Summary
Positioning the nucleus is essential for the formation of polarized cells, pronuclear migration, cell division, cell migration and the organization of specialized syncytia such as mammalian skeletal muscles. Proteins that are required for nuclear positioning also function during chromosome movement and pairing in meiosis. Defects in these processes lead to human diseases including laminopathies. To properly position the nucleus or move chromosomes within the nucleus, the cell must specify the outer surface of the nucleus and transfer forces across both membranes of the nuclear envelope. KASH proteins are specifically recruited to the outer nuclear membrane by SUN proteins, which reside in the inner nuclear membrane. KASH and SUN proteins physically interact in the perinuclear space, forming a bridge across the two membranes of the nuclear envelope. The divergent N-terminal domains of KASH proteins extend from the surface of the nucleus into the cytoplasm and interact with the cytoskeleton, whereas the N-termini of SUN proteins extend into the nucleoplasm to interact with the lamina or chromatin. The bridge of SUN and KASH across the nuclear envelope functions to transfer forces that are generated in the cytoplasm into the nucleoplasm during nuclear migration, nuclear anchorage, centrosome attachment, intermediate-filament association and telomere clustering.
Keywords: KASH, SUN, Bouquet, Nuclear anchorage, Nuclear envelope, Nuclear positioning
Introduction
Most cell biologists envision the nucleus sitting passively in the middle of the cell. However, the nucleus is usually precisely and actively positioned. Moving and anchoring the nucleus to a specific location in the cytoplasm is essential for the formation of polarized cells, pronuclear migration, cell division, cell migration, differentiation and the organization of specialized syncytia (Fig. 1).
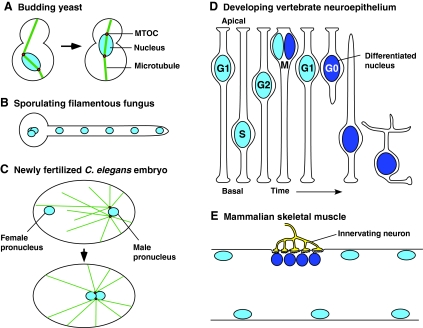
Fig. 1.
Selected examples of nuclear positioning are shown. Nuclei are light blue, differentiated nuclei are dark blue, microtubule organizing centers (MTOCs) are red, and microtubules are green. Arrows represent development over time. (A) In budding yeast, the nucleus must be positioned to the bud neck prior to mitosis. (B) In sporulating filamentous fungi, nuclei are evenly spaced in the syncytia. (C) In a newly fertilized C. elegans embryo, the male (right) and female (left) pronuclei must migrate towards one another before the first mitotic event. (D) In the developing vertebrate neuroepithelium, nuclei migrate basally and then apically, to where they divide. Differentiated cells often leave the pseudostratified epithelium, which requires additional nuclear migration events. (E) In a mammalian skeletal muscle, nuclei are spaced out evenly, except for specialized nuclei at the neuromuscular junction (innervating neuron is yellow).
In budding yeast, microtubules and actin filaments function together to move the nucleus into the bud neck prior to mitosis, whereas, in filamentous fungi, dynein and its associated proteins keep nuclei spaced evenly apart during hyphal growth (Fig. 1A,B) (reviewed by Pearson and Bloom, 2004; Xiang and Fischer, 2004). In early animal development, male and female pronuclei migrate towards one another in the large one-cell zygote. In most cases, the male pronucleus is attached to the centrosomes and their associated microtubule asters. As the asters grow, the male pronucleus is pushed towards the center of the cell. Next, the female pronucleus interacts with the microtubule aster and translocates towards the male pronucleus (Fig. 1C) (reviewed by Reinsch and Gonczy, 1998).
Another example of nuclear migration in development is the interkinetic nuclear migration in the pseudostratified neural epithelium, first described by Sauer (Sauer, 1935). These cells remain attached to both the apical and basal surface of the tissue, but the nucleus migrates from the apical surface to the basal surface and back to the apical surface, where it divides (Fig. 1D) (reviewed by Baye and Link, 2008). The apical division helps regulate development; differentiated cells may leave the neuroepithelium after the apical division (Del Bene et al., 2008). Finally, in the specialized syncytia of the mammalian skeletal-muscle myotube, hundreds of nuclei are evenly spaced at the periphery of the cell and a few transcriptionally specialized nuclei cluster under the neuromuscular junction (Fig. 1E) (Bruusgaard et al., 2003).
Depending on the cell type, all three components of the cytoskeleton (microtubules, actin filaments and intermediate filaments) can function either alone or together to position nuclei. Two common threads underlie the mechanisms of nuclear positioning. First, the nucleus must communicate with the cytoskeleton. Second, forces that are generated in the cytoplasm must be transferred across the nuclear envelope to the nuclear matrix, which is the structural element of the nucleus. Because of the fluid properties of membranes, it is thought that, if a molecular machine were to simply pull on the outer nuclear membrane (ONM) without connecting to the inner nuclear membrane (INM), the machine would simply pull an endoplasmic-reticulum (ER) tubule away from the nuclear envelope. It has recently become apparent that the bridges that are used to transfer forces from the cytoskeleton across the nuclear envelope to position nuclei are the same as those that are used to move meiotic chromosomes and organize chromatin. The mechanisms of these force-transferring bridges are the focus of this Commentary.
To understand how forces are transferred across the nuclear envelope, two fundamental cell-biological questions must be addressed. How are proteins targeted specifically to the ONM and not the contiguous ER? And how is force transferred across the two membranes of the nuclear envelope from the cytoskeleton to the nuclear matrix? The answers involve the KASH (Klarsicht, ANC-1, Syne homology) and SUN (Sad1 and UNC-84) proteins, which form a bridge across the two membranes of the nuclear envelope, directly linking the cytoplasm to the nuclear lamina. In this Commentary, the current model of the targeting of proteins specifically to the ONM and how a bridge across the nuclear envelope is built to transfer forces between the cytoplasm and nucleoplasm will be discussed. The proposed mechanisms of how ONM proteins interact with the cytoskeleton and how the INM interacts with the lamina and chromosomes will also be summarized. Finally, the recent literature as to how these mechanisms might be involved in human disease is reviewed.
Systems in which to study nuclear migration and anchorage
Many important genes that regulate nuclear migration were first identified in genetic screens in yeast, filamentous fungi, the nematode Caenorhabditis elegans and the fly Drosophila melanogaster. These include the nud (nuclear distribution) genes in Aspergillus nidulans, most of which regulate dynein, and the KASH and SUN proteins in C. elegans. Importantly, these screens have identified the essential functions of proteins that are involved in nuclear positioning. For example, the study of null alleles of anc-1 in C. elegans and its orthologs (Syne-1 and Syne-2) in mice has unambiguously shown that the products of these genes are required to anchor nuclei to specific locations in specialized syncytial cells (Starr and Han, 2002; Zhang et al., 2007b). The contributions of model genetic systems in assigning functions have been thoroughly reviewed in Journal of Cell Science (Wilhelmsen et al., 2006) and elsewhere (Fischer, 1999; Morris, 2003; Starr and Fischer, 2005; Starr and Han, 2005; Xiang and Fischer, 2004). However, a weakness of invertebrate model systems is that the nuclear migrations take place in a limited number of tissues and at specific times in development, making them difficult to study or manipulate in real time. The recent establishment of two tissue-culture models of nuclear migration will certainly complement the findings that are gained from model organisms.
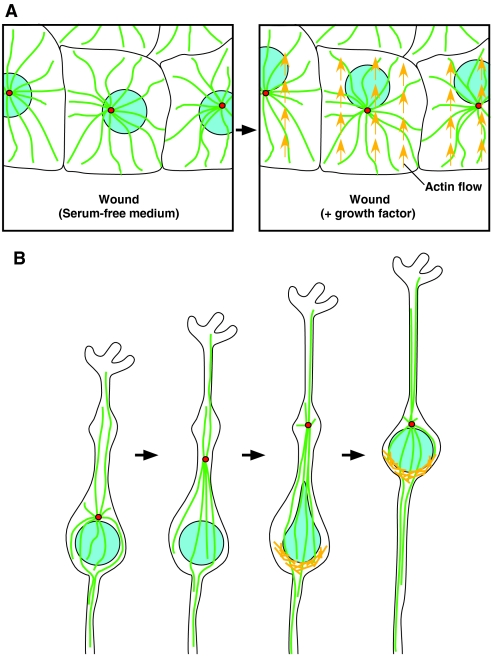
As seen in both a wound-healing polarization assay in fibroblasts and a neuronal-precursor migration system (Gomes et al., 2005; Tsai et al., 2007), nuclear migration depends on both microtubule and actin dynamics, although to different extents (Fig. 2). When NIH-3T3 fibroblasts are starved and wounded, bordering cells will polarize, but not migrate, after the addition of the growth factor lysophosphatidic acid (LPA). The newly polarized cells have their centrosomes facing the wound and their nuclei behind the centrosomes. A time-lapse microscopy study demonstrated that the centrosomes remain anchored in the middle of the cell while the nucleus actively migrates behind the centrosomes (Gomes et al., 2005) (Fig. 2A). This rearward nuclear movement is an active process, as actin, myosin and the small G-protein Cdc42 are required and nuclear movement is coupled with retrograde actin flow (Gomes et al., 2005).
Fig. 2.
Mammalian cell-culture model systems for nuclear migration. (A) NIH-3T3 cells polarize towards a wound edge prior to migration. In response to the addition of a growth factor, the nucleus (blue) migrates away from the wound edge in conjunction with actin flow (yellow arrows), whereas the centrosomes (red) and microtubules (green) remain stationary in the center of the cell. (B) Migrating neuronal precursors in culture. The centrosome (red) migrates forwards at a constant rate into a swelling. The microtubules (green) begin to pull on the nucleus (blue) and the nucleus jumps forwards in large steps with the assistance of actin-myosin contraction (yellow bars) behind the nucleus.
Neuronal precursors migrate through the cortex of the brain to form the complex layering that is characteristic of the mammalian brain. Failure of nuclear migration in the neuronal precursors leads to lissencephaly, which is derived from the Greek words for `smooth brain' because neurons fail to populate the cortex (Lambert de Rouvroit and Goffinet, 2001). Studies using time-lapse imaging of neuronal precursors in culture have demonstrated that the leading process and the centrosome move forwards at a nearly constant rate – the centrosome and Golgi move forwards into a small swelling, well ahead of the nucleus (Fig. 2B). The nucleus stretches towards the centrosome in a LIS1 (PAFAH1B1)- and dynein-dependent manner, and catches up to the centrosome when it jumps forward in a saltatory manner that is dependent on actin–myosin-II contraction behind the nucleus (Fig. 2B) (Bellion et al., 2005; Schaar and McConnell, 2005; Tsai et al., 2007; Xie et al., 2007). In both of these systems, the roles of KASH and SUN proteins remain to be determined. It is intriguing to speculate that KASH proteins anchor the nucleus to the retrograde actin flow during fibroblast polarization or to microtubules during neuronal migration.
The nuclear-envelope bridging model
Understanding how the nucleus interacts with the cytoskeleton is essential to understanding the mechanisms of nuclear positioning and of chromosome movement within the nucleus. The nuclear envelope separates the nucleoplasm from the cytoplasm and consists of the ONM and the INM, two membranes that are separated by 30-50 nm (reviewed by Gruenbaum et al., 2005; Stewart et al., 2007). The ONM and INM are continuous with one another at the nuclear pore and the ONM is continuous with the ER, effectively making the nuclear envelope a specialized extension of the ER. The nuclear lamina underlies the INM, providing a structural scaffold to the nuclear envelope. As all the membranes of the nuclear envelope are contiguous with each other as well as with the ER, specific mechanisms exist to target proteins to either the ONM or the INM. Furthermore, as membranes are fluid, structural elements must bridge the nuclear envelope to transfer forces from the cytoskeleton to the nuclear lamina. Not surprisingly, understanding the basic mechanisms of cellular trafficking that targets proteins specifically to the ONM or INM has been instrumental in forming the current nuclear-envelope bridging model that is presented here.
In the current model, KASH proteins are recruited to the ONM by interacting with the INM SUN proteins in the perinuclear space (Figs 3, 4). As a result, KASH and SUN proteins bridge the nuclear envelope to connect the cytoskeleton to nuclear structural elements (Crisp et al., 2006; McGee et al., 2006; Padmakumar et al., 2005). Force that is transferred across the nuclear envelope must be distributed in the lamina or chromatin. In Schizosaccharomyces pombe, one possible mechanism for this dispersion is via Ima1, which links chromatin to the INM close to where the Kms2-Sad1 KASH-SUN complex transfers forces from the spindle pole body (SPB), across the nuclear envelope, through kinetochores to centromeres. King et al. (King et al., 2008) suggest that, if the KASH-SUN bridge is acting as the bolt, Ima1 and associated chromatin act as the nut to distribute the forces along the inside of the INM (Fig. 4D). Ima1 homologs (e.g. NET5) (Schirmer et al., 2003) or lamin itself might play a similar role for the KASH-SUN bridge in higher organisms.
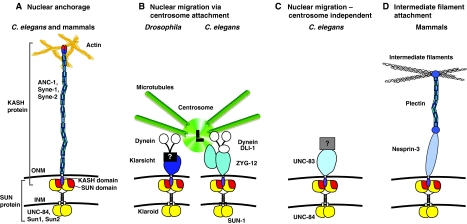
Fig. 3.
The nuclear-envelope bridge, and roles of KASH proteins connecting the ONM to the cytoskeleton. SUN proteins (yellow and red) dimerize at the INM and recruit KASH proteins (different shades of blue) to the ONM. The central link of the bridge occurs in the perinuclear space, where the KASH domain (purple) of KASH proteins directly interacts with two domains in the SUN protein, the SUN domain (red) and a less-conserved domain (yellow). The large cytoplasmic domains of KASH proteins (shades of blue) then extend away from the ONM into the cytoplasm to interact with the cytoskeleton. (A) One class of KASH proteins (including ANC-1, Syne-1 and Syne-2) connect the ONM to actin filaments (tan) to anchor nuclei. (B) Klarsicht and ZYG-12 connect the ONM to centrosomes (green). Both function through dynein (white). ZYG-12 in the ONM dimerizes with KASH-less ZYG-12 in the centrosome. (C) UNC-83 and UNC-84 mediate nuclear migration in a centrosome-independent mechanism that remains unknown. (D) Nesprin-3 links the ONM to intermediate filaments (gray) through plectin. See text for more details.
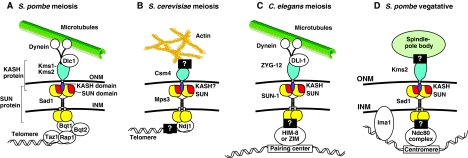
Fig. 4.
The KASH-SUN nuclear-envelope bridge transfers forces to move chromosomes. SUN proteins (yellow and red) at the INM interact with KASH proteins (blue and purple) at the ONM. The N-terminal nucleoplasmic domain of SUN proteins (bottom) interacts with chromosome-binding proteins. KASH proteins extend away from the ONM into the cytoplasm to interact with the cytoskeleton. (A) Kms1-Kms2 and Sad1 move telomeres along the INM by transferring forces that are generated by dynein on microtubules. (B) Csm4 and Mps3 move telomeres along the INM by transferring forces that are generated by the actin cytoskeleton. (C) ZYG-12 and SUN-1 target pairing centers of meiotic chromosomes to the INM and might move them to facilitate pairing by transferring forces from dynein and microtubules. (D) Kms2 and Sad1 connect the SPB to the nucleus. The forces generated by the SPB are then spread along the chromatin through the centromere and neighboring Ima1-heterochromatin complexes. See text for details.
SUN proteins at the INM
SUN proteins make up the INM half of the nuclear-envelope bridge (Figs 3, 4; Table 1). SUN proteins were first defined through molecular characterization of the C. elegans gene unc-84 (Malone et al., 1999). Mutations in unc-84 disrupt nuclear migration in three tissues – embryonic hypodermal and intestinal cells, and larval P-cells – and disrupt nuclear anchorage in all post-embryonic syncytial cells (Malone et al., 1999; Starr et al., 2001). UNC-84 is an integral membrane component of the nuclear envelope and contains an ∼175-residue C-terminal domain that is homologous with the C-terminus of the S. pombe protein Sad1 (Hagan and Yanmagida, 1995; Malone et al., 1999). To fit the definition of a SUN protein, a protein must have three characteristics (reviewed by Tzur et al., 2006b; Worman and Gundersen, 2006): first, it must have at least one transmembrane domain; second, it must localize to the INM, although in yeast, SUN proteins localize predominantly to the SPB (perhaps at the location of the INM that is associated with the SPB) and at lower levels elsewhere in the nuclear envelope (Bupp et al., 2007; Tran et al., 2001); and third, it must contain a C-terminal domain with homology to other SUN proteins that extends into the perinuclear space of the ER. SUN proteins fitting this definition are conserved across eukaryotes (Table 1) and are even found in the basal eukaryote flagellate Giardia lamblia (Jaspersen et al., 2006; Mans et al., 2004).
Table 1.
Functions of SUN and KASH proteins
| SUN proteins | Characteristics and function(s) at the INM | References |
|---|---|---|
| Mammals | ||
| Sun1 | Telomere attachment in meiosis and gametogenesis; nuclear-envelope integrity; recruit KASH proteins | (Crisp et al., 2006; Ding et al., 2007; Haque et al., 2006; Padmakumar et al., 2005) |
| Sun2 | Nuclear-envelope integrity; recruit KASH proteins | (Crisp et al., 2006; Hodzic et al., 2004) |
| Sun3 | Testis specific; ER localized; unknown function | (Crisp et al., 2006) |
| Spag4 | Testis specific; not at nuclear envelope; unknown function | (Shao et al., 1999) |
| C. elegans | ||
| UNC-84 | Nuclear positioning; recruit ANC-1 and UNC-83 in somatic cells | (Malone et al., 1999; Starr and Han, 2002; Starr et al., 2001) |
| SUN-1 (matefin) | Centrosome attachment; apoptosis; chromosome pairing in meiosis; recruit ZYG-12 and CED-4 | (Malone et al., 2003; Penkner et al., 2007; Tzur et al., 2006a) |
| D. melanogaster | ||
| Klaroid | Recruit Klarsicht in eye disc; nuclear migration in eye disc | (Kracklauer et al., 2007) |
| CG6589 | Unknown | (Kracklauer et al., 2007) |
| Dictyostelium | ||
| Sun-1 | Centrosome attachment; genome stability | (Xiong et al., 2008) |
| S. pombe | ||
| Sad1 | Spindle architecture; recruit Kms1 | (Chikashige et al., 2006; Hagan and Yanmagida, 1995) |
| S. cerevisiae | ||
| Mps3 | SPB duplication; attachment of telomeres to nuclear envelope; recruit Csm4 | (Conrad et al., 2008; Jaspersen et al., 2006; Koszul et al., 2008) |
| KASH proteins | Known function(s) at the ONM | References |
| Mammals | ||
| Syne-1 (Nesprin-1) | Anchorage of nuclei to actin filaments | (Apel et al., 2000; Zhang et al., 2001; Zhang et al., 2007b) |
| Syne-2 (Nesprin-2) | Anchorage of nuclei to actin filaments | (Apel et al., 2000; Zhang et al., 2007b; Zhen et al., 2002) |
| Nesprin-3 | Intermediate-filament interactions with the nucleus | (Ketema et al., 2007; Wilhelmsen et al., 2005) |
| Nesprin-4 | Unknown; endocrine specific | (Crisp and Burke, 2008) |
| C. elegans | ||
| ANC-1 | Anchorage of nuclei to actin filaments | (Starr and Han, 2002) |
| UNC-83 | Nuclear migration | (McGee et al., 2006; Starr et al., 2001) |
| ZYG-12 | Attachment of centrosomes to nuclei | (Malone et al., 2003) |
| D. melanogaster | ||
| Klarsicht | Nuclear migration and centrosome attachment | (Mosley-Bishop et al., 1999; Patterson et al., 2004) |
| MSP-300 | Muscle development (?); nuclear anchorage (?) | (Rosenberg-Hasson et al., 1996; Technau and Roth, 2008; Xie and Fischer, 2008; Yu et al., 2006) |
| S. pombe | ||
| Kms1 | Meiotic chromosome movement and pairing via dynein | (Chikashige et al., 2006; Miki et al., 2004) |
| Kms2 | Meiotic and mitotic chromosome movement | (Chikashige et al., 2006; King et al., 2008; Miki et al., 2004) |
| S. cerevisiae | ||
| Csm4 | Meiotic actin-driven telomere movements and pairing | (Conrad et al., 2008; Koszul et al., 2008) |
The relationships between SUN proteins from different species are not clear. For example, the SUN domain of UNC-84 is closest to human Sun1 in sequence, but it is almost as closely related to human Sun2. Furthermore, the SUN domain of C. elegans SUN-1 is equally related to all four mammalian SUN proteins [for a detailed genomic analysis, see the supplementary data figure S2 of Jaspersen et al. (Jaspersen et al., 2006)]. The nucleoplasmic N-termini of SUN proteins are not obviously conserved. Some SUN proteins are dependent on nuclear lamins for localization (Lee et al., 2002), whereas others are not (Haque et al., 2006; Fridkin et al., 2004). Higher organisms have multiple SUN proteins, which are expressed in a tissue-specific manner (Crisp et al., 2006; Ding et al., 2007; Hodzic et al., 2004; Kracklauer et al., 2007; Malone et al., 2003; Padmakumar et al., 2005). For example, in C. elegans, SUN-1 (also known as matefin) is expressed in the germ line and early embryo, whereas UNC-84 is expressed in most somatic cells from the 24-cell-stage embryo through to adulthood (Fridkin et al., 2004; Lee et al., 2002).
The precise topologies and mechanism(s) of targeting SUN proteins to the INM remain to be elucidated. Current genetic and biochemical evidence suggests that SUN proteins form dimers or multimers. Consistent with this model, most SUN proteins have short coiled-coil domains in their perinuclear-space domains (Crisp et al., 2006; Haque et al., 2006; Malone et al., 1999). The coiled-coil domains of human Sun1 form immobile oligomeric complexes through disulfide bonds (Lu et al., 2008).
SUN proteins have been implicated in a wide variety of important cellular functions (Table 1; Figs 3, 4). For example, C. elegans UNC-84 and S. pombe Sad1 function in nuclear positioning (Malone et al., 1999; Tran et al., 2001), whereas SUN proteins in budding and fission yeast play essential roles in SPB duplication and spindle architecture, respectively (Hagan and Yanmagida, 1995; Jaspersen et al., 2006). SUN-1 in C. elegans recruits CED-4 to the nuclear envelope during apoptosis (Tzur et al., 2006a) and is required for centrosome attachment to the nucleus, as is Klaroid in Drosophila (Fig. 3B) (Kracklauer et al., 2007; Malone et al., 2003). SUN proteins in humans and Dictyostelium discoideum function to maintain the even spacing between the INM and ONM (Crisp et al., 2006; Xiong et al., 2008). Finally, SUN proteins are required in a number of systems to attach telomeres or pairing centers to the nuclear envelope during meiosis (Fig. 4) (Chikashige et al., 2006; Conrad et al., 2008; Ding et al., 2007; Koszul et al., 2008; Penkner et al., 2007).
How can SUN proteins be involved in so many different cellular functions? The current model (Figs 3, 4) is that SUN proteins function at the INM to recruit KASH proteins to the ONM, forming a bridge across the nuclear envelope. Then, a number of different KASH proteins that are positioned in the ONM and extend into the cytoplasm can function in a variety of cellular processes. A single SUN protein can interact with multiple KASH proteins. For example, UNC-84 interacts with ANC-1 to anchor nuclei to the actin cytoskeleton and then with UNC-83 during nuclear migration (McGee et al., 2006; Starr and Han, 2002; Starr et al., 2001). Many cells have both UNC-83 and ANC-1 on their nuclear envelope at the same time, so the SUN protein must be able to regulate its interactions with different KASH domains. The AAA+ (ATPases associated with diverse cellular activities) ATPase torsinA has recently been shown to bind to KASH domains and regulate their localization to the nuclear envelope (Nery et al., 2008). Thus, torsinA is a leading candidate to regulate SUN proteins as they switch their interactions between different KASH proteins. Furthermore, different SUN proteins are likely to interact with different partners in the nucleoplasm.
KASH proteins at the ONM
KASH proteins make up the ONM half of the nuclear-envelope bridge and connect the ONM to the cytoskeleton (Table 1). The term KASH was coined when it was first noted that Drosophila Klarsicht, C. elegans ANC-1, and human Syne-1 and Syne-2 all have a conserved C-terminal domain (Starr and Han, 2002). Klarsicht is a component of the nuclear envelope that is required for nuclear migration and centrosome attachment to the nucleus in the developing fly eye disc and is thought to coordinate opposing forces from kinesin and dynein microtubule motors (Fig. 3B) (Mosley-Bishop et al., 1999; Patterson et al., 2004; Welte, 2004). ANC-1 anchors nuclei in syncytial cells by tethering the ONM to the actin cytoskeleton (Fig. 3A) (Starr and Han, 2002). Two mammalian orthologs of ANC-1 have been independently identified by six groups, and were originally called Syne-1 and Syne-2, but are also frequently referred to as Nesprin-1 and Nesprin-2, respectively (Apel et al., 2000; Cottrell et al., 2004; Gough et al., 2003; Mislow et al., 2002; Padmakumar et al., 2004; Zhang et al., 2001; Zhen et al., 2002). A Drosophila ortholog, MSP-300, has also been identified, but its function is not clear (Technau and Roth, 2008; Xie and Fischer, 2008; Yu et al., 2006). Since then, KASH domains have been found in a number of different proteins across eukaryotes (Table 1).
To fit the definition of a KASH protein (Table 1) (reviewed by Starr and Fischer, 2005; Wilhelmsen et al., 2006), a protein should meet four criteria. First, KASH proteins have a domain at their C-termini that consists of a single transmembrane domain that is followed by fewer than 35 residues before the C-terminus. Thus, KASH proteins are C-tail-anchored integral membrane proteins that are inserted into membranes post-translationally (see Box 1). The level of similarity between KASH domains may be very weak, suggesting that many KASH proteins remain to be found. For example, the C-terminus of C. elegans ZYG-12 poorly aligns with other KASH domains, and S. cerevisiae Csm4 has no sequence similarity to other known KASH domains, but these two KASH proteins meet all the other criteria listed here (Conrad et al., 2008; Koszul et al., 2008; Starr and Fischer, 2005). Second, KASH proteins localize specifically to the ONM with the small C-terminus in the perinuclear space and the large divergent N-terminal domain extending into the cytoplasm. KASH domains are both necessary and sufficient to localize KASH proteins to the ONM (Fischer et al., 2004; Grady et al., 2005; Guo et al., 2005; McGee et al., 2006; Starr and Han, 2002; Zhang et al., 2001; Zhang et al., 2007b). To date, only KASH proteins have been shown to localize exclusively to the ONM and not to the contiguous ER membrane. KASH proteins often exist in many isoforms, some of which are KASH-less and function at locations away from the ONM (Guo et al., 2005; Zhang et al., 2002; Zhang et al., 2001). Third, KASH proteins require SUN proteins for localization to the nuclear envelope (Crisp et al., 2006; Padmakumar et al., 2005; Starr and Han, 2002; Starr et al., 2001). KASH domains directly interact with SUN proteins, through both the SUN domain and a less-conserved domain, in the perinuclear space (Crisp et al., 2006; Haque et al., 2006; McGee et al., 2006; Padmakumar et al., 2005). How the two KASH-interacting domains of SUN proteins function to bind to KASH peptides will require detailed structural studies. Fourth, The N-terminal domains of KASH proteins are not conserved, but do interact with a variety of components of the cytoskeleton to mediate multiple cellular processes (Fig. 3).
Box 1. Targeting KASH and other C-tail-anchored proteins to membranes
Tail-anchored proteins contain cytosolic N-termini and a single transmembrane domain very close to their C-termini. SNAREs, components of the Sec61 translocon, apoptosis regulators of the Bcl family, the TOM complex for mitochondrial import, cytochrome b5 and KASH proteins are examples of tail-anchored proteins (reviewed by Borgese et al., 2007). In fact, bioinformatics studies predict that there are about 325 genes that encode tail-anchored proteins in the human genome (Kalbfleisch et al., 2007). As the transmembrane domain of a tail-anchored protein remains in the ribosome until translation is complete, it is not presented to the signal-recognition particle (SRP) that is used by most other integral membrane proteins to target to the ER membrane. Instead, tail-anchored proteins are inserted into membranes post-translationally and independently of SRP and Sec61 (the translocon) activity. Much progress has recently been made on the mechanisms of membrane targeting of tail-anchored proteins. Biochemical purification of the transmembrane-domain recognition complex identified the cytosolic factor TRC40 (Asna-1) as a key component of the tail-anchored targeting machinery (Stefanovic and Hegde, 2007). The yeast homolog Get3 also recognizes transmembrane domains of tail-anchored proteins (Schuldiner et al., 2008). The Get3-tail-anchored complex is recruited to the ER membrane by the Get1-Get2 receptor complex (Schuldiner et al., 2008). With the establishment of cell-free assays to study tail-anchored protein insertion into membranes, rapid progress should be made on the elucidation of the mechanism of membrane targeting of tail-anchored proteins, including KASH proteins, to the ONM.
Mechanisms of nuclear positioning
Once at the ONM, how do KASH proteins connect the nuclear envelope to the cytoskeleton? Other than the KASH domain itself, KASH proteins are not homologous to one another, although many of them contain divergent spectrin repeats and are predicted to be highly α-helical (reviewed by Starr and Fischer, 2005; Wilhelmsen et al., 2006). Therefore, the different cytoplasmic domains of KASH proteins are free to interact with a variety of proteins. The known KASH-interacting proteins include actin filaments, microtubule motors and regulators, and plectin, which binds intermediate filaments. There are at least four mechanisms, discussed in detail below, in which KASH proteins function to position nuclei (Fig. 3).
C. elegans ANC-1 and its mammalian orthologs Syne-1 and Syne-2 are giant proteins of nearly a megadalton that consist of an N-terminal actin-binding domain, a large rope-like domain of mostly divergent spectrin repeats and a C-terminal KASH domain. ANC-1 and Syne proteins are thought to function to directly tether the ONM to the actin cytoskeleton (Fig. 3A) (Padmakumar et al., 2004; Starr and Han, 2002; Zhang et al., 2002; Zhen et al., 2002). Some data suggest that shorter isoforms of Syne proteins, some KASH-less, exist that may localize to the INM and possibly contribute to nuclear positioning (Warren et al., 2005). Loss-of-function mutations in ANC-1 or Syne-1 lead to unanchored nuclei, which form large clumps in syncytial cells (Starr, 2007; Starr and Han, 2002; Zhang et al., 2007b). This nuclear-anchorage defect can also be phenocopied by a dominant-negative approach in which overexpression of the KASH domain of ANC-1 or Syne-1 displaces endogenous KASH proteins from the nuclear envelope (Grady et al., 2005; Starr and Han, 2002). Both an analogous dominant-negative construct and a morpholino against syne2 have been used to show that zebrafish KASH proteins are involved in nuclear migration in the developing retina (Tsujikawa et al., 2007; Del Bene et al., 2008).
Syne-1, Syne-2 and ANC-1 have similar domains and lengths to dystrophin. The KASH domain of ANC-1 and of the Syne proteins allows them and their SUN partners to bridge the double membrane of the nuclear envelope, whereas dystrophin and its partners only transfer forces across a single plasma membrane (Starr and Han, 2003). The Syne proteins also are involved in mediating the stiffness of the global cytoskeleton. Tissue-culture cells that overexpress the dominant-negative construct show a decrease in mechanical stiffness, even far from the nucleus (Stewart-Hutchinson et al., 2008). These data lead to an interesting hypothesis that Syne proteins, the actin network and the nuclear-envelope bridge might directly transmit mechanical stimuli from the plasma membrane to the chromatin, thereby affecting the transcription of certain genes (Gieni and Hendzel, 2008).
KASH proteins also mediate nuclear migration through both centrosome-dependent and centrosome-independent mechanisms. Drosophila Klarsicht functions during nuclear migration in pseudostratified developing photoreceptor cells to attach the nucleus to centrosomes (Fig. 3B). In klarsicht or klaroid mutants, the centrosome migrates normally to the apical surface of the eye disc immediately after the morphogenic front passes, but nuclei fail to attach to the centrosome and remain basal (Kracklauer et al., 2007; Patterson et al., 2004). Klarsicht is thought to function through dynein (Gross et al., 2000; Welte et al., 1998), and mutations in the dynein regulators dynactin, Bic-D and LIS1 also disrupt nuclear migration (Swan et al., 1999; Whited et al., 2004). However, the exact mechanism of how Klarsicht interacts with dynein is unknown.
C. elegans ZYG-12 functions during pronuclear migration to attach centrosomes to nuclei (Fig. 3B). In zyg-12 or sun-1 mutant embryos, both centrosomes detach from the nucleus and pronuclear migration fails, leading to lethality (Malone et al., 2003). ZYG-12 on the ONM is thought to bind to isoforms of ZYG-12 that lack the KASH domain and bind to the centrosome (Malone et al., 2003). ZYG-12 also interacts with the dynein light chain, suggesting that dynein attaches centrosomes to the nuclear envelope (Malone et al., 2003). Nuclear migration events that occur later in C. elegans development apparently occur by a centrosome-attachment-independent manner (Fig. 3C). Mutations in unc-83 or unc-84 disrupt nuclear migration in a variety of tissues, but the centrosomes remain attached to mis-positioned nuclei (Lee et al., 2002; Starr et al., 2001). The exact mechanism of how the KASH protein UNC-83 interacts with the cytoskeleton, and whether the centrosome plays a role, are open questions.
The mammalian KASH protein Nesprin-3 links nuclei to intermediate filaments through plectin (Fig. 3D) (Ketema et al., 2007; Wilhelmsen et al., 2005). The cellular function of connecting the ONM to intermediate filaments by Nesprin-3 remains to be determined, although intermediate filaments have been implicated in nuclear positioning. Mice with knockout alleles of desmin, the major skeletal-muscle component of intermediate filaments, have a striking nuclear-anchorage defect (Ralston et al., 2006). In addition, the intermediate-filament vimentin is often found closely associated with nuclei, and mutations in vimentin lead to defects in nuclear morphology (Toivola et al., 2005).
Moving chromosomes within the nucleus
An exciting recent development in the research field of the KASH-SUN nuclear-envelope bridge has been the finding that the nuclear-envelope bridge is not only used to transfer forces from the cytoplasm to move the entire nucleus, but that it is also used to transfer forces that are generated in the cytoplasm to move individual chromosomes within the nucleus. Chromosomes are not randomly localized in the nucleus; often, loci are associated with the nuclear periphery to regulate transcription (reviewed by Akhtar and Gasser, 2007). In meiosis, homologous chromosomes must move to the synapse with one another. As originally described by Eisen (Eisen, 1900), dramatic rearrangements of meiotic chromosomes in many systems at the leptotene-to-zygotene transition result in all the telomeres associating with the INM and clustering together to form the bouquet (reviewed by Harper et al., 2004; Scherthan, 2001; Zickler and Kleckner, 1998), which contributes to meiotic chromosome pairing. The forces to move telomeres and chromosomes in the nucleus are generated in the cytoplasm (reviewed by Chikashige et al., 2007).
KASH and SUN proteins were implicated in chromosome movement when Chikashige et al. (Chikashige et al., 2006) identified Bqt1 and Bqt2 in fission yeast and showed that these meiotic-specific proteins connect telomeres to Sad1 on the nucleoplasmic face of the INM (Fig. 4A). The foci of Sad1-associated telomeres move towards the SPB in a microtubule-dependent manner and probably function through Kms1 (Chikashige et al., 2006). Kms1 fits the criteria of a KASH protein – it has a homologous KASH domain and its interaction with Sad1 is required for localization to the nuclear envelope (Miki et al., 2004). The cytoplasmic domain of Kms1 interacts with dynein components (Miki et al., 2004). Thus, Kms1 and Sad1 have been proposed to form a bridge that transfers forces that are generated by dynein along microtubules in the cytoplasm to telomeres in the nucleus (Chikashige et al., 2006).
Subsequently, SUN proteins have been shown to localize telomeres (or pairing centers in C. elegans) to the nuclear periphery during meiosis in budding yeast, C. elegans and mice (Bhalla and Dernburg, 2008; Bupp et al., 2007; Conrad et al., 2007; Ding et al., 2007; Penkner et al., 2007). In contrast to fission yeast, telomere movements in budding yeast are dependent on actin dynamics (Koszul et al., 2008). No KASH protein has been implicated to work with the SUN protein Mps3 in budding yeast, but Csm4 fits the criteria of a KASH protein. Csm4 localizes to the nuclear envelope (although it is not known whether it localizes to the ONM) in an Mps3-dependent manner (Conrad et al., 2008; Koszul et al., 2008). It is a tail-anchored protein, but the C-terminus contains no homology to other KASH domains. On the basis of these criteria, Csm4 is the first KASH protein to be identified in budding yeast and it can be hypothesized that its cytoplasmic domain is likely to interact with a component of the actin cytoskeleton (Fig. 4B). In C. elegans, HIM-8 and the ZIM proteins bind to pairing centers of each chromosome and then localize to SUN-1 and ZYG-12 foci at the nuclear envelope of meiotic prophase nuclei (Bhalla and Dernburg, 2008; Penkner et al., 2007; Phillips et al., 2005). Thus, ZYG-12 and SUN-1 transfer forces that are generated by dynein on microtubules to chromosomes within the nucleus (Fig. 4C). The cytoskeletal components that are used to move mouse telomeres along the INM through Sun1 remain to be identified. Mouse Sun2 is also associated with telomeres at the INM of meiotic cells, but its functional role is unknown (Schmitt et al., 2007).
KASH-SUN complexes also function during interphase to help organize chromatin. For example, in S. pombe, the Kms2-Sad1 nuclear-envelope bridge connects the SPB to centromeric heterochromatin through the Ndc80 kinetochore complex (Fig. 4D) (King et al., 2008). The S. cerevisiae Mps3 SUN protein is also required for telomere association with the INM in mitotic cells (Bupp et al., 2007). Although not yet demonstrated, KASH-SUN complexes are likely to play similar roles in higher eukaryotes.
Possible links between Syne proteins and disease
Nuclear-envelope components have been implicated in over 20 diseases, termed laminopathies, that include muscular dystrophies, neuropathies, lipodystrophies and premature-aging disorders (reviewed by Mounkes et al., 2003; Worman and Bonne, 2007). Disrupting nuclear migration in the developing brain probably results in lissencephaly, a severe psychomotor retardation disease (Lambert de Rouvroit and Goffinet, 2001). It would not be surprising if mutations in KASH and SUN proteins were involved in the progression of lissencephaly, laminopathies and other neuromuscular diseases.
Recently, Syne-1 was linked to a neurological disease in a group of French-Canadian families. Mutations in Syne-1 were found to be causative of autosomal recessive cerebellar ataxia type 1 (ARCA-1; also known as recessive ataxia of Beauce), a late-onset ataxia with a slow progression (Gros-Louis et al., 2007). It remains to be seen whether ARCA-1 mutations in Syne-1 disrupt nuclear-envelope-associated functions of Syne proteins. Further studies are therefore required to understand whether and how nuclear positioning contributes to ARCA-1.
Other studies point to a role for Syne proteins in the pathogenesis of Emery-Dreifuss muscular dystrophy (EDMD). Heterozygous mutations have been found in Syne-1 and Syne-2 from EDMD patients with wild-type lamin and emerin genes. The fibroblasts from such patients show changes in nuclear morphology that phenocopy Syne-1-siRNA-transfected fibroblasts (Zhang et al., 2007a). Syne proteins bind emerin in vitro and emerin mutations causing EDMD disrupt this interaction. These data suggest that Syne proteins function with emerin and that disrupting this interaction causes phenotypes that are indicative of an uncoupling of the nucleus and cytoskeleton, leading to the progression of EDMD (Wheeler et al., 2007; Zhang et al., 2007a). It remains to be determined whether mutations in KASH or SUN proteins are causative for EDMD.
Finally, Syne-2 appears to play a role in the progression of the premature-aging disease progeria. In a study by Kandert et al. (Kandert et al., 2007), fibroblasts from a patient with progeria had nuclei with abnormal morphologies, including blebs between the INM and ONM; these blebs are similar to those seen in other cell lines in which interactions between SUN and KASH proteins have been disrupted (Crisp et al., 2006). Furthermore, Syne-2 localization is correlated with the severity of the nuclear-morphology defects – the more severe the defect, the less Syne-2 is observed at the nuclear envelope (Kandert et al., 2007). Thus, one probable function of Syne-2 is to `safeguard' the structural integrity of the nuclear envelope (Kandert et al., 2007). It is not known, however, whether mutations in SUN or KASH proteins are a cause of progeria. On the basis of these examples, defects in SUN-KASH protein interactions are likely to have an important role in the progression of a variety of laminopathies.
Perspectives
Ten years ago, the ONM was thought to be a homogenous extension of the ER; since then, it has become clear that the ONM is a specialized membrane. KASH proteins localize specifically to the ONM, specifying the ONM as the docking site for the nucleus with the cytoskeleton. Studies from many different laboratories have led to the nuclear-envelope bridging model, as presented here. We now believe that KASH proteins are targeted specifically to the ONM by SUN proteins at the INM, forming a bridge to transfer forces across the nuclear envelope. This nuclear-envelope bridge functions in a variety of essential cellular processes, including nuclear migration, nuclear anchorage, centrosome attachment to the nucleus, apoptosis and chromosome movements, and disruptions of this nuclear-envelope bridge can lead to human disease.
Many unanswered questions remain about the nuclear-envelope bridge. Most KASH proteins have not been proven to be on the ONM, as traditional immunofluorescence experiments lack the resolution necessary to distinguish ONM from INM. New imaging techniques, such as structured illumination microscopy, could be used to address this issue (Schermelleh et al., 2008). Given the importance of the central link of this bridge, much more needs to be understood about how KASH and SUN proteins interact. What is the stoichiometry of SUN proteins to KASH proteins? How do these proteins interact with one another across a double-membrane structure? Answers to these questions will probably require protein-structure data. How do SUN proteins switch interactions between different KASH proteins? What proteins are involved in the regulation of this switch?
Besides the bridge itself, the cellular mechanisms that are described in Figs 3 and 4 remain to be fully elucidated. Many of the players that link the nucleoplasmic domain of SUN proteins to the nuclear lamina and chromosomes are unknown. Also, mechanisms of how KASH proteins function are not completely understood. For example, how does UNC-83 function during nuclear migration? Furthermore, there are likely to be multiple KASH proteins that remain to be identified. Given the weak homology of the KASH domains in Csm4 or ZYG-12, this daunting task is unlikely to be solved using bioinformatics tools. A more fruitful approach to find more divergent KASH-domain-containing proteins might be through investigating proteins that interact with SUN proteins. Using a split-ubiquitin yeast two-hybrid assay, we have recently identified KDP-1 as a new, essential KASH protein in C. elegans (M. McGee and D.A.S., unpublished data). Finally, recent developments in the field suggest that KASH proteins at the ONM have a global effect on the cytoskeleton of the cell (Gieni and Hendzel, 2008; Stewart-Hutchinson et al., 2008), raising the exciting possibility that KASH proteins are involved in direct mechanotransduction of signals from the plasma membrane to the chromatin. Thus, the roles of KASH and SUN proteins and the nuclear-envelope bridge keep expanding.
I thank Sean Burgess, Abdul Barakat, Carol Erickson, Josh Morgan and all the members of the Starr lab for their insightful comments on the manuscript. Studies in the Starr lab are supported by a grant from the National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Akhtar, A. and Gasser, S. M. (2007). The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8, 507-517. [DOI] [PubMed] [Google Scholar]
- Apel, E. D., Lewis, R. M., Grady, R. M. and Sanes, J. R. (2000). Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275, 31986-31995. [DOI] [PubMed] [Google Scholar]
- Baye, L. M. and Link, B. A. (2008). Nuclear migration during retinal development. Brain Res. 1192, 29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion, A., Baudoin, J. P., Alvarez, C., Bornens, M. and Metin, C. (2005). Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla, N. and Dernburg, A. F. (2008). Prelude to a division. Annu. Rev. Cell Dev. Biol. 24, 397-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese, N., Brambillasca, S. and Colombo, S. (2007). How tails guide tail-anchored proteins to their destinations. Curr. Opin. Cell Biol. 19, 368-375. [DOI] [PubMed] [Google Scholar]
- Bruusgaard, J. C., Liestol, K., Ekmark, M., Kollstad, K. and Gundersen, K. (2003). Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 551, 467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupp, J. M., Martin, A. E., Stensrud, E. S. and Jaspersen, S. L. (2007). Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J. Cell Biol. 179, 845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige, Y., Tsutsumi, C., Yamane, M., Okamasa, K., Haraguchi, T. and Hiraoka, Y. (2006). Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125, 59-69. [DOI] [PubMed] [Google Scholar]
- Chikashige, Y., Haraguchi, T. and Hiraoka, Y. (2007). Another way to move chromosomes. Chromosoma 116, 497-505. [DOI] [PubMed] [Google Scholar]
- Conrad, M. N., Lee, C. Y., Wilkerson, J. L. and Dresser, M. E. (2007). MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 104, 8863-8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, M. N., Lee, C. Y., Chao, G., Shinohara, M., Kosaka, H., Shinohara, A., Conchello, J. A. and Dresser, M. E. (2008). Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133, 1175-1187. [DOI] [PubMed] [Google Scholar]
- Cottrell, J. R., Borok, E., Horvath, T. L. and Nedivi, E. (2004). CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron 44, 677-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp, M. and Burke, B. (2008). The nuclear envelope as an integrator of nuclear and cytoplasmic architecture. FEBS Lett. 582, 2023-2032. [DOI] [PubMed] [Google Scholar]
- Crisp, M., Liu, Q., Roux, K., Rattner, J. B., Shanahan, C., Burke, B., Stahl, P. D. and Hodzic, D. (2006). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene, F., Wehman, A. M., Link, B. A. and Baier, H. (2008). Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell 134, 1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X., Xu, R., Yu, J., Xu, T., Zhuang, Y. and Han, M. (2007). SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863-872. [DOI] [PubMed] [Google Scholar]
- Eisen, G. (1900). The spermatogenesis of batrachoseps. J. Morphol. 18, 1-131. [Google Scholar]
- Fischer, J. A., Acosta, S., Kenny, A., Cater, C., Robinson, C. and Hook, J. (2004). Drosophila klarsicht has distinct subcellular localization domains for nuclear envelope and microtubule localization in the eye. Genetics 168, 1385-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, R. (1999). Nuclear movement in filamentous fungi. FEMS Microbiol. Rev. 23, 39-68. [DOI] [PubMed] [Google Scholar]
- Fridkin, A., Mills, E., Margalit, A., Neufeld, E., Lee, K. K., Feinstein, N., Cohen, M., Wilson, K. L. and Gruenbaum, Y. (2004). Matefin, a Caenorhabditis elegans germ line-specific SUN-domain nuclear membrane protein, is essential for early embryonic and germ cell development. Proc. Natl. Acad. Sci. USA 101, 6987-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieni, R. S. and Hendzel, M. J. (2008). Mechanotransduction from the ECM to the genome: are the pieces now in place? J. Cell Biochem. 104, 1964-1987. [DOI] [PubMed] [Google Scholar]
- Gomes, E. R., Jani, S. and Gundersen, G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451-463. [DOI] [PubMed] [Google Scholar]
- Gough, L. L., Fan, J., Chu, S., Winnick, S. and Beck, K. A. (2003). Golgi localization of Syne-1. Mol. Biol. Cell 14, 2410-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, R. M., Starr, D. A., Ackerman, G. L., Sanes, J. R. and Han, M. (2005). Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 102, 4359-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis, F., Dupre, N., Dion, P., Fox, M. A., Laurent, S., Verreault, S., Sanes, J. R., Bouchard, J. P. and Rouleau, G. A. (2007). Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat. Genet. 39, 80-85. [DOI] [PubMed] [Google Scholar]
- Gross, S. P., Welte, M. A., Block, S. M. and Wieschaus, E. F. (2000). Dynein-mediated cargo transport in vivo: a switch controls travel distance. J. Cell Biol. 148, 945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum, Y., Margalit, A., Goldman, R. D., Shumaker, D. K. and Wilson, K. L. (2005). The nuclear lamina comes of age. Nat. Rev. Mol. Cell. Biol. 6, 21-31. [DOI] [PubMed] [Google Scholar]
- Guo, Y., Jangi, S. and Welte, M. A. (2005). Organelle-specific control of intracellular transport: distinctly targeted isoforms of the regulator klar. Mol. Biol. Cell 16, 1406-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I. and Yanmagida, M. (1995). The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129, 1033-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, F., Lloyd, D. J., Smallwood, D. T., Dent, C. L., Shanahan, C. M., Fry, A. M., Trembath, R. C. and Shackleton, S. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, L., Golubovskaya, I. and Cande, W. Z. (2004). A bouquet of chromosomes. J. Cell Sci. 117, 4025-4032. [DOI] [PubMed] [Google Scholar]
- Hodzic, D. M., Yeater, D. B., Bengtsson, L., Otto, H. and Stahl, P. D. (2004). Sun2 is a novel mammalian inner nuclear membrane protein. J. Biol. Chem. 279, 25805-25812. [DOI] [PubMed] [Google Scholar]
- Jaspersen, S. L., Martin, A. E., Glazko, G., Giddings, T. H., Jr, Morgan, G., Mushegian, A. and Winey, M. (2006). The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J. Cell Biol. 174, 665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbfleisch, T., Cambon, A. and Wattenberg, B. W. (2007). A bioinformatics approach to identifying tail-anchored proteins in the human genome. Traffic 8, 1687-1694. [DOI] [PubMed] [Google Scholar]
- Kandert, S., Luke, Y., Kleinhenz, T., Neumann, S., Lu, W., Jaeger, V. M., Munck, M., Wehnert, M., Muller, C. R., Zhou, Z. et al. (2007). Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum. Mol. Genet. 16, 2944-2959. [DOI] [PubMed] [Google Scholar]
- Ketema, M., Wilhelmsen, K., Kuikman, I., Janssen, H., Hodzic, D. and Sonnenberg, A. (2007). Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J. Cell Sci. 120, 3384-3394. [DOI] [PubMed] [Google Scholar]
- King, M. C., Drivas, T. G. and Blobel, G. (2008). A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell 134, 427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszul, R., Kim, K. P., Prentiss, M., Kleckner, N. and Kameoka, S. (2008). Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133, 1188-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracklauer, M. P., Banks, S. M. L., Xie, X., Wu, Y. and Fischer, J. A. (2007). Drosophila klaroid encodes a SUN domain protein required for klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly 1, 75-85. [DOI] [PubMed] [Google Scholar]
- Lambert de Rouvroit, C. and Goffinet, A. M. (2001). Neuronal migration. Mech. Dev. 105, 47-56. [DOI] [PubMed] [Google Scholar]
- Lee, K. K., Starr, D. A., Cohen, M., Liu, J., Han, M., Wilson, K. L. and Gruenbaum, Y. (2002). Lamin-dependent localization of UNC-84, a protein required for nuclear migration in C. elegans. Mol. Biol. Cell 13, 892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, W., Gotzmann, J., Sironi, L., Jaeger, V. M., Schneider, M., Luke, Y., Uhlen, M., Szigyarto, C. A., Brachner, A., Ellenberg, J. et al. (2008). Sun1 forms immobile macromolecular assemblies at the nuclear envelope. Biochim. Biophys. Acta 1783, 2415-2426. [DOI] [PubMed] [Google Scholar]
- Malone, C. J., Fixsen, W. D., Horvitz, H. R. and Han, M. (1999). UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126, 3171-3181. [DOI] [PubMed] [Google Scholar]
- Malone, C. J., Misner, L., Le Bot, N., Tsai, M. C., Campbell, J. M., Ahringer, J. and White, J. G. (2003). The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 115, 825-836. [DOI] [PubMed] [Google Scholar]
- Mans, B. J., Anantharaman, V., Aravind, L. and Koonin, E. V. (2004). Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle 3, 1612-1637. [DOI] [PubMed] [Google Scholar]
- McGee, M. D., Rillo, R., Anderson, A. S. and Starr, D. A. (2006). UNC-83 is a KASH protein required for nuclear migration and is recruited to the outer nuclear membrane by a physical interaction with the SUN protein UNC-84. Mol. Biol. Cell 17, 1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, F., Kurabayashi, A., Tange, Y., Okazaki, K., Shimanuki, M. and Niwa, O. (2004). Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol. Genet. Genomics 270, 449-461. [DOI] [PubMed] [Google Scholar]
- Mislow, J. M., Kim, M. S., Davis, D. B. and McNally, E. M. (2002). Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C. J. Cell Sci. 115, 61-70. [DOI] [PubMed] [Google Scholar]
- Morris, N. R. (2003). Nuclear positioning: the means is at the ends. Curr. Opin. Cell Biol. 15, 54-59. [DOI] [PubMed] [Google Scholar]
- Mosley-Bishop, K. L., Li, Q., Patterson, L. and Fischer, J. A. (1999). Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr. Biol. 9, 1211-1220. [DOI] [PubMed] [Google Scholar]
- Mounkes, L., Kozlov, S., Burke, B. and Stewart, C. L. (2003). The laminopathies: nuclear structure meets disease. Curr. Opin. Genet. Dev. 13, 223-230. [DOI] [PubMed] [Google Scholar]
- Nery, F. C., Zeng, J., Niland, B. P., Hewett, J., Farley, J., Irimia, D., Li, Y., Wiche, G., Sonnenberg, A. and Breakefield, X. O. (2008). TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J. Cell Sci. 121, 3476-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmakumar, V. C., Abraham, S., Braune, S., Noegel, A. A., Tunggal, B., Karakesisoglou, I. and Korenbaum, E. (2004). Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp. Cell Res. 295, 330-339. [DOI] [PubMed] [Google Scholar]
- Padmakumar, V. C., Libotte, T., Lu, W., Zaim, H., Abraham, S., Noegel, A. A., Gotzmann, J., Foisner, R. and Karakesisoglou, I. (2005). The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118, 3419-3430. [DOI] [PubMed] [Google Scholar]
- Patterson, K., Molofsky, A. B., Robinson, C., Acosta, S., Cater, C. and Fischer, J. A. (2004). The functions of klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol. Biol. Cell 15, 600-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, C. G. and Bloom, K. (2004). Dynamic microtubules lead the way for spindle positioning. Nat. Rev. Mol. Cell. Biol. 5, 481-492. [DOI] [PubMed] [Google Scholar]
- Penkner, A., Tang, L., Novatchkova, M., Ladurner, M., Fridkin, A., Gruenbaum, Y., Schweizer, D., Loidl, J. and Jantsch, V. (2007). The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell 12, 873-885. [DOI] [PubMed] [Google Scholar]
- Phillips, C. M., Wong, C., Bhalla, N., Carlton, P. M., Weiser, P., Meneely, P. M. and Dernburg, A. F. (2005). HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123, 1051-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston, E., Lu, Z., Biscocho, N., Soumaka, E., Mavroidis, M., Prats, C., Lomo, T., Capetanaki, Y. and Ploug, T. (2006). Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J. Cell Physiol. 209, 874-882. [DOI] [PubMed] [Google Scholar]
- Reinsch, S. and Gonczy, P. (1998). Mechanisms of nuclear positioning. J. Cell Sci. 111, 2283-2295. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Hasson, Y., Renert-Pasca, M. and Volk, T. (1996). A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech. Dev. 60, 83-94. [DOI] [PubMed] [Google Scholar]
- Sauer, F. C. (1935). Mitosis in the neural tube. J. Comp. Neurol. 62, 377-405. [Google Scholar]
- Schaar, B. T. and McConnell, S. K. (2005). Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. USA 102, 13652-13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh, L., Carlton, P. M., Haase, S., Shao, L., Winoto, L., Kner, P., Burke, B., Cardoso, M. C., Agard, D. A., Gustafsson, M. G. et al. (2008). Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan, H. (2001). A bouquet makes ends meet. Nat. Rev. Mol. Cell. Biol. 2, 621-627. [DOI] [PubMed] [Google Scholar]
- Schirmer, E. C., Florens, L., Guan, T., Yates, J. R., 3rd and Gerace, L. (2003). Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301, 1380-1382. [DOI] [PubMed] [Google Scholar]
- Schmitt, J., Benavente, R., Hodzic, D., Hoog, C., Stewart, C. L. and Alsheimer, M. (2007). Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc. Natl. Acad. Sci. USA. 104, 7426-7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner, M., Metz, J., Schmid, V., Denic, V., Rakwalska, M., Schmitt, H. D., Schwappach, B. and Weissman, J. S. (2008). The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, X., Tarnasky, H. A., Lee, J. P., Oko, R. and van der Hoorn, F. A. (1999). Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev. Biol. 211, 109-123. [DOI] [PubMed] [Google Scholar]
- Starr, D. A. (2007). Communication between the cytoskeleton and the nuclear envelope to position the nucleus. Mol. Biosyst. 3, 583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, D. A. and Han, M. (2002). Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406-409. [DOI] [PubMed] [Google Scholar]
- Starr, D. A. and Han, M. (2003). ANChors away: an actin based mechanism of nuclear positioning. J. Cell Sci. 116, 211-216. [DOI] [PubMed] [Google Scholar]
- Starr, D. A. and Fischer, J. A. (2005). KASH 'n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. BioEssays 27, 1136-1146. [DOI] [PubMed] [Google Scholar]
- Starr, D. A. and Han, M. (2005). A genetic approach to study the role of nuclear envelope components in nuclear positioning. Novartis Found. Symp. 264, 208-219; discussion 219-230. [PubMed] [Google Scholar]
- Starr, D. A., Hermann, G. J., Malone, C. J., Fixsen, W., Priess, J. R., Horvitz, H. R. and Han, M. (2001). unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development 128, 5039-5050. [DOI] [PubMed] [Google Scholar]
- Stefanovic, S. and Hegde, R. S. (2007). Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128, 1147-1159. [DOI] [PubMed] [Google Scholar]
- Stewart, C. L., Roux, K. J. and Burke, B. (2007). Blurring the boundary: the nuclear envelope extends its reach. Science 318, 1408-1412. [DOI] [PubMed] [Google Scholar]
- Stewart-Hutchinson, P. J., Hale, C. M., Wirtz, D. and Hodzic, D. (2008). Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 314, 1892-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan, A., Nguyen, T. and Suter, B. (1999). Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat. Cell Biol. 1, 444-449. [DOI] [PubMed] [Google Scholar]
- Technau, M. and Roth, S. (2008). The Drosophila KASH domain proteins Msp-300 and Klarsicht and the SUN domain protein Klaroid have no essential function during oogenesis. Fly 2, 82-91. [DOI] [PubMed] [Google Scholar]
- Toivola, D. M., Tao, G. Z., Habtezion, A., Liao, J. and Omary, M. B. (2005). Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15, 608-617. [DOI] [PubMed] [Google Scholar]
- Tran, P. T., Marsh, L., Doye, V., Inoue, S. and Chang, F. (2001). A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 153, 397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, J. W., Bremner, K. H. and Vallee, R. B. (2007). Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970-979. [DOI] [PubMed] [Google Scholar]
- Tsujikawa, M., Omori, Y., Biyanwila, J. and Malicki, J. (2007). Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc. Natl. Acad. Sci. USA 104, 14819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur, Y. B., Margalit, A., Melamed-Book, N. and Gruenbaum, Y. (2006a). Matefin/SUN-1 is a nuclear envelope receptor for CED-4 during Caenorhabditis elegans apoptosis. Proc. Natl. Acad. Sci. USA 103, 13397-13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur, Y. B., Wilson, K. L. and Gruenbaum, Y. (2006b). SUN-domain proteins: `Velcro' that links the nucleoskeleton to the cytoskeleton. Nat. Rev. Mol. Cell. Biol. 7, 782-788. [DOI] [PubMed] [Google Scholar]
- Warren, D. T., Zhang, Q., Weissberg, P. L. and Shanahan, C. M. (2005). Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev. Mol. Med. 7, 1-15. [DOI] [PubMed] [Google Scholar]
- Welte, M. A. (2004). Bidirectional transport along microtubules. Curr. Biol. 14, R525-R537. [DOI] [PubMed] [Google Scholar]
- Welte, M. A., Gross, S. P., Postner, M., Block, S. M. and Wieschaus, E. F. (1998). Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547-557. [DOI] [PubMed] [Google Scholar]
- Wheeler, M. A., Davies, J. D., Zhang, Q., Emerson, L. J., Hunt, J., Shanahan, C. M. and Ellis, J. A. (2007). Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp. Cell Res. 313, 2845-2857. [DOI] [PubMed] [Google Scholar]
- Whited, J. L., Cassell, A., Brouillette, M. and Garrity, P. A. (2004). Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development 131, 4677-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen, K., Litjens, S. H., Kuikman, I., Tshimbalanga, N., Janssen, H., van den Bout, I., Raymond, K. and Sonnenberg, A. (2005). Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 171, 799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen, K., Ketema, M., Truong, H. and Sonnenberg, A. (2006). KASH-domain proteins in nuclear migration, anchorage and other processes. J. Cell Sci. 119, 5021-5029. [DOI] [PubMed] [Google Scholar]
- Worman, H. J. and Gundersen, G. G. (2006). Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 16, 67-69. [DOI] [PubMed] [Google Scholar]
- Worman, H. J. and Bonne, G. (2007). “Laminopathies”: a wide spectrum of human diseases. Exp. Cell Res. 313, 2121-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, X. and Fischer, R. (2004). Nuclear migration and positioning in filamentous fungi. Fungal Genet. Biol. 41, 411-419. [DOI] [PubMed] [Google Scholar]
- Xie, X. and Fischer, J. A. (2008). On the roles of the Drosophila KASH domain proteins Msp-300 and Klarsicht. Fly 2, 74-81. [DOI] [PubMed] [Google Scholar]
- Xie, Z., Moy, L. Y., Sanada, K., Zhou, Y., Buchman, J. J. and Tsai, L. H. (2007). Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron 56, 79-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, H., Rivero, F., Euteneuer, U., Mondal, S., Mana-Capelli, S., Larochelle, D., Vogel, A., Gassen, B. and Noegel, A. A. (2008). Dictyostelium sun-1 connects the centrosome to chromatin and ensures genome stability. Traffic 9, 708-724. [DOI] [PubMed] [Google Scholar]
- Yu, J., Starr, D. A., Wu, X., Parkhurst, S. M., Zhuang, Y., Xu, T., Xu, R. and Han, M. (2006). The KASH domain protein MSP-300 plays an essential role in nuclear anchoring during Drosophila oogenesis. Dev. Biol. 289, 336-345. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Skepper, J. N., Yang, F., Davies, J. D., Hegyi, L., Roberts, R. G., Weissberg, P. L., Ellis, J. A. and Shanahan, C. M. (2001). Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 114, 4485-4498. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Ragnauth, C., Greener, M. J., Shanahan, C. M. and Roberts, R. G. (2002). The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80, 473-481. [PubMed] [Google Scholar]
- Zhang, Q., Bethmann, C., Worth, N. F., Davies, J. D., Wasner, C., Feuer, A., Ragnauth, C. D., Yi, Q., Mellad, J. A., Warren, D. T. et al. (2007a). Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, 2816-2833. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Xu, R., Zhu, B., Yang, X., Ding, X., Duan, S., Xu, T., Zhuang, Y. and Han, M. (2007b). Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development 134, 901-908. [DOI] [PubMed] [Google Scholar]
- Zhen, Y. Y., Libotte, T., Munck, M., Noegel, A. A. and Korenbaum, E. (2002). NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell Sci. 115, 3207-3222. [DOI] [PubMed] [Google Scholar]
- Zickler, D. and Kleckner, N. (1998). The leptotene-zygotene transition of meiosis. Annu. Rev. Genet. 32, 619-697. [DOI] [PubMed] [Google Scholar]