were used as a surrogate for waist circumference and †impared glucose tolerance was used interchangeably with impaired fasting glucose. BG Blood glucose; HDL High density lipoprotein; N/A Not available; NCEP ATP III National Cholesterol Education Program’s Adult Treatment Panel III; NHANES III Third National Health and Nutrition Examination Survey
There is a need to define the metabolic syndrome for children and adolescents. Many features of this syndrome track from childhood into adulthood (20); therefore, the definition should include similar components. Early identification of the clustering of risk factors should help target prevention efforts in high-risk individuals. Lack of a clear definition will continue to impede our ability to determine the prevalence of the syndrome in children and to establish the natural history of the disease, including identifying predictors and risk factors. In many paediatric studies to date looking at the metabolic syndrome, BMI percentiles have been used as a surrogate for waist circumference, and IGT has been used interchangeably with impaired fasting glucose (Table 1).
EPIDEMIOLOGY
There have been several studies looking at the prevalence of the metabolic syndrome in the paediatric population. Unfortunately, a uniform definition of the condition has not been established, making direct comparisons of these studies difficult.
The overall prevalence of the metabolic syndrome among North American children and adolescents is between 3% and 4% (8,10,11). Prevalence rates are higher in boys (6.1%) than in girls (2.1%) (8). Forty-one per cent of adolescents have one and 14.2% have two features of the metabolic syndrome (8). The most common abnormality is high triglycerides and low HDL-C and the least common abnormality is impaired fasting glucose (1.5%) (8).
The prevalence of the metabolic syndrome among obese children and adolescents (BMI at or above the 95th percentile) is dramatically higher than their non-obese counterparts: 28.7% versus 6.1% (BMI between the 85th and 95th percentiles). The metabolic syndrome is virtually absent in children with a BMI less than the 85th percentile (0.1%). Close to 90% of obese children and adolescents have at least one feature of the metabolic syndrome and over one-half (56%) meet two criteria (8). In a more recent study where BMI rather than waist circumference was used as a measure of central adiposity, the prevalence of metabolic syndrome reached 50% among children and adolescents with a BMI at or above the 97th percentile (9).
A clustering of risk factors associated with the metabolic syndrome has been demonstrated in certain populations, including Native Canadians/Americans, Mexican Americans, Japanese Americans, Micronesians, Caucasians, East Asians and Asian Indians (21,22). Sung et al (22) demonstrated that 50% of obese nine- to 12-year-old Hong Kong Chinese children had at least two of the three cardiovascular risk factors of dyslipidemia, hypertension and hyperinsulinemia. According to the third National Health and Nutrition Examination Survey (8), the prevalence of the metabolic syndrome is higher among Hispanic children (5.6%), in particular, Mexican Americans, and it is lower in African American children and adolescents (2.0%). The prevalence among Caucasian adolescents was 4.8% in the survey (8).
THE LINK BETWEEN OBESITY AND INSULIN RESISTANCE
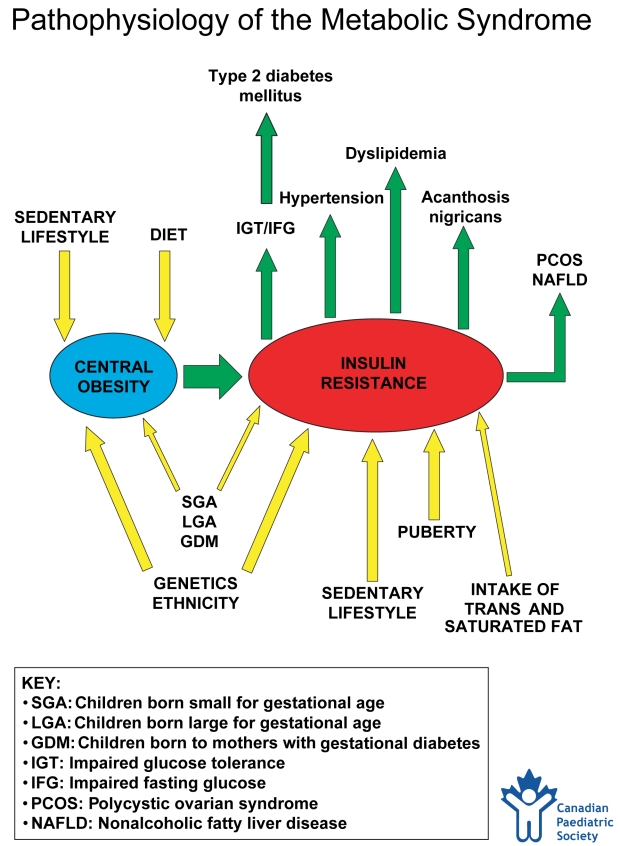
Central obesity leading to insulin resistance is the underlying abnormality of the metabolic syndrome (Figure 1) (23–25). Obesity is the most common cause of insulin resistance in children (26). Children in the top quartile for fasting insulin and for BMI are 3.6 and 11.7 times more likely, respectively, to have the features of metabolic syndrome (27). Higher baseline insulin levels are predictive of clustering of the features of high triglycerides, low HDL-C and high systolic blood pressure at six-year follow-up (11). When insulin levels are elevated in childhood, they remain so in adulthood, leading to a higher prevalence of obesity, hypertension and dyslipidemia (20).
Figure 1.
Pathophysiology of the metabolic syndrome. In the assessment of a child or adolescent with central obesity, the physician must consider the complex interplay between factors that contribute to obesity and insulin resistance (incoming arrows) in addition to the metabolic consequences of insulin resistance (outgoing arrows). This diagram can be copied and used with parents to help them understand the interrelationship of all of these factors. Created by HJ Dean, S Hadjiyannakis and EAC Sellers. This chart has been reviewed and approved by the Canadian Paediatric Society (January 2005)
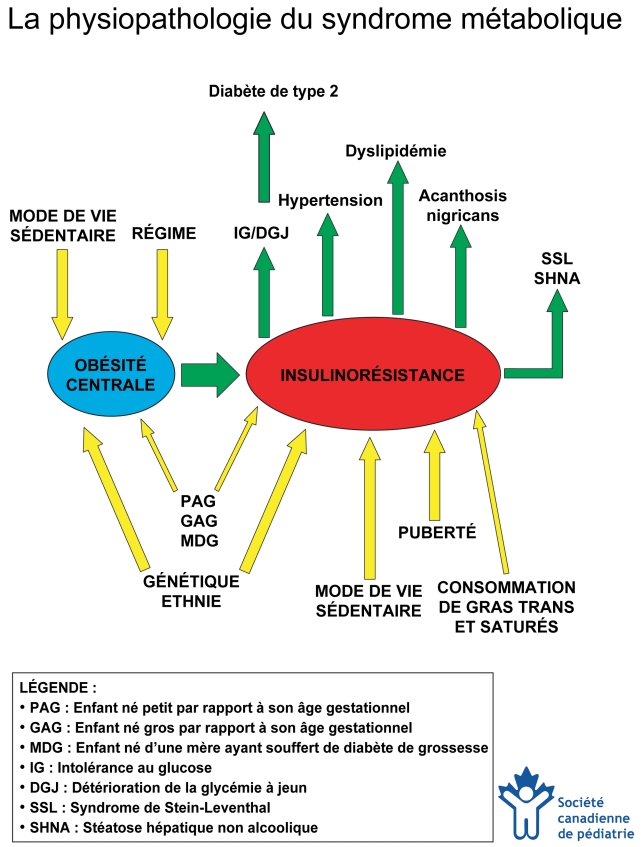
Figure 1) Physiopathologie du syndrome métabolique. Dans l’évaluation d’un enfant ou d’un adolescent atteint d’obésité centrale, le médecin doit tenir compte des interactions complexes entre des facteurs qui contribuent à l’obésité et à l’insulinorésistance (flèches entrantes) en plus des conséquences métaboliques de l’insulinorésistance (flèches sortantes). Ce diagramme peut être copié et utilisé auprès des parents afin de les aider à comprendre les interactions entre tous ces facteurs. Créé par HJ Dean, S Hadjiyannakis et EAC Sellers. Ce diagramme a été révisé et approuvé par la Société canadienne de pédiatrie (janvier 2005).
The distribution of fat plays an important role in the development of insulin resistance. A preponderance of fat in the abdomen, upper body and trunk (android obesity) is predictive of diabetes and CVD (28). Abdominal fat is located in two major components; subcutaneous and visceral, with increased visceral fat resulting in worsening of insulin resistance. Waist circumference is a surrogate measure for visceral fat. Central adiposity measured by waist circumference in children has been linked to adverse lipid profiles (high triglycerides and low HDL-C), and higher fasting insulin levels and blood pressure, independent of weight and height (29–32). In addition to an increase in visceral fat, accumulation of lipids in muscle cells also decreases insulin sensitivity because skeletal muscle is a primary site of insulin action (33).
RISK FACTORS
Factors that contribute to insulin resistance include obesity (central adiposity), a sedentary lifestyle, age, onset of puberty, genetics (family history of T2DM, high-risk ethnic background), intrauterine environment (small for gestational age [SGA], large for gestational age [LGA] and gestational diabetes) and diet.
Puberty is associated with a 30% decrease in insulin sensitivity during the progression from Tanner stage II to IV (34). Therefore, the clinical manifestations of the metabolic syndrome often present peripubertally.
Features of the metabolic syndrome aggregate in families. Children of obese parents are five times more likely to be obese (35). Family studies show that genetic factors account for about 50% of the variance in intra-abdominal fat even after adjusting for age, sex and total body fat (36). The children of individuals with T2DM have insulin resistance even before the onset of puberty (37,38). Children of hypertensive parents also have more insulin resistance and higher blood pressure, serum cholesterol and triglyceride levels than control subjects (39,40). In Mexican Americans, a parental phenotype of T2DM is the most predictive factor for the development of metabolic syndrome (12).
Recent studies suggest that the risk for metabolic syndrome may originate in utero in some individuals. A continuum of increased risk for CVD, T2DM, insulin resistance and hypertension in individuals born SGA has been established (41). This is seen more commonly in SGA babies who undergo rapid postnatal weight gain (42). LGA infants are also at risk for metabolic syndrome, obesity and T2DM (43,44). Gestational diabetes often gives rise to macrosomia in the fetus and represents an increased risk for obesity and T2DM in offspring (45,46). The metabolic syndrome in pregnancy is itself an independent predictor of macrosomia, which in turn increases the probability of metabolic syndrome in the children (47).
Insulin resistance has also been implicated in polycystic ovarian syndrome (PCOS) and nonalcoholic fatty liver disease (NAFLD). Individuals with PCOS and/or NAFLD are at increased risk for the metabolic syndrome given the shared underlying presence of insulin resistance.
PCOS is one of the most common endocrine diseases of young women, affecting up to 10% of women of reproductive age (48–50). It is defined by ovulatory dysfunction; clinical evidence of hyperandrogenism (hirsutism, acne, androgenic alopecia) and/or hyperandrogenemia; and by the exclusion of related disorders, such as hyperprolactinemia, thyroid disorders and nonclassic adrenal hyperplasia (51). Women and adolescents with PCOS have a greater frequency and degree of insulin resistance than weight-matched control subjects (52,53). The insulin resistance itself is linked to the ovarian and adrenal hyperandrogenemia seen in this syndrome (54). PCOS has been associated with IGT, T2DM, hypertension and dyslipidemia. Insulin resistance is the major risk factor for all of these conditions (55–57).
NAFLD is an increasingly recognized condition that may progress to end-stage liver disease. The pathological picture resembles that of alcohol-induced liver injury (58,59). Central obesity, T2DM and hyperlipidemia frequently coexist with NAFLD (58–62). NAFLD affects 2.6% of children and 22.5% to 52.8% of obese children (60–63). NAFLD is often the cause of asymptomatic elevation in aminotranferases (alanine aminotransferase level greater than aspartate aminotransferase level) (62). Insulin resistance is the most reproducible factor implicated in the development of NAFLD (62,63).
WHO SHOULD BE SCREENED?
Screening for features of the metabolic syndrome should be done in obese children and youth (BMI at or above the 95th percentile) in the presence of the risk factors mentioned above. Screening should include an assessment of BMI (64), waist circumference (18), blood pressure and a fasting lipid profile. A fasting blood glucose and/or a 2 h oral glucose tolerance test should be performed in obese children 10 years of age or older with two or more risk factors, in accordance with the Canadian Diabetes Association’s 2003 clinical practice guidelines (65).
TREATMENT
Insulin resistance should be targeted for treatment through exercise and dietary interventions. The role of pharmacotherapeutic agents remains unclear. All five components of the metabolic syndrome improve with even modest amounts of weight loss achieved through diet and exercise.
It is well known that regular physical activity can reduce insulin resistance, improve glucose intolerance, reduce the risk of T2DM and reduce CVD risk. Ku et al (66) demonstrated in prepubertal five- to 11-year-old children that increased physical activity was related to greater insulin sensitivity, independent of body composition and race. Kang et al (67) showed that eight months of high-intensity physical training in obese adolescents improved fasting plasma triglycerides, low density lipoprotein particle size and diastolic blood pressure.
Very few studies have been conducted in children relating diet to insulin resistance. Dietary modifications, such as decreasing consumption of fat and simple sugars and increasing consumption of omega-3 fatty acids (eg, fish oil, flaxseed oil), help to decrease triglyceride levels (68). A 12-week study (69) in overweight adolescents showed that a low-carbohydrate diet resulted in greater weight loss than a low-fat diet (9.9±9.3 kg versus 4.1±4.9 kg, respectively) and mild, although not significant, improvements in HDL-C and triglyceride levels. A six-month study (70) in obese adolescents showed that a low glycemic load diet versus a low-fat diet resulted in a greater decrease in BMI (−1.3±0.7 versus 0.7±0.5, respectively) and fat mass (−3.0±1.6 versus 1.8±1.0 kg, respectively). Insulin resistance increased over time in both groups, but less so in the group following the low glycemic load diet (70). However, further studies are needed before such dietary interventions are recommended.
Family-based behavioural interventions for obese children have been associated with reductions in total cholesterol, increases in HDL-C, reductions in insulin resistance and a return of ovulatory cycles in girls with PCOS (71–73).
In very high-risk insulin-resistant children, pharmacotherapy may be indicated. A double-blind randomized controlled (74) trial using metformin for six months in obese, insulin-resistant youth with a family history of T2DM demonstrated significant improvements in glucose tolerance and fasting insulin levels. However, the study was small and of relatively short duration. Further studies looking at the use of metformin and other insulin sensitizers are necessary. Other agents that may prove beneficial are thiazolidinediones.
CONCLUSIONS
Nearly 30% of obese (BMI at or above the 95th percentile) paediatric patients meet the criteria for the metabolic syndrome and 90% of obese children have at least one feature of the syndrome. These children may be at a significantly increased risk for the development of T2DM and CVD. The features of the metabolic syndrome are driven by the presence of abdominal obesity and insulin resistance. A uniform definition of the metabolic syndrome for paediatric patients needs to be created and comprehensive screening of obese children should be considered. Early intervention targeting insulin resistance may help attenuate the disease process.
REFERENCES
- 1.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 2.Isoma B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary artery disease, cardiovascular disease and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: Application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–7. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 5.Hanson RL, Imperatore G, Vennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–7. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 6.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle aged-men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 8.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–94. Arch Pediatr Adolesc Med. 2003;57:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 9.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Srinivasan SR, Elkasabany A, Berenson GS. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: The Bogalusa Heart Study. Am J Epidemiol. 1999;150:667–74. doi: 10.1093/oxfordjournals.aje.a010069. [DOI] [PubMed] [Google Scholar]
- 11.Raitakari OT, Porkka KV, Ronnemaa T, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The Cardiovascular Risk in Young Finns Study. Diabetologia. 1995;38:1042–50. doi: 10.1007/BF00402173. [DOI] [PubMed] [Google Scholar]
- 12.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–13. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 13.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 15.Fagot-Campagna A, Saaddine JB, Flegal KM, Beckles GL. Diabetes, impaired fasting glucose and elevated HbA1c in U.S. adolescents: The Third National Health and Nutrition Examination Survey. Diabetes Care. 2001;24:834–7. doi: 10.2337/diacare.24.5.834. [DOI] [PubMed] [Google Scholar]
- 16.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation of the European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 19.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts and different goals. Endocrin Metab Clin N Am. 2004;33:283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: The Bogalusa Heart Study. Diabetes. 2000;49:1042–8. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 21.Zimmet PZ, McCarty DJ, de Courten MP. The global epidemiology of non-insulin dependant diabetes mellitus and the metabolic syndrome. J Diabetes Complications. 1997;11:60–8. doi: 10.1016/s1056-8727(96)00090-6. [DOI] [PubMed] [Google Scholar]
- 22.Sung RY, Tong PC, Yu CW, et al. High prevalence of insulin resistance and metabolic syndrome in overweight/obese preadolescent Hong Kong Chinese children aged 9–12 years. Diabetes Care. 2003;26:250–1. doi: 10.2337/diacare.26.1.250. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Insulin resistance and compensatory hyperinsulinemia: Role in hypertension, dysplipidemia and coronary heart disease. Am Heart J. 1991;121:1283–8. doi: 10.1016/0002-8703(91)90434-j. [DOI] [PubMed] [Google Scholar]
- 24.Liese AD, Mayer-Davis EJ, Tyroler HA, et al. Development of the multiple metabolic syndrome in the ARIC cohort: Joint contribution of insulin, BMI and WHR. Atherosclerosis risk in communities. Ann Epidemiol. 1997;7:407–16. doi: 10.1016/s1047-2797(97)00047-1. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Muscelli E, Stern MP, Haffner SM. Differential impact of insulin and obesity on cardiovascular risk in non-diabetic subjects. Int J Obes Relat Metab Disord. 1996;20:7–14. [PubMed] [Google Scholar]
- 26.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: Associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–7. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan SR, Myers L, Berenson GM. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: The Bogalusa Heart Study. Diabetes. 2002;51:204–9. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 28.Vague J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. 1956. Nutrition. 1999;15:89–90. doi: 10.1016/s0899-9007(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 29.Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: The Bogalusa Heart Study. Am J Clin Nutr. 1999;69:308–17. doi: 10.1093/ajcn/69.2.308. [DOI] [PubMed] [Google Scholar]
- 30.Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198–205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- 31.Maffeis C, Petrobelli A, Grezzani A, Provera S, Tato L. Waist circumference and cardiovascular disease risk factors in prepubertal children. Obes Res. 2001;9:179–87. doi: 10.1038/oby.2001.19. [DOI] [PubMed] [Google Scholar]
- 32.Mei Z, Grummer-Strawn LM, Petrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:975–85. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 33.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–7. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: A defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab. 1991;72:277–82. doi: 10.1210/jcem-72-2-277. [DOI] [PubMed] [Google Scholar]
- 35.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 36.Bouchard C, Despres JP, Mauriege P. Genetic and non-genetic determinants of regional fat distribution. Endocr Rev. 1993;34:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan SR, Elkasabani A, Dalferes ER, Jr, Bao W, Berenson GS. Characteristics of young offspring of type 2 diabetic parents in a biracial (black-white) community based sample: The Bogalusa Heart Study. Metabolism. 1998;47:998–1004. doi: 10.1016/s0026-0495(98)90358-4. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan SR, Frontini MG, Berenson GS. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to adulthood in offspring of parents with type 2 diabetes: The Bogalusa Heart Study. Metabolism. 2003;52:443–50. doi: 10.1053/meta.2003.50065. [DOI] [PubMed] [Google Scholar]
- 39.Grunfeld B, Balzareti M, Romo M, Gimenez M, Gutman R. Hyperinsulinemia in normotensive offspring of hypertensive parents. Hypertension. 1994;23:12–5. doi: 10.1161/01.hyp.23.1_suppl.i12. [DOI] [PubMed] [Google Scholar]
- 40.Vlasakova Z, Pelikanova T, Karasova L, Skibova J. Insulin secretion, sensitivity and metabolic profile of young, healthy offspring of hypertensive parents. Metabolism. 2004;53:469–75. doi: 10.1016/j.metabol.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–74. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 42.Wilkin TJ, Metcalfe BS, Murphy MJ, Kirby J, Jeffrey AN, Voss LD. The relative contributions of birth weight, weight change and current weight to insulin resistance in contemporary 5-year olds: The Early Bird Study. Diabetes. 2002;51:3468–72. doi: 10.2337/diabetes.51.12.3468. [DOI] [PubMed] [Google Scholar]
- 43.Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–50. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- 44.Murtaugh MA, Jacobs DR, Moran A, Steinberger J, Sinaiko AR. Relation of birth weight to fasting insulin, insulin resistance and body size in adolescents. Diabetes Care. 2003;26:187–92. doi: 10.2337/diacare.26.1.187. [DOI] [PubMed] [Google Scholar]
- 45.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring; follow up research in the Pima Indians. J Matern Fetal Med. 2000;9:86–8. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 46.Sobngwi E, Boudou P, Mauvais-Jarvis F, et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361:1861–5. doi: 10.1016/S0140-6736(03)13505-2. [DOI] [PubMed] [Google Scholar]
- 47.Bo S, Menato G, Gallo ML, et al. Mild gestational hyperglycemia, the metabolic syndrome and adverse neonatal outcomes. Acta Obstet Gynecol Scand. 2004;83:335–40. doi: 10.1111/j.0001-6349.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 48.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 49.Frank S. Polycystic ovarian syndrome. N Engl J Med. 1995;333:853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 50.Scarpitta AM, Sinagra D. Polycysytic ovarian syndrome and endocrine and metabolic disease. Gynecol Endocrinol. 2000;14:392–5. doi: 10.3109/09513590009167709. [DOI] [PubMed] [Google Scholar]
- 51.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Hasseltine F, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell; 1992. pp. 377–84. [Google Scholar]
- 52.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;28:165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 53.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2000;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 54.Dunaif A. Insulin resistance and ovarian dysfunction. In: Moller D, editor. Insulin Resistance. New York: John Wiley; 1993. pp. 301–25. [Google Scholar]
- 55.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: Roles of insulin resistance and beta cell dysfunction and risk of cardiovascular disease. J Clin Endocr Metab. 2000;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 56.Dunaif A. Insulin resistance and the polycystic ovarian syndrome: Mechanism and implications for pathogenesis. Endocrin Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 57.Orio F, Jr, Palomba S, Spinelli L, et al. The cardiovascular risk of young women with polycystic ovary syndrome: An observational, analytical, prospective case-control study. J Clin Endocrinol Metab. 2004;89:3696–701. doi: 10.1210/jc.2003-032049. Erratum in 2004;89:5621. [DOI] [PubMed] [Google Scholar]
- 58.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 59.Scaffner F, Thasler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283–98. [PubMed] [Google Scholar]
- 60.Tominaga K, Kurata JH, Chen YK, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–9. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 61.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–32. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 62.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 63.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 64.National Centre for Health Statistics: National Health and Nutrition Examination Survey . CDC growth charts: Body mass index for age. 2000 < www.cdc.gov/growthcharts> (Version current at December 10, 2004)
- 65.Canadian Diabetes Association, Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(Suppl 2):S1–152. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Ku CY, Gower BA, Hunter GR, Goran MI. Racial differences in insulin secretion and sensitivity in prepubertal chidlren: Role of physical fitness and physical activity. Obes Res. 2000;8:506–15. doi: 10.1038/oby.2000.63. [DOI] [PubMed] [Google Scholar]
- 67.Kang HS, Gutin B, Barbeau P, et al. Physical training improves insulin resistance syndrome markers in obese adolescents. Med Sci Sports Exerc. 2002;34:1920–7. doi: 10.1097/00005768-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Williams CL, Hayman LL, Daniels SR, et al. Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106:143–60. doi: 10.1161/01.cir.0000019555.61092.9e. Erratum in 2002;106:1178. [DOI] [PubMed] [Google Scholar]
- 69.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142:253–8. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 70.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157:773–9. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 71.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101:554–70. [PubMed] [Google Scholar]
- 72.Wisotsky W, Swencionis C. Cognitive-behavioral approaches in the management of obesity. Adolesc Med. 2003;14:37–48. [PubMed] [Google Scholar]
- 73.Pasquali R, Antenucci D, Casimirri F, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68:173–9. doi: 10.1210/jcem-68-1-173. [DOI] [PubMed] [Google Scholar]
- 74.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:e55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]