Summary
Elucidating the mechanisms by which eukaryotic cells coordinate environmental signals with intracellular `fate' decisions, such as apoptosis, remains one of the important challenges facing cell biologists. It has recently emerged that the dynamic nature of the actin cytoskeleton is an important factor in the linkage of sensation of extracellular stimuli to signalling mechanisms that regulate programmed cell death. In yeast, actin has been shown to play a role in the regulation of apoptosis as cells prepare themselves for quiescence in the face of nutritional exhaustion, by facilitating the shutdown of Ras-cAMP-PKA pathway activity. Here, we demonstrate that the loss of Whi2p function, a protein known to influence cell cycle exit under conditions of nutritional stress, leads to cell death in yeast that displays the hallmarks of actin-mediated apoptosis. We show that actin-mediated apoptosis occurs as a result of inappropriate Ras-cAMP-PKA activity in Δwhi2 cells. Cells lacking Whi2p function exhibit an aberrant accumulation of activated Ras2 at the mitochondria in response to nutritional depletion. This study provides evidence that the shutdown of cAMP-PKA signalling activity in wild-type cells involves Whi2p-dependent targeting of Ras2p to the vacuole for proteolysis. We also demonstrate for the first time that Whi2p-dependent regulation of cAMP-PKA signalling plays a physiological role in the differentiation of yeast colonies by facilitating elaboration of distinct zones of cell death.
Keywords: PKA, ROS, Ras, Actin, Mitochondria, Yeast
Introduction
The coordination of proliferation and stress response with nutritional availability is a central requirement of all cells. In yeast cells grown in liquid culture in the presence of glucose this is illustrated by a predictable pattern of growth. Initially, cells divide during a period of logarithmic growth, at which stage energy is produced preferentially via the fermentation of glucose. At this stage, stress response mechanisms and respiration are actively repressed. Log phase growth is followed by a dramatic series of events that occurs as cells sense the exhaustion of their nutritional supply, termed the diauxic shift. Changes that occur during the diauxic shift include a reduction in protein production and the elevation of stress response pathway activity (Werner-Washburne et al., 1993). In the postdiauxic phase of growth, cells resume growth at a slower rate and metabolise nonfermentable carbon sources. Finally, cultures enter the stationary, or postmitotic phase of growth, during which growth is arrested and cells become quiescent (Gray et al., 2004; Herman, 2002).
In the budding yeast Saccharomyces cerevisiae, the Ras-cAMP-PKA signalling pathway is an essential component required by the culture to navigate through the diauxic shift successfully. During this transition, the Ras-cAMP-PKA pathway must be shut down to allow cell cycle exit and a full stress response (Reinders et al., 1998). The production of cAMP is catalysed by adenylate cyclase via two apparently independent routes. The first route signals through the G-protein-coupled receptor, Gpr1p, and is involved in a transient burst of cAMP-PKA activity in response to glucose addition (Thevelein et al., 2005). A second mechanism employs the activation of Ras and adenylyl cyclase-associated proteins (Srv2p/CAP), and is involved in the response to glucose depletion (Toda et al., 1985). The action of adenylate cyclase and subsequent elevation of cAMP leads to the activation of protein kinase A (PKA) (Thevelein, 1992). Three A kinase catalytic subunits encoded by TPK1, TPK2 and TPK3 are found in S. cerevisiae. These subunits do display functional redundancy in response to activation by cAMP. However, unique activities have also been demonstrated for particular PKA subunits (Robertson et al., 2000; Robertson and Fink, 1998). An example of this was recently demonstrated when the loss of Tpk3p resulted in a significant reduction in respiratory function (Chevtzoff et al., 2005).
Ras pathway activity is an important component in the regulation of cellular longevity in eukaryotic cells. The expression of a constitutively active form of Ras has been shown to result in the accumulation of reactive oxygen species (ROS) and a reduction in the life span in yeast and mammalian cells (Heeren et al., 2004; Hlavata et al., 2008; Serrano et al., 1997). The ability of the Ras-cAMP-PKA pathway to regulate mitochondrial function and ROS production also appears to have been harnessed by a fungal apoptotic response. This has been demonstrated in both the fungal pathogen Candida albicans (Phillips et al., 2006; Phillips et al., 2003) and the budding yeast S. cerevisiae (Gourlay and Ayscough, 2005; Gourlay and Ayscough, 2006). The Ras-cAMP-PKA pathway therefore provides a good potential link from environmental sensing to the processes of apoptosis and ageing in yeast.
Recent evidence has demonstrated that the actin cytoskeleton plays an important role in regulating yeast cell death during the diauxic shift via interactions with the Ras-cAMP-PKA signalling pathway (Gourlay and Ayscough, 2005; Gourlay and Ayscough, 2006). Mutations in the actin regulatory genes SLA1 or END3 lead to the retardation of actin dynamics and the formation of large aggregates of filamentous actin as cells enter the diauxic shift (Gourlay and Ayscough, 2005). The formation of stable actin structures at this stage of growth has been shown to trigger hyperactivation of Ras signalling (Gourlay and Ayscough, 2006). The increase in Ras activity leads to significant and inappropriate elevation of the secondary messenger cAMP, which triggers a PKA-dependent loss of mitochondrial function (Gourlay and Ayscough, 2006). Aberrant mitochondrial function in actin aggregate-containing cells leads to the production of large quantities of ROS and cell death.
Aggregates of actin have been shown to arise in cells lacking the WHI2 gene, which plays a role in linking proliferation and stress response to environmental sensing mechanisms (Care et al., 2004; Radcliffe et al., 1997b). The loss of Whi2p function gives rise to a number of phenotypes including a failure to elicit an appropriate stress response, the continuation of cell cycle activity preventing entry into G0, and a weak activation of storage carbohydrate production (Mountain and Sudbery, 1990a; Mountain and Sudbery, 1990b; Radcliffe et al., 1997a; Saul and Sudbery, 1985). Whi2p is known to function within the general stress response pathway as it is required for full activation of genes under the control of stress response elements (STRE). Whi2p has been shown to physically interact with the redundant C-terminal domain plasma membrane phosphatases, Psr1p and Psr2p (Kaida et al., 2002). It has been proposed that Whi2p acts through Psr1p and Psr2p to elicit a full response to environmental stresses, as Δpsr1Δpsr2 cells present a similar set of phenotypes to those found in Δwhi2 cells (Kaida et al., 2002).
Our results demonstrated that defects in actin organisation can trigger apoptosis in Δwhi2 cells, and that this process occurs as a result of Ras-cAMP-PKA activity. The formation of actin aggregates, mitochondrial dysfunction and ROS build-up, as well as cell viability, could be rescued by treatment with the actin depolymerising drug latrunculin-A. Overexpression of PDE2 or deletion of the PKA subunit TPK3, was also sufficient to prevent actin-mediated apoptosis (ActMAp) occurring in these cells. Our data argue that the localisation of activated Ras2p to the mitochondria during nutritional depletion, leads to unregulated cell killing in Δwhi2 cultures. We present evidence to suggest that the regulation of Ras-cAMP-PKA activity plays an important role in linking environmental stress sensing to cell death regulation and colony patterning in budding yeast.
Results
Loss of Whi2p function leads to actin aggregation, nuclear fragmentation and loss of mitochondrial DNA during diauxic shift
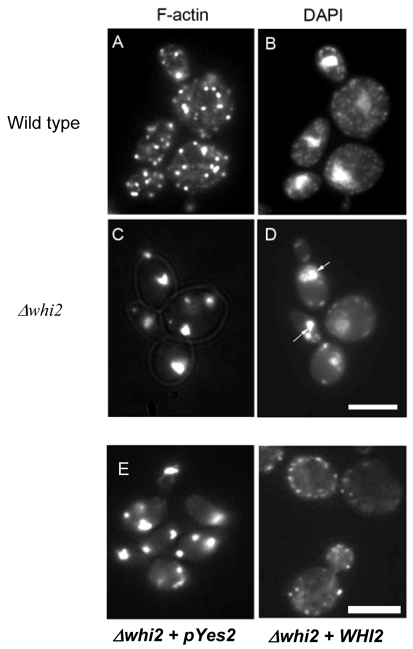
It had been reported previously that the actin cytoskeleton of Δwhi2 cells appeared to be aggregated after 2 days of growth in rich media containing glucose (Care et al., 2004). We hypothesise that the timing of actin aggregate formation is critical for the elaboration of its toxicity. We therefore assessed at what stage Δwhi2 cells started to accumulate cytoplasmic aggregates of filamentous actin (F-actin). Therefore wild-type and Δwhi2 cells were cultured in rich YPAD medium, as described in Materials and Methods. Samples were processed for rhodamine-phalloidin staining during the log phase of growth, after 24 hours when cells enter the diauxic phase, and after 2 days when cell numbers had stopped increasing (early stationary phase). During the log phase the actin cytoskeleton appeared normal in wild-type and Δwhi2 cells, displaying a typical pattern of cytoplasmic cables and cortical patches (data not shown). In wild-type cells after 24 hours of growth, the actin cytoskeleton appeared as punctate cortical patches and disorganised actin cables throughout the cell (Fig. 1A). At the same time point, however, Δwhi2 cells exhibited severe aggregation of the F-actin cytoskeleton (Fig. 1C). These aggregates were also observed in samples taken after 2 days of growth. This suggests that actin aggregates appear in Δwhi2 cells at an earlier time point than has previously been described. We also co-stained samples with DAPI to highlight nuclear and mitochondrial DNA (mtDNA) content. This revealed that Δwhi2 cells displayed far fewer mtDNA than was observed in the wild type (Fig. 1B,D). In addition, the nuclear DNA in Δwhi2 cells was highly fragmented (Fig. 1D), which is a strong indicator of apoptosis. In order to confirm that the loss of Whi2p function was responsible for the formation of actin aggregates we introduced an expression vector carrying the full-length WHI2 gene. The presence of an empty control vector still led to aggregation in Δwhi2 cells; however, the restored expression of WHI2 clearly prevented the formation of large F-actin clumps (Fig. 1E).
Fig. 1.
Actin aggregation and mitochondrial DNA loss in Δwhi2 cells. Wild-type (A,B) and Δwhi2 (C,D) cells were grown for 24 hours to diauxic shift in YPAD media before being processed for F-actin and DNA staining using rhodamine-phalloidin and DAPI, respectively. Fragmented genomic DNA is highlighted by arrows (D). As a control experiment Δwhi2 cells containing either an empty pYes2 expression vector, or pYes2 + WHI2 were grown for 24 hours to diauxic shift and processed for F-actin staining (E). Scale bars: 10 μm.
Aberrant mitochondrial function and ROS accumulation are observed in Δwhi2 cells
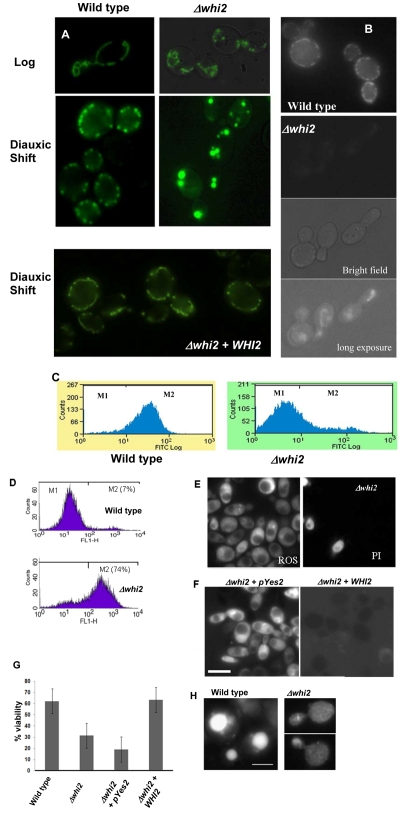
The formation of actin aggregates during the diauxic shift was shown to demarcate a cascade of events described as actin-mediated apoptosis (ActMAp) in cells lacking the actin regulatory function of the Sla1p-End3p proteins (Gourlay and Ayscough, 2005; Gourlay and Ayscough, 2006). ActMAp in yeast leads to the loss of mitochondrial membrane potential, an increase in ROS level and loss of viability. We therefore set out to establish whether ActMAp was occurring in Δwhi2 cells. First, we examined the mitochondrial morphology using a targeted GFP probe (described in Materials and Methods). In wild-type cells grown to late log phase (18 hours of growth), the mitochondria in dividing cells appeared as a cortical network of punctate and tubular membranes that spanned mother and daughter cells (Fig. 2A). However, in cells lacking Whi2p, the mitochondrial membranes appeared as highly fragmented structures when compared with the wild type (Fig. 2A). Inheritance of mitochondrial membranes did not appear to be affected and could be found in the buds of all dividing cells (data not shown). After 24 hours of growth, as cells entered the diauxic shift, the mitochondria typically appeared fragmented in wild-type cells but were still found at the cell cortex and were evenly distributed (Fig. 2A). At the same stage, Δwhi2 mitochondria appeared as ball- or grape-like structures that were larger and far fewer in number those found in wild-type cells (Fig. 2A). The loss of Whi2p function was clearly responsible for the aberrant mitochondrial morphologies observed as the re-expression of WHI2 clearly restored a wild-type morphology (Fig. 2A).
Fig. 2.
Δwhi2 cells possess dysfunctional mitochondria, accumulate ROS and exhibit markers of apoptosis. We grew wild-type and Δwhi2 cells that expressed a mitochondria-targeted GFP protein for 18 hours to late log phase and 24 hours to diauxic shift in YPAD media and visualised by fluorescence microscopy. As a control experiment WHI2 was re-introduced into Δwhi2 cells on a pYes2 plasmid, and mitochondria were visualised after 24 hours of growth to diauxic shift. Mitochondria were visualised using a targeted GFP protein as above (A). Mitochondrial membrane potential was assessed in wild-type and Δwhi2 cells grown to diauxic shift using the fluorescent indicator dye DiOC6. The accumulation of DiOC6 at the mitochondria is dependent upon the presence of a membrane potential. Cells were visualised by fluoresence microscopy (B); however as Δwhi2 cells have reduced mitochondrial membrane potential and so fail to take up much dye the cells are also shown as bright field and long exposure fluorescence images (B). DiOC6 uptake was also assessed within wild-type and Δwhi2 cells cultured for 24 hours to diauxic shift using flow cytometry as described in Materials and Methods (C). ROS accumulation was assayed by flow cytometry in wild-type and Δwhi2 cells grown to diauxic shift using the dye H2DCF-DA (D). The same cells were co-stained with propidium iodide and H2DCF-DA to assess the level of necrosis in Δwhi2 cultures (E). As a control experiment, Δwhi2 cells containing either an empty pYes2 expression vector or pYes2 + WHI2 were grown for 24 hours to diauxic shift and ROS production assessed using H2DCF-DA (F). The effect of deletion of the WHI2 gene on culture viability after growth to diauxic shift was assessed. The effect of re-introducing the WHI2 gene on culture viability was also determined (G). Apoptotic yeast cells often exhibit a loss of vacuolar membrane integrity, which can be visualised using a GFP-Pep4 fusion protein. In wild-type cells GFP-Pep4 is retained in the vacuole whereas in cells that have lost vacuolar membrane integrity it is found distributed throughout the cell (H). Scale bars: 10 μm.
Mitochondrial function was further assessed using the membrane-potential-sensitive stain DiOC6. At low concentrations this dye specifically stains the mitochondrial membranes in a manner that depends on them exhibiting a membrane potential. Wild-type cells displayed a similar pattern of staining to that observed with the targeted GFP molecule (Fig. 2B) indicating that these mitochondria were healthy and possessed a membrane potential. By contrast, the mitochondrial membranes present in Δwhi2 cells could not be visualised with DiOC6 under the same conditions (Fig. 2B). However, the mitochondria of Δwhi2 cells could be visualised with DiOC6 by using very long exposure times, and appeared as rounded, highly fragmented, structures akin to those seen using the targeted GFP molecule (Fig. 2B). These data demonstrate that Δwhi2 mitochondria exhibit a reduced membrane potential. Observations were confirmed using flow cytometry to assess mitochondrial membrane potential in wild-type and Δwhi2 cell populations (Fig. 2C). In these experiments a dramatic peak shift was observed in Δwhi2 cells when compared with the wild type, indicating a drop in fluorescence and confirming a lack of mitochondrial DiOC6 accumulation.
Our previous studies have linked actin aggregation to the production of ROS from the mitochondria (Gourlay and Ayscough, 2005; Gourlay and Ayscough, 2006). We therefore assessed ROS accumulation in wild-type and Δwhi2 cells grown for 24 hours to diauxic shift. Earlier studies have shown that wild-type yeast cells accumulate ROS in a small proportion of the population. In line with this we observed that approximately 7% of cells appeared in the M2, high ROS, fraction when assessed by flow cytometry (Fig. 2D). In Δwhi2 cells, there was a dramatic shift in the population of cells displaying ROS accumulation, with 74% appearing in the M2 fraction (Fig. 2D). Cell-death-displaying markers of apoptosis should also be accompanied by the maintenance of plasma membrane integrity, suggesting that cells have died from within as opposed to suffering a necrotic fate. This is typically assessed by the ability of cells to take up the dye propidium iodide, which will only enter cells with compromised plasma membranes. We therefore co-labelled Δwhi2 cells grown for 24 hours to diauxic shift with the ROS indicator H2-DCFDA and propidium iodide before observation using fluorescence microscopy (Fig. 2E). The results clearly showed that only a small number of ROS-producing Δwhi2 cells take up propidium iodide, indicating that the majority of Δwhi2 cells showed high ROS and intact plasma membranes. In order to establish whether the loss of Whi2p function led to ROS production we stained Δwhi2 cells that contained a vector to restore WHI2 expression and were grown for 24 hours to diauxic shift, with H2-DCFDA (Fig. 2F). Although Δwhi2 cells containing a control empty vector still fluoresced brightly, and hence contained high levels of ROS, the re-expression of WHI2 gave rise to cells with a low fluorescence signal, indicating low ROS production (Fig. 2F). We then assessed the proportion of viable cells within wild-type and Δwhi2 cell populations in cultures grown for 24 hours to the diauxic shift phase of growth. As expected there was a significant loss of viability observed in Δwhi2 cell populations when compared with the wild type (Fig. 2G). The expression of WHI2 from plasmid was sufficient to restore viability within the culture to wild-type levels, confirming loss of Whi2p function as the root cause of cell death (Fig. 2G).
Apoptosis in yeast is often associated with the loss of vacuolar membrane integrity, which can be assessed using a fluorescently labelled vacuolar-specific protease such as Pep4p. When the vacuolar membrane is compromised during the apoptotic process, Pep4-GFP can be seen to leave the vacuole and diffuse into the cytoplasm. We assessed vacuolar membrane integrity in wild-type and Δwhi2 cells using this approach (Fig. 2H). In wild-type cells, Pep4-GFP was observed exclusively within the vacuolar compartment as expected. However, in Δwhi2 cells the fluorescent protease was found distributed throughout the cytoplasm, indicating a loss of vacuolar membrane integrity.
Apoptosis in Δwhi2 cells is triggered by actin aggregation
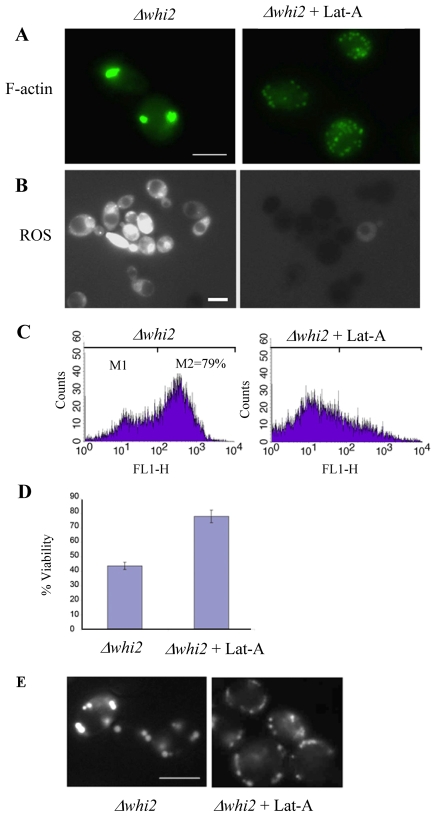
The cell death phenotypes observed in Δwhi2 cells suggest that ActMAp is taking place as these cells navigate the diauxic shift. To test whether actin aggregation is indeed the trigger of these cell death events we made use of the actin monomer-binding drug latrunculin A. Latrunculin A binds to actin monomers with a 1:1 stochiometry, and if used at high doses causes complete, but reversible, depolymerisation of the F-actin cytoskeleton (Ayscough et al., 1997). When used at lower doses, however, this drug can be used to inhibit actin aggregate formation, presumably by slowing the addition of actin monomers to filaments and restoring a steady state between addition and removal of monomers to filaments (Gourlay and Ayscough, 2006). We found that a concentration of 2.6 μM latrunculin A added to the media at time of culture inoculation was sufficient to completely inhibit the formation of actin aggregates at diauxic shift in Δwhi2 cell populations (Fig. 3A). Cells grown in the presence of 2.6 μM latrunculin A reached similar densities to Δwhi2 cultures lacking the drug (data not shown). We then investigated the effect of latrunculin A addition on the accumulation of ROS in Δwhi2 cells. We observed by fluorescence microscopy that the presence of latrunculin A also led to a dramatic reduction in the accumulation of ROS normally observed in Δwhi2 cell populations (Fig. 3B). This result was confirmed using flow cytometry, which showed a significant reduction in the number of cells displaying high fluorescence in the presence of the ROS indicator H2-DCFDA. As expected, the prevention of actin aggregate production during the diauxic shift and the consequential reduction in ROS production led to a significant increase in culture viability when compared with untreated samples (Fig. 3D). In addition, the formation of rounded-up mitochondria observed in Δwhi2 cells, was inhibited by the presence of latrunculin A (Fig. 3E).
Fig. 3.
Inhibition of actin aggregation prevents apoptosis in Δwhi2 cells. Cells lacking Whi2p were grown for 24 hours in YPAD to diauxic shift with or without the presence of the actin monomer-binding drug latrunculin-A. These cells were then prepared for F-actin staining (A) or ROS assessment with H2DCF-DA (B) and visualised using fluorescence microscopy. ROS accumulation was also assayed in these cells using flow cytometry (C). The effect of latrunculin-A presence on Δwhi2 culture viability was assessed after 24 hours of growth (D). Mitochondrial morphology was examined in Δwhi2 cells grown with and without latrunculin-A using a targeted GFP molecule (E). Scale bars: 10 μm.
ActMAp in Δwhi2 cells occurs as a result of Ras-cAMP-PKA pathway activity
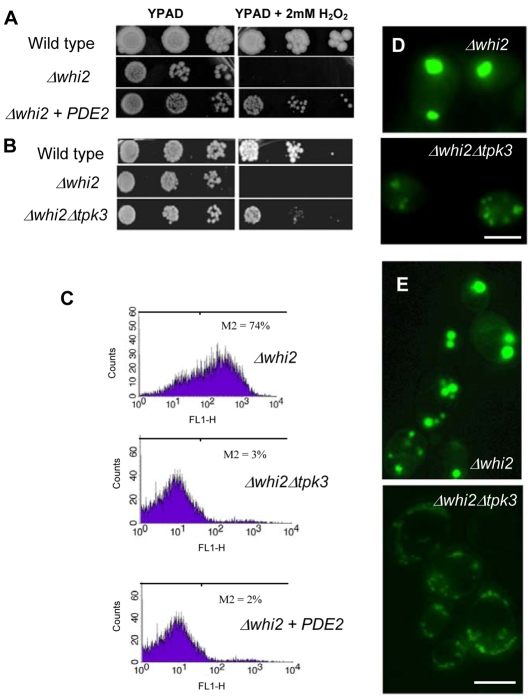
The formation of actin aggregates and the ensuing cell death has been shown to occur as a result of hyperactivation of the Ras-cAMP-PKA signalling cascade (Chevtzoff et al., 2005; Gourlay and Ayscough, 2006). To test whether this is the case in Δwhi2 cell populations, we overexpressed the high affinity cAMP phosphodiesterase PDE2, which functions to regulate Ras-cAMP-PKA activity by hydrolysing the secondary messenger cAMP to AMP. The overexpression of PDE2 was sufficient to rescue sensitivity to H2O2 observed in Δwhi2 cells (Fig. 4A). The synthesis of cAMP leads to the activation of PKA, of which there are three possible subunits in S. cerevisiae, Tpk1p, Tpk2p and Tpk3p. It has been reported that TPK3 plays a major role in regulating mitochondrial function (Chevtzoff et al., 2005) and is necessary for ROS production during ActMAp (Gourlay and Ayscough, 2006). We therefore generated a double mutant strain lacking both WHI2 and TPK3. As was observed for PDE2 overexpression, Δwhi2Δtpk3 cells displayed an increased resistance to H2O2 when compared with Δwhi2 cells (Fig. 4B). As was expected the overexpression of PDE2, or deletion of TPK3 also led to a complete rescue of ROS build-up observed in Δwhi2 cells (Fig. 4C). The re-introduction of TPK3 on a plasmid into Δwhi2Δtpk3 cells was also shown to restore the production of ROS (data not shown). The aggregation of actin was also relieved to some extent but aggregation was still evident (Fig. 4D). This is in line with the probable enhancement of aggregates caused by elevated ROS levels (Dalle-Donne et al., 2001). The formation of enlarged rounded-up mitochondria observed in Δwhi2 cells was also inhibited by the loss of Tpk3p activity (Fig. 4E).
Fig. 4.
ROS production and mitochondrial dysfunction occurs as a result of elevated cAMP-PKA activity in Δwhi2 cells. We examined the effect of overexpressing the high affinity phosphodiesterase PDE2 (A), and deletion of the PKA subunit TPK3 (B), on Δwhi2 sensitivity to 2 mM H2O2 when grown on YPAD agar plates. The effect of PDE2 overexpression and TPK3 deletion on ROS accumulation was also assessed by flow cytometry using H2DCF-DA (C). Further experiments investigated the impact of TPK3 deletion on the accumulation of actin aggregates (D) and mitochondrial morphology (E) observed in Δwhi2 cells grown to diauxic shift. Scale bars: 10 μm.
Whi2p is required for the deactivation and degradation of Ras2p upon nutritional depletion
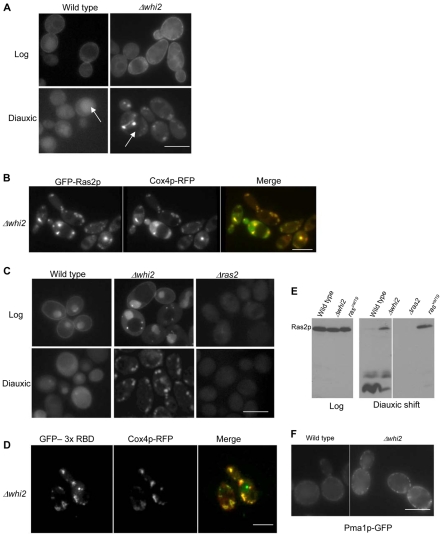
The evidence presented indicates that loss of Whi2p function leads to activation of the Ras-cAMP-PKA signalling cascade as cells enter the diauxic phase of growth. We therefore investigated the effects of loss of Whi2p function on the localisation and activation of Ras2p using a variety of techniques. A GFP-Ras2p fusion protein was used to identify its intracellular localisation during culture. During the log phase of growth, Ras2p was localised predominately to the plasma membrane in wild-type and Δwhi2 cells (Fig. 5A). The fact that Ras2p can localise to the plasma membrane indicates that it receives appropriate post-translational modifications during log phase growth in both wild-type and mutant cells (Dong et al., 2003). In contrast to the log phase staining pattern, GFP-Ras2p was found to be largely cytoplasmic as wild-type cells entered the diauxic shift (Fig. 5A); however, a strong GFP signal was also evident within the vacuole (Fig. 5A, arrow). Surprisingly, upon entry to the diauxic shift GFP-Ras2p appeared to occupy discrete intracellular foci in Δwhi2 cells (Fig. 5A). In addition, the vacuolar GFP signal that was so prevalent in the wild type was not present at all in Δwhi2 cells (Fig. 5A, arrow). The compartmentalised GFP-Ras2p staining pattern observed in Δwhi2 cells appeared similar to the aberrant mitochondrial morphology phenotype described above (Fig. 2A). To investigate this further we coexpressed an RFP-labelled Cox4p fusion protein, which specifically labels the mitochondrial compartment, and GFP-Ras2p in Δwhi2 cells (Fig. 5B). The extensive colocalisation of GFP-Ras2p with Cox4p-RFP clearly demonstrates that GFP-Ras2p localises to the mitochondria in Δwhi2 cells navigating the diauxic shift. These observations demonstrate that the loss of Whi2p function has a profound effect on Ras2p trafficking during the diauxic shift. It is worth noting that the constitutively active RAS2val19 protein was observed, by immunolocalisation, to associate with the plasma membrane and not the mitochondria during both log and diauxic growth (data not shown).
Fig. 5.
Analysis of Ras2p localisation and activation in Δwhi2 cells. GFP-Ras2p was localised in wild-type cells during log and diauxic phases of growth in wild-type and Δwhi2 cells. Arrows indicate the location of the vacuole in these cells (A). GFP-Ras2p and Cox4p-RFP were colocalised in Δwhi2 cells grown for 24 hours to the diauxic phase (B). GTP-bound active Ras proteins were detected using a probe consisting of three Raf 1-binding domains fused to GFP. 3xRBD-GFP localisation was examined in wild-type, Δwhi2 and control Δras2 cells at log and diauxic shift phases of culture (C). 3xRBD-GFP and Cox4p-RFP were colocalised in Δwhi2 cells grown for 24 hours to the diauxic phase (D). Total protein was isolated from wild-type, Δwhi2, Δras2 and RAS2val19 strains at the time points indicated and western blots probed for Ras2p (E). The integral plasma membrane protein Pma1p was localised in wild-type and Δwhi2 cells grown for 24 hours to diauxic shift (F). Scale bars: 10 μm.
It is well reported that the Ras-binding domain (RBD) from the human Raf1 protein binds exclusively to GTP-bound or activated Ras (de Rooij and Bos, 1997). The similarity between mammalian and yeast Ras proteins means that the human RBD can also be used to differentiate between active and inactive Ras in yeast (Colombo et al., 2004; Gourlay and Ayscough, 2006). To determine whether the mitochondria-localised Ras2p was present in an active state we made use of a newly developed construct that expresses GFP fused to three sequential RBD domains. This construct allows the assessment of activated Ras in live cell imaging experiments (Augsten et al., 2006). As expected the GFP-RBD probe was localised to the plasma membrane in wild-type log cells (Fig. 5C) indicating the presence of GTP-bound or active Ras. Surprisingly the RBD probe was also found to accumulate within the nucleus (Fig. 5C). As a control we expressed this construct in cells lacking Ras2p and observed a diffuse staining during the log phase of growth, indicating that both nuclear and plasma membrane staining occur as a result of activated forms of Ras. The significance of the nuclear accumulation of this probe in log phase cells is currently under investigation. During diauxic shift the GFP-RBD signal appeared diffuse in wild-type cells and in cells deleted for the RAS2 gene. In Δwhi2 cells the GFP-RBD signal was localised to the plasma membrane and nuclear compartments as had been observed in wild-type cells, but also to small punctuate foci within the cytoplasm (Fig. 5C). During the diauxic shift, and in contrast to wild-type cells, the RBD signal occupied the same intracellular compartment as was observed for GFP-Ras2p (Fig. 5B). These data suggest that Ras2p is in an activated state when localised to the mitochondria in Δwhi2 cells. To confirm this we performed in vivo colocalisation studies using Cox4p-RFP, which demonstrated that the active Ras signal was indeed residing in the mitochondrial compartment (Fig. 5D).
A strong GFP signal was observed in the vacuole of wild-type cells at diauxic shift (Fig. 5A, arrow). We therefore investigated the possibility that Ras2p is targeted for vacuolar proteolysis during this period of growth. Ras2p levels were examined in protein extracts prepared from wild-type and Δwhi2 cultures grown to log phase or diauxic shift (Fig. 5E). During log phase the levels of Ras2p were identical in both strains and there appeared to be no obvious degradation. At diauxic shift, however, in samples prepared from wild-type cultures there was a pronounced reduction in the level of full-length Ras2p, and the appearance of numerous smaller fragments indicative of proteolysis (Fig. 5E, arrows). By contrast, the levels of full-length Ras2p remained significantly higher in Δwhi2 samples, and the level of proteolytic fragments were reduced accordingly (Fig. 5E). These data are consistent with the lack of vacuolar GFP signal observed in Δwhi2 cells. No signal was detected in extracts from Δras2 cells, demonstrating the specificity of the antibody used (Fig. 5E). Interestingly, Ras2p was also not degraded in a mutant expressing the constitutively active RAS2val19 allele. This result raises the possibility that degradation depends on the de-activation of Ras2p.
In order to establish whether the localisation of Ras2p to the mitochondria in cells lacking Whi2p function was representative of a general trafficking defect of proteins to the plasma membrane, we investigated the localisation of the integral plasma membrane protein Pma1p (Fig. 5F). We observed that Pma1p was localised correctly to the plasma membrane in Δwhi2 cells, although the signal appeared much brighter when compared with the wild type (Fig. 5F), which may occur as a result of endocytosis defects associated with the Δwhi2 mutation (Care et al., 2004).
cAMP signalling plays a role in yeast colony differentiation
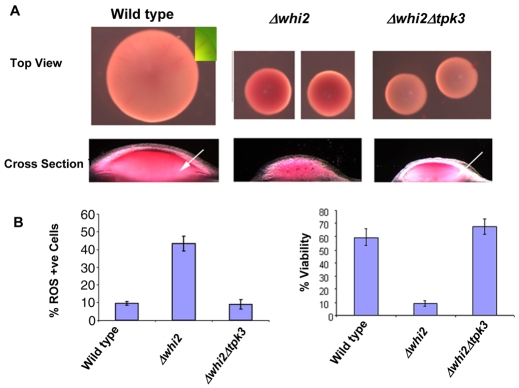
In pursuit of a physiological role for ActMAp in yeast cells we wished to assess whether the loss of Whi2p function and the resultant elevation in cell death had an impact on early colony formation and patterning. To achieve this we made use of the viability indicator dye phloxine B, which has been widely used as a marker of reduced cell viability and death in yeasts (Middelhoven et al., 1976; Peck et al., 1997). Phloxine B has been used in previous studies to assess viability within S. cerevisiae colonies (Cannon et al., 1986). We grew wild-type, Δwhi2 and Δwhi2Δtpk3 cells on YPAD plates containing phloxine B for 5 days to allow colonies to form. Wild-type colonies appeared pink after this stage over the entire surface, with a zone of exclusion apparent at the growing edge. By contrast, Δwhi2 cells appeared a much darker red, indicating a higher level of phloxine B uptake, inferring increased cell death within the colony (Fig. 6A). Colonies formed by Δwhi2 cells were much smaller than those of wild-type cells, although this may be a consequence of the smaller cell size observed in this mutant. Interestingly, colonies formed from Δwhi2Δtpk3 cells exhibited low levels of phloxine B uptake indicating reduced levels of cell death (Fig. 6A). To obtain further information, colonies from each yeast strain were bisected through the medial plane and observed under a light microscope as described in Materials and Methods. This method offers minimal disruption to the organisation of the colony and involves no treatments such as freezing, dehydration or fixation that may lead to cell death and misleading phloxine B uptake after dissection. Wild-type cells displayed phloxine B staining over the surface of the colony with more intense staining at the centre (Fig. 6A). Zones of phloxine B clearance on either side of the central region were apparent and had significantly less phloxine B staining (Fig. 6A, arrow). The appearance of `zones' or patterning of phloxine B uptake was less apparent in Δwhi2 colonies, which appeared red throughout, indicating a homogeneous distribution of cell death (Fig. 6B). Strikingly the deletion of TPK3 in a Δwhi2 background dramatically reduced phloxine B uptake observed in Δwhi2 colonies (Fig. 6A). Colonies formed from Δwhi2Δtpk3 cells also appeared to exhibit distinct phloxine B exclusion zones, as had been noted in the wild type (Fig. 6A, arrow). The phloxine B patterns observed in these cells was highly reproducible and apparent in approximately 100 colonies observed for each strain. ROS production in these colonies was also assessed by flow cytometry (Fig. 6B). As expected Δwhi2 colonies exhibited a much higher percentage of ROS-producing cells than was observed in colonies formed from either wild-type or Δwhi2Δtpk3 cells. In agreement with the phloxine B data, viability within Δwhi2 colonies was greatly decreased when compared with the wild type (Fig. 6B). The reduction in colony viability was restored when TPK3 was deleted in the Δwhi2 background (Fig. 6B).
Fig. 6.
The effect of Δwhi2 and cAMP elevation on cell death within S. cerevisiae colonies. We wished to assess whether the loss of Whi2p function had an impact on the patterns of cell death observed in emerging colonies. Colonies were grown from single cells on YPAD containing phloxine B, which accumulates within dead cells and stains them red. Colonies formed from wild type, Δwhi2 and Δwhi2Δtpk3 were observed from above and in cross section after dissection. Arrows refer to zones of reduced phloxine B uptake (A). ROS accumulation and cell viability within these colonies were assessed, as described in Materials and Methods (B).
Discussion
The budding yeast S. cerevisiae has been successfully utilised as a model for studying a variety of eukaryotic cellular processes. In recent years this has been extended to include mechanisms of programmed cell death and apoptosis (for recent reviews, see Buttner et al., 2006; Gourlay et al., 2006; Madeo et al., 2002; Madeo et al., 2004), processes long thought to reside exclusively within complex multicellular organisms. One explanation as to why apoptotic mechanisms have developed in yeasts is that they do not commonly exist as unicellular entities but as multicellular formations, such as colonies or biofilms, that are capable of communication and simple differentiation (Palkova, 2004). It follows that complex and predictable patterns of growth, within a colony for example, require regulatory mechanisms to control both proliferation and cell death in response to nutrient availability and exposure to environmental stress.
In line with this hypothesis it should be possible to identify mutations that both render cells unable to respond to their environment and to commit them to a programme of cell death. We believe that the data generated in this study, coupled with previously published work on the Δwhi2 mutation, provide evidence to support the above hypothesis.
Cells lacking Whi2p function fail to enter a stationary phase and do not elicit a full stress response, suggesting that they are unable to coordinate the nutritional status of their environment with appropriate signalling events. In addition, the Whi2p protein is known to play an important role in regulating the cellular response to glucose depletion in cultured yeast cells. In line with this the shutdown of WHI2 expression has been shown to coincide with entry into the diauxic shift. Whi2p also has a role in coordinating proliferation with nutritional status, as whi2 mutant cell lines have been shown to continue dividing at a stage when their comparative wild-type strain has exited the cell cycle. Cells lacking Whi2p are also well documented to suffer from a chronic inability to respond to a variety of stress stimuli such as heat, osmotic shock, oxidative stress and nutrient depletion (Mountain and Sudbery, 1990a; Radcliffe et al., 1997a; Saul and Sudbery, 1985). The observation that Δwhi2 cells display actin aggregates during entry into the diauxic shift, led us to investigate whether these cells underwent apoptosis. In previous studies we demonstrated that the presence of such actin formations during the diauxic shift led to the hyperactivation of the Ras signalling cascade, severe mitochondrial dysfunction, ROS accumulation and a loss of viability (Gourlay and Ayscough, 2006). In line with our hypothesis that actin aggregation correlates with known markers of apoptosis, the mitochondria in Δwhi2 cells exhibited very low membrane potentials, were severely fragmented and produced large amounts of ROS during the period of growth when actin aggregates developed. The mtDNA content of Δwhi2 was also severely depleted when compared with wild-type cells.
The influence of actin dynamics on cAMP-PKA signalling activity has recently been noted in both S. cerevisiae and C. albicans (Gourlay and Ayscough, 2006; Wolyniak and Sundstrom, 2007). In addition, the activity of Ras-cAMP-PKA signalling is known to be a critical component in the regulation of mitochondrial activity, stress response and cell cycle arrest in response to nutritional status (Hlavata et al., 2003; Hlavata et al., 2008). In line with the apoptotic phenotypes observed in Δwhi2 cells being a consequence of inappropriate Ras-cAMP-PKA pathway activity, ROS accumulation could be prevented by the overexpression of the negative regulator, PDE2. As the only known function of PDE2 in yeast is as a high affinity cAMP phosphodiesterase, we suggest that cAMP elevation in Δwhi2, leading to aberrant PKA activity is the major causative factor leading to mitochondrial-derived ROS build-up and death. We had previously identified Tpk3p as the major kinase responsible for mitochondrial dysfunction leading to ROS build-up in actin-aggregating cells. In agreement with this, the deletion of TPK3 in Δwhi2 cells both prevented the accumulation of ROS and reduced mitochondrial fragmentation. Further evidence of the involvement of Whi2p in the regulation of mitochondrial function and cell death has come from a very recent study. Here, spontaneous whi2 mutations were found to arise in response to the deletion of the mitochondrial fission gene FIS1 (Cheng et al., 2008). The loss of Whi2p function was able to compensate for respiratory defects found in Δfis1 cells but also gave rise to a propensity for cell death under conditions of stress, such as nutritional depletion. Although the authors did not investigate the nature of cell death within these strains, in light of the data we have presented here we would predict that Ras-cAMP-PKA-dependent apoptosis plays an important role.
An important question arising from these studies is why do mutations that affect the stability of the actin cytoskeleton lead to the inappropriate activation of Ras signalling during nutrient depletion? During the transition to diauxic shift it is essential that Ras-cAMP-PKA signalling is reduced to allow cell cycle arrest, a full stress response and the initiation of storage carbohydrate formation. Here we have presented evidence to suggest that Ras2p is a target for proteolysis as cells sense nutritional depletion and enter the diauxic phase of growth. It seems logical to conclude that the degradation of Ras2p upon nutritional depletion forms part of the mechanism to effectively shut down Ras-cAMP-PKA pathway activity during this phase of growth. In support of this, the translocation of Ras2p from the plasma membrane to the mitochondria in Δwhi2 cells during the diauxic shift results in a failure to either degrade or deactivate the protein. The actin cytoskeleton is essential for efficient vesicle trafficking from the plasma membrane to the vacuole (Girao et al., 2008). It seems plausible that mutants affecting actin dynamics may lead to a failure to shut down Ras activity as a result of vesicle trafficking defects that facilitate Ras2p movement to the vacuole for degradation (Fig. 7). Evidence to support a role for vesicle trafficking in the regulation of Ras2p comes from a previous study that convincingly demonstrated that the disruption of the Class C VPS complex leads to mitochondrial localisation of Ras2p and respiratory defects (Wang and Deschenes, 2006). In addition we have recently demonstrated that mutations in the class C VPS machinery also result in the accumulation of ROS, formation of actin aggregates and failure to degrade Ras2p during the diauxic shift in a manner similar to that observed for Δwhi2 cells (data not shown). Interestingly, K-Ras has also been observed relocating from the plasma membrane to the mitochondrial outer membrane in response to PKC-mediated phosphorylation in mammalian cells (Bivona et al., 2006). In this study, mitochondria-localised phospho-K-Ras was shown to have a pro-apoptotic effect, which was attributed to an interaction with the anti-apoptotic factor Bcl-XL. It will be of interest to establish whether the mitochondrial localisation of Ras2p is important for the promotion of apoptosis in yeast, and whether this compartmentalisation involves phosphoregulation.
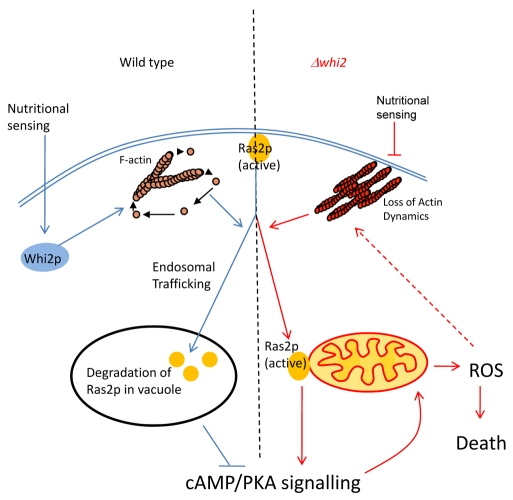
Fig. 7.
Model depicting the regulation of Ras activity in wild-type and Δwhi2 cells. In wild-type cells Whi2p functions to integrate nutritional sensing with maintenance of the dynamic nature of the cytoskeleton. Functional actin dynamics are required for the appropriate tracking and degradation of Ras2p, which in turn facilitates the shutdown of cAMP-PKA signalling and an appropriate cellular response. By contrast, the loss of Whi2p function leads to the loss of coordination between nutritional sensing and actin regulation. The stabilisation of F-actin structures results in the failure to correctly traffic Ras2p to the vacuole. Ras2p in turn becomes localised to the mitochondrial surface in an active form. The failure to shut down Ras signalling leads to mitochondrial dysfunction, the accumulation of damaging ROS and cell death.
As is the case with many unicellular organisms, yeasts normally exist as multicellular entities such as colonies or within biofilms. Recent data have demonstrated a role for ammonia signalling and apoptosis in the maintenance of yeast colonies grown for 14 days. The loss of Sok2p, a protein involved in sensing the ammonia signal, prevented the establishment of zones of apoptosis, which in turn had an impact on further colony development (Vachova and Palkova, 2005). We investigated whether the loss of Whi2p function also influenced the formation of colonies by using growth media containing the dye phloxine B. In wild-type colonies we observed that phloxine B accumulated as a cap, and within discrete radial spokes (highlighted in Fig. 6A insert), indicating an increased level of cell death within discrete compartments. The compartmentalisation of colonies in this way reflects the fact that S. cerevisiae colonies are not homogeneous collections of cells but undergo simple differentiation. Strikingly, colonies formed from Δwhi2 cells exhibited much more widespread and intense phloxine B uptake within the colony, exhibiting greatly reduced clearance zones at its periphery. These data suggest that cAMP signalling plays an important role in regulating S. cerevisiae colony differentiation. Colonies formed from Δwhi2 cells also had elevated levels of ROS when compared with wild-type cells. In agreement with phloxine B uptake acting as an indicator of colony fitness, the viability of samples taken from the centre of Δwhi2 colonies was dramatically reduced compared with the wild type. In light of the information gained from experiments in liquid culture, these data argue that Whi2p has a similar function within colonies, and that the inability of Δwhi2 to respond to environmental signalling cues has a detrimental effect on colony viability. Interestingly, we were able to reduce the level of phloxine B uptake and ROS production in colonies formed from double mutant cells lacking both Tpk3p and Whi2p function. Double mutant Δwhi2Δtpk3 colonies also exhibited wild-type levels of viability at the time point sampled, implicating cAMP-PKA activity in regulating cell death within colonies. These data also imply that, as we observed in liquid culture, cAMP-PKA pathway activity is elevated in Δwhi2 cells leading to Tpk3p-dependent ROS production and loss of viability.
The data provided in this study consolidate previous research on the Whi2p protein with recent findings that the dynamic nature of the actin cytoskeleton can modulate Ras-cAMP-PKA function. They also provide further proof of principle that environmental sensing systems in yeast are linked to signalling mechanisms that can determine multiple cell fate decisions. In this case the loss of Whi2p function renders cells unable to enter cell cycle arrest, elicit a full stress response, or appropriately modulate mitochondrial function in the face of nutritional exhaustion. We have shown that the consequence of this failure to correctly coordinate environmental sensing with major signalling events leads to an alternative cell fate decision, cell death that resembles apoptosis. We have also shown that this mechanism plays a physiologically relevant role in determining cell death and the pattern of differentiation in growing colonies. The data presented in this paper also add weight to our belief that the dynamic status of the actin cytoskeleton, which may become reduced within ageing or damaged cells, is tightly linked to signalling mechanisms that regulate cell death decisions in yeast and other higher eukaryotic systems.
Materials and Methods
Yeast strains, plasmids, media and growth conditions
Yeast strains used in this study are listed in Table 1. Unless stated otherwise cells were grown in a rotary shaker at 30°C in liquid YPD medium (1% yeast extract, 2% bactopeptone, 2% glucose supplemented with 40 μg/ml adenine).
Table 1.
Yeast strains used in this study
| Strain | Relevant genotype | Origin |
|---|---|---|
| CGY188 | Mata ade2-1can1-100 his-11,15 leu2-3,112 trp1-1 ura3 [psi+] GAL1 ssd1-d | (Care et al., 2004) |
| CGY189 | Mata ade2-1can1-100 his-11,15 leu2-3,112 | (Care et al., 2004) |
| trp1-1 ura3 [psi+] GAL1 ssd1-d Δwhi2::HIS4 | ||
| CGY498b | Mata ade2-1can1-100 his-11,15 leu2-3,112trp1-1 ura3 [psi+] GAL1 ssd1-d Δwhi2::HIS4 Δtpk3:: URA3 | This study |
| CGY371 | Mata uras3-52 leu2 his4-539 Δras2::LEU2 | (Pichova et al., 1997) |
| CGY372 | Mata uras3-52 leu2 his4-539 RAS2ala18val19::LEU2 | (Pichova et al., 1997) |
Oligonucleotides designed for the targeted disruption of TPK3 were as follows: delTPK3–5′-ATGTATGTTGATCCGATGAACAACAATGAAATCAGGAAATTAAGCATTACCAGCTGAAGCTTCGTACGC-3′ and delTPK3–5′-TTAAAATTCTTTCATTAAATCCATATATGGATCCTCCCCTTGAATTCGCATAGGCCACTAGTGGATCTG-3′. The plasmid used for overexpression of PDE2 has been described previously (Gourlay and Ayscough, 2005). The plasmids used to express Ras2-GFP and GFP-Pma1 have been described previously (Wang and Deschenes, 2006). The plasmid pWHIM3 used to re-express WHI2 has been previously characterised (Radcliffe et al., 1997b). Expression of WHI2 from pWHIM3 was achieved by growing cells in YPD medium plus 1% glucose and 1% galactose. To obtain pYX212-EGFP-RBD-3 we used the following strategy. The pEGFP-C2 construct [kindly provided by I. Rubio (Jena, Germany), and expressing the fusion eGfp-RBD-3 (Augsten et al., 2006)] was digested with NheI, and the pYX expression vectors, pYX212 and pYX242, were digested with EcoRI. Filling in of the 3′-recessed ends was done using the Klenow fragment of DNA polymerase I. The blunted DNA was then purified using the JETSORB Gel Extraction Kit and digested with SalI. Finally, the fragment containing the fusion EGFP-RBD-3 was purified using the JETSORB Gel Extraction Kit and ligated with the linearised vectors.
Assessment of ROS content
Cells were grown in a rotary shaker at 30°C in liquid YPD medium (1% yeast extract, 2% bactopeptone, 2% glucose supplemented with 40 μg/ml adenine) to a density of 2×108 cells/ml in the presence of 5 μg/ml 2′,7′-dichloro dihydrofluorescein diacetate (H2-DCFDA) obtained from Invitrogen (Molecular Probes). Prior to analysis, cultures were sonicated and then diluted to a density of 1×106 cells/ml in 50 mM Tris-HCl, pH 7.5. Fluorescence data from 10,000 cells per sample were then collected using a Becton-Dickinson FACScaliber Flow Cytometer and analysed using Cell Quest Pro software. FACS parameters were set at excitation and emission settings of 304 and 551 nm (filter FL-1), respectively. All experiments were performed at least three times.
Viability assays
Assays were carried out as previously described (Gourlay and Ayscough, 2005; Herker et al., 2004). Cells were grown in liquid YPD medium and their number determined using a haemocytometer. Serial dilutions were plated onto YPD agar plates, and the surviving colonies were counted. The percentage of viable cells was determined by dividing the number of surviving colonies by the calculated number of plated colonies.
Fluorescence microscopy
Rhodamine-phalloidin and DAPI staining were performed as previously described for F-actin (Gourlay et al., 2004). Cells were viewed with an Olympus IX-81 fluorescence microscope with a 150 W xenon-mercury lamp and an Olympus 150X Plan NeoFluor oil-immersion objective. GFP-RFP colocalisation studies were performed using an Optosplit II Image Splitter (Cairn Scientific). Images were captured using a Hammamatsu ORCA AG digital camera using Olympus Cell R software.
Mitochondrial morphology and membrane potential
Mitochondria were visualised by using the membrane-potential-sensitive dye 3,3′-dihexyloxacarbocyanine iodide (DiOC6) obtained from Molecular Probes at a concentration of 20 ng/ml, as previously described (Simon et al., 1997). GFP-labelled mitochondria were visualised using the plasmids pVTU100U-mtGFP and pYX122-mtGFP (Westermann and Neupert, 2000).
Analysis of cell death in yeast colonies
Between 10 and 20 cells were plated onto 9-cm YPAD agar plates containing 10 μM phloxine B (Sigma). Colonies were grown for 5 days before being viewed using a Leica M2FLIII microscope and documented with a Leica DC300F colour camera. To visualise a cross section through the colony, a glass coverslip was inserted directly, bisecting the colony, and removed from the agar. Dissected colonies were viewed and documented immediately. All images were taken using the same illumination conditions and exposure times. To examine ROS build-up and viability, cells were removed from the centre of 5-day-old colonies. Viability was assessed as described above; for ROS cell viability, cells were resuspended in Tris pH 7.5 plus dihydrorhodamine 123 (DHR123) (Molecular Probes) for 2 hours before FACS analysis, as described above.
Thanks to Gary Robinson and Will Kotiadis for critical reading of this manuscript. We are thankful to Ignacio Rubio (Friedrich Schiller-University Jena) for his kind gift of the RBD-GFP construct. This work was sponsored by a Medical Research Council (MRC) career development fellowship to C.W.G. (ref. no. 78573). Deposited in PMC for release after 6 months.
References
- Augsten, M., Pusch, R., Biskup, C., Rennert, K., Wittig, U., Beyer, K., Blume, A., Wetzker, R., Friedrich, K. and Rubio, I. (2006). Live-cell imaging of endogenous Ras-GTP illustrates predominant Ras activation at the plasma membrane. EMBO Rep. 7, 46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough, K. R., Stryker, J., Pokala, N., Sanders, M., Crews, P. and Drubin, D. G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona, T. G., Quatela, S. E., Bodemann, B. O., Ahearn, I. M., Soskis, M. J., Mor, A., Miura, J., Wiener, H. H., Wright, L., Saba, S. G. et al. (2006). PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell 21, 481-493. [DOI] [PubMed] [Google Scholar]
- Buttner, S., Eisenberg, T., Herker, E., Carmona-Gutierrez, D., Kroemer, G. and Madeo, F. (2006). Why yeast cells can undergo apoptosis: death in times of peace, love, and war. J. Cell Biol. 175, 521-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, J. F., Gibbs, J. B. and Tatchell, K. (1986). Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics 113, 247-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care, A., Vousden, K. A., Binley, K. M., Radcliffe, P., Trevethick, J., Mannazzu, I. and Sudbery, P. E. (2004). A synthetic lethal screen identifies a role for the cortical actin patch/endocytosis complex in the response to nutrient deprivation in Saccharomyces cerevisiae. Genetics 166, 707-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W. C., Teng, X., Park, H. K., Tucker, C. M., Dunham, M. J. and Hardwick, J. M. (2008). Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 15, 1838-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevtzoff, C., Vallortigara, J., Averet, N., Rigoulet, M. and Devin, A. (2005). The yeast cAMP protein kinase Tpk3p is involved in the regulation of mitochondrial enzymatic content during growth. Biochim. Biophys. Acta 1706, 117-125. [DOI] [PubMed] [Google Scholar]
- Colombo, S., Ronchetti, D., Thevelein, J. M., Winderickx, J. and Martegani, E. (2004). Activation state of the Ras2 protein and glucose-induced signaling in Saccharomyces cerevisiae. J. Biol. Chem. 279, 46715-46722. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne, I., Rossi, R., Milzani, A., Di Simplicio, P. and Colombo, R. (2001). The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 31, 1624-1632. [DOI] [PubMed] [Google Scholar]
- de Rooij, J. and Bos, J. L. (1997). Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene 14, 623-625. [DOI] [PubMed] [Google Scholar]
- Dong, X., Mitchell, D. A., Lobo, S., Zhao, L., Bartels, D. J. and Deschenes, R. J. (2003). Palmitoylation and plasma membrane localization of Ras2p by a nonclassical trafficking pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 23, 6574-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao, H., Geli, M. I. and Idrissi, F. Z. (2008). Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 582, 2112-2119. [DOI] [PubMed] [Google Scholar]
- Gourlay, C. W. and Ayscough, K. R. (2005). Identification of an upstream regulatory pathway controlling actin-mediated apoptosis in yeast. J. Cell Sci. 118, 2119-2132. [DOI] [PubMed] [Google Scholar]
- Gourlay, C. W. and Ayscough, K. R. (2006). Actin-induced hyperactivation of the Ras signaling pathway leads to apoptosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 6487-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay, C. W., Carpp, L. N., Timpson, P., Winder, S. J. and Ayscough, K. R. (2004). A role for the actin cytoskeleton in cell death and aging in yeast. J. Cell Biol. 164, 803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay, C. W., Du, W. and Ayscough, K. R. (2006). Apoptosis in yeast-mechanisms and benefits to a unicellular organism. Mol. Microbiol. 62, 1515-1521. [DOI] [PubMed] [Google Scholar]
- Gray, J. V., Petsko, G. A., Johnston, G. C., Ringe, D., Singer, R. A. and Werner-Washburne, M. (2004). “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 68, 187-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren, G., Jarolim, S., Laun, P., Rinnerthaler, M., Stolze, K., Perrone, G. G., Kohlwein, S. D., Nohl, H., Dawes, I. W. and Breitenbach, M. (2004). The role of respiration, reactive oxygen species and oxidative stress in mother cell-specific ageing of yeast strains defective in the RAS signalling pathway. FEMS Yeast Res. 5, 157-167. [DOI] [PubMed] [Google Scholar]
- Herker, E., Jungwirth, H., Lehmann, K. A., Maldener, C., Frohlich, K. U., Wissing, S., Buttner, S., Fehr, M., Sigrist, S. and Madeo, F. (2004). Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164, 501-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, P. K. (2002). Stationary phase in yeast. Curr. Opin. Microbiol. 5, 602-607. [DOI] [PubMed] [Google Scholar]
- Hlavata, L., Aguilaniu, H., Pichova, A. and Nystrom, T. (2003). The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 22, 3337-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavata, L., Nachin, L., Jezek, P. and Nystrom, T. (2008). Elevated Ras/protein kinase A activity in Saccharomyces cerevisiae reduces proliferation rate and lifespan by two different reactive oxygen species-dependent routes. Aging Cell 7, 148-157. [DOI] [PubMed] [Google Scholar]
- Kaida, D., Yashiroda, H., Toh-e, A. and Kikuchi, Y. (2002). Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells 7, 543-552. [DOI] [PubMed] [Google Scholar]
- Madeo, F., Engelhardt, S., Herker, E., Lehmann, N., Maldener, C., Proksch, A., Wissing, S. and Frohlich, K. U. (2002). Apoptosis in yeast: a new model system with applications in cell biology and medicine. Curr. Genet. 41, 208-216. [DOI] [PubMed] [Google Scholar]
- Madeo, F., Herker, E., Wissing, S., Jungwirth, H., Eisenberg, T. and Frohlich, K. U. (2004). Apoptosis in yeast. Curr. Opin. Microbiol. 7, 655-660. [DOI] [PubMed] [Google Scholar]
- Middelhoven, W. J., Broekhuizen, B. and van Eijk, J. (1976). Detection, with the dye phloxine B, of yeast mutants unable to utilize nitrogenous substances as the sole nitrogen source. J. Bacteriol. 128, 851-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain, H. A. and Sudbery, P. E. (1990a). Regulation of the Saccharomyces cerevisiae WHI2 gene. J. Gen. Microbiol. 136, 727-732. [DOI] [PubMed] [Google Scholar]
- Mountain, H. A. and Sudbery, P. E. (1990b). The relationship of growth rate and catabolite repression with WHI2 expression and cell size in Saccharomyces cerevisiae. J. Gen. Microbiol. 136, 733-737. [DOI] [PubMed] [Google Scholar]
- Palkova, Z. (2004). Multicellular microorganisms: laboratory versus nature. EMBO Rep. 5, 470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, V. M., Fuge, E. K., Padilla, P. A., Gomez, M. A. and Werner-Washburne, M. (1997). Yeast bcy1 mutants with stationary phase-specific defects. Curr. Genet. 32, 83-92. [DOI] [PubMed] [Google Scholar]
- Phillips, A. J., Sudbery, I. and Ramsdale, M. (2003). Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. USA 100, 14327-14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, A. J., Crowe, J. D. and Ramsdale, M. (2006). Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 103, 726-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichova, A., Vondrakova, D. and Breitenbach, M. (1997). Mutants in the Saccharomyces cerevisiae RAS2 gene influence life span, cytoskeleton, and regulation of mitosis. Can. J. Microbiol. 43, 774-781. [DOI] [PubMed] [Google Scholar]
- Radcliffe, P., Trevethick, J., Tyers, M. and Sudbery, P. (1997a). Deregulation of CLN1 and CLN2 in the Saccharomyces cerevisiae whi2 mutant. Yeast 13, 707-715. [DOI] [PubMed] [Google Scholar]
- Radcliffe, P. A., Binley, K. M., Trevethick, J., Hall, M. and Sudbery, P. E. (1997b). Filamentous growth of the budding yeast Saccharomyces cerevisiae induced by overexpression of the WHi2 gene. Microbiology 143, 1867-1876. [DOI] [PubMed] [Google Scholar]
- Reinders, A., Burckert, N., Boller, T., Wiemken, A. and De Virgilio, C. (1998). Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 12, 2943-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, L. S. and Fink, G. R. (1998). The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95, 13783-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, L. S., Causton, H. C., Young, R. A. and Fink, G. R. (2000). The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. USA 97, 5984-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul, D. J. and Sudbery, P. E. (1985). Molecular cloning of WHI2, a gene involved in the regulation of cell proliferation in Saccharomyces cerevisiae. J. Gen. Microbiol. 131, 1797-1806. [DOI] [PubMed] [Google Scholar]
- Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. and Lowe, S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593-602. [DOI] [PubMed] [Google Scholar]
- Simon, V. R., Karmon, S. L. and Pon, L. A. (1997). Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell Motil. Cytoskeleton 37, 199-210. [DOI] [PubMed] [Google Scholar]
- Thevelein, J. M. (1992). The RAS-adenylate cyclase pathway and cell cycle control in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 62, 109-130. [DOI] [PubMed] [Google Scholar]
- Thevelein, J. M., Gelade, R., Holsbeeks, I., Lagatie, O., Popova, Y., Rolland, F., Stolz, F., Van de Velde, S., Van Dijck, P., Vandormael, P. et al. (2005). Nutrient sensing systems for rapid activation of the protein kinase A pathway in yeast. Biochem. Soc. Trans. 33, 253-256. [DOI] [PubMed] [Google Scholar]
- Toda, T., Uno, I., Ishikawa, T., Powers, S., Kataoka, T., Broek, D., Cameron, S., Broach, J., Matsumoto, K. and Wigler, M. (1985). In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40, 27-36. [DOI] [PubMed] [Google Scholar]
- Vachova, L. and Palkova, Z. (2005). Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia. J. Cell Biol. 169, 711-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. and Deschenes, R. J. (2006). Plasma membrane localization of Ras requires class C Vps proteins and functional mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 3243-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne, M., Braun, E., Johnston, G. C. and Singer, R. A. (1993). Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol. Rev. 57, 383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann, B. and Neupert, W. (2000). Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast 16, 1421-1427. [DOI] [PubMed] [Google Scholar]
- Wolyniak, M. J. and Sundstrom, P. (2007). Role of actin cytoskeletal dynamics in activation of the cyclic AMP pathway and HWP1 gene expression in Candida albicans. Eukaryot. Cell 6, 1824-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]