Summary
Signaling through the IGF-I receptor by locally synthesized IGF-I or IGF-II is crucial for normal skeletal development and for bone remodeling. Osteogenesis is primarily regulated by bone morphogenetic proteins (BMPs), which activate gene expression programs driven by bone-specific transcription factors. In a mesenchymal stem cell model of osteoblast commitment and differentiation controlled by BMP2, we show that an inhibitor of PI3-kinase or a dominant-negative Akt were as potent in preventing osteoblast differentiation as the IGF binding protein IGFBP5, whereas a Mek inhibitor was ineffective. Conversely, an adenovirus encoding an inducible-active Akt was able to overcome the blockade of differentiation caused by IGFBP5 or the PI3-kinase inhibitor, and could restore normal osteogenesis. Inhibition of PI3-kinase or Akt did not block BMP2-mediated signaling, because the Smad-responsive genes Sox9 and JunB were induced normally under all experimental conditions. When activated during different stages of osteoblast maturation, dominant-negative Akt prevented accumulation of bone-specific alkaline phosphatase and reduced mineralization, and more significantly inhibited the longitudinal growth of metatarsal bones in primary culture by interfering with both chondrocyte and osteoblast development and function. We conclude that an intact IGF-induced PI3-kinase–Akt signaling cascade is essential for BMP2-activated osteoblast differentiation and maturation, bone development and growth, and suggest that manipulation of this pathway could facilitate bone remodeling and fracture repair.
Keywords: Bone development, Bone morphogenetic factors, Insulin-like growth factors, PI3-kinase–Akt pathway, Akt, Osteoblast
Introduction
Bone remodeling occurs throughout life to maintain bone mass and integrity, and involves the dynamic interplay of two opposing processes: resorption by osteoclasts and deposition by osteoblasts (Hadjidakis and Androulakis, 2006; Khosla et al., 2008; Raisz, 2005; Zaidi, 2007). In the adult skeleton, both phases of remodeling are coupled temporally and spatially, and take place within a specialized environment termed the bone multicellular unit (Khosla et al., 2008; Raisz, 2005; Zaidi, 2007). Bone remodeling also requires regulated interactions between local and systemically derived signals mediated by hormones, growth factors and cytokines, and genetically-defined hierarchical programs of bone-specific transcription factors (Raisz, 2005; Zaidi, 2007). Among growth factors with positive actions on bone formation are the bone morphogenetic proteins (BMPs) (Li and Cao, 2006), and the insulin-like growth factors (IGFs) (Li and Cao, 2006; Raisz, 2005; Zaidi, 2007).
BMPs are central regulators of osteoblast differentiation, and were named originally for their ability to promote ectopic bone formation (Wozney, 1992). Like other members of the TGFβ superfamily, BMPs signal through heteromeric Type I and Type II serine-threonine kinase receptors, and activate the intracellular signaling molecules, Smad1, Smad5 and Smad8, through their serine phosphorylation (Herpin and Cunningham, 2007). Activated Smad proteins form heterodimers with the co-Smad, Smad4, and translocate to the nucleus, where they regulate target gene transcription (Herpin and Cunningham, 2007). BMP2 stimulates transcription of Runx2, the master regulator of osteoblast commitment (Lian et al., 2006), and BMP2-activated Smad proteins also collaborate with Runx2 to induce other genes in differentiating osteoblasts, including osterix (Osx/Sp7), another bone-specific transcription factor (Lian et al., 2006).
The IGF family consists of two secreted growth factors, IGF-I and IGF-II (official protein symbols IGF1 and IGF2), two receptors and six high-affinity binding proteins. Actions of both IGFs are mediated by the IGF-I receptor, a ligand-activated tyrosine protein kinase that uses a series of intracellular adaptor molecules, including the insulin receptor substrate proteins IRS1 and IRS2, to engage downstream signaling pathways (Nakae et al., 2001). IGF binding proteins function primarily to modulate the bioavailability of IGFs, but might have other IGF-independent effects (Bach et al., 2005; Duan and Xu, 2005). Studies in experimental animals have concluded that action of IGF is essential for normal bone formation, growth and maintenance. Mice globally lacking the IGF-I receptor have retarded skeletal development accompanied by delayed ossification, as well as other severe systemic defects that contribute to their neonatal death (Liu et al., 1993). Targeted loss of the IGF-I receptor exclusively in osteoblasts also has a bone phenotype, in which total trabecular thickness and number were reduced because of a decline in mineral apposition rate (Zhang et al., 2002). In agreement with these conclusions, individual knockouts of IRS1 and IRS2 also caused osteopenia, with defects seen in both cortical and trabecular bone (Akune et al., 2002; Ogata et al., 2000).
In contrast to the deficits secondary to loss of IGF signaling, increased expression of IGF-I appears to stimulate bone growth and mineralization. Targeting IGF-I to mature osteoblasts in transgenic mice caused enhanced bone formation and mineralization, and resulted in increased trabecular bone volume (Zhao et al., 2000). Targeting IGF-I to osteoblast precursors also gave rise to a robust bone phenotype in mice, and led to increases in femur length, cortical width and cross-sectional area (Jiang et al., 2006). Thus, regardless of the timing of IGF-I overexpression in bone of transgenic mice, net bone formation and mass were enhanced. Therefore, based on several types of evidence, IGF action via the IGF-I receptor is crucial for normal bone development and mineralization.
IGF-mediated stimulation of the IGF-I receptor triggers receptor autophosphorylation to create docking sites at phosphorylated tyrosine residues for adaptor molecules (Nakae et al., 2001). This initiates a series of protein-protein interactions that lead to activation of intracellular signal transduction pathways (Nakae et al., 2001). Although several signaling pathways mediate IGF action in bone, as well as in other tissues (Giustina et al., 2008), a growing literature supports the idea that the PI3-kinase–Akt network is critical for both osteoblast differentiation and bone growth (Fujita et al., 2004; Ghosh-Choudhury et al., 2002; Liu et al., 2007; Osyczka and Leboy, 2005; Peng et al., 2003; Raucci et al., 2008), yet the biochemical or molecular mechanisms through which the IGF-stimulated PI3-kinase–Akt pathway increases osteoblast development and function have not been elucidated. Fujita and colleagues have postulated an interaction with Runx2, because the PI3-kinase inhibitor LY294002 reduced both its DNA-binding activity and its ability to stimulate target gene transcription (Fujita et al., 2004). Qiao and co-workers have reached similar conclusions (Qiao et al., 2004), whereas others have suggested collaboration at the level of nuclear translocation of BMP2-stimulated Smad proteins (Ghosh-Choudhury et al., 2002).
Here, we define a key role for the IGF-activated PI3-kinase–Akt pathway in BMP-mediated osteoblast differentiation of uncommitted mesenchymal precursor cells and their subsequent maturation. We also find that Akt-regulated signaling is crucial for longitudinal bone growth and that it exerts positive actions on both chondrocyte and osteoblast differentiation and function in developing bone. Based on these observations, we conclude that the IGF-stimulated PI3-kinase–Akt pathway is a central component in an interactive osteogenic signaling network that is necessary for both bone development and remodeling.
Results
Inhibition of the PI3-kinase–Akt pathway blocks BMP2-mediated osteoblast differentiation
We previously demonstrated that both BMP2-stimulated osteoblast differentiation of mouse mesenchymal stem cells and growth and mineralization of mouse metatarsal bones, could be blocked by IGFBP5 (Mukherjee and Rotwein, 2008). In these studies, the inhibitory effects of IGFBP5 on osteogenesis appeared to depend on its ability to bind IGF-I with high affinity, thereby sequestering IGF-I from its cell-surface receptor, and leading to impaired IGF-I receptor activity (Mukherjee and Rotwein, 2008). Since an IGF-I analog with diminished affinity for IGFBPs but normal affinity for the IGF-I receptor could restore BMP2-mediated osteogenesis in the presence of otherwise inhibitory concentrations of IGFBP5 (Mukherjee and Rotwein, 2008), our results indicated that sustained IGF action is required for osteoblast differentiation and bone growth. The focus of current experiments was to identify and characterize the pertinent IGF-activated signaling pathways.
To define the IGF-mediated mechanisms involved in regulation of osteoblast development and function, we first incubated confluent C3H10T1/2 mesenchymal stem cells with recombinant BMP2 in osteogenic medium. Under these conditions, BMP2 treatment was accompanied by the rapid and sustained stimulation of intracellular signaling via BMP receptors, as indicated by serine phosphorylation of Smad1, Smad5 and Smad8 in protein extracts observed by day 1 and maintained for up to 7 days (Fig. 1A), and by rapid and sustained upregulation of Dlx5 and Runx2 mRNA (Fig. 1B), two osteoblast-specific transcription factors whose genes are well-known targets of BMP2 (Lee et al., 2003; Phimphilai et al., 2006). Subsequent events included accumulation of transcripts encoding the bone transcription factor osterix (Osx), and for the secreted osteoblast protein osteocalcin (Ocn) (Fig. 1B), followed by activity of bone-specific alkaline phosphatase, and mineralization of extracellular matrix, the latter measured by Alizarin red staining (Fig. 1C,D). None of these biological effects were observed in cells incubated in osteogenic medium without BMP2 (Fig. 1A-D).
Fig. 1.
BMP2 promotes osteoblast differentiation. Results are shown of experiments in which C3H10T1/2 cells were incubated in osteogenic media (OM) without or with BMP2 (200 ng/ml) for up to 7 days. (A) Immunoblots of whole-cell protein lysates for serine-phosphorylated Smad1, Smad5 and Smad8 (pSmad1,5,8), total Smads, Akt phosphorylated at Ser473 (pAktS473) and total Akt. (B) Results of RT-PCR assays showing expression of osteoblast-specific genes Dlx5, Runx2, osterix (Osx) and osteocalcin (Ocn), and control gene S17 after incubation for up to 7 days in osteogenic medium with or without BMP2. (C) Representative images of qualitative alkaline phosphatase (AP) staining in cells after incubation in osteogenic medium with or without BMP2 for 7 days. (D) Measurement of mineralization assessed by Alizarin red staining 7 days after incubation in osteogenic medium with or without BMP2.
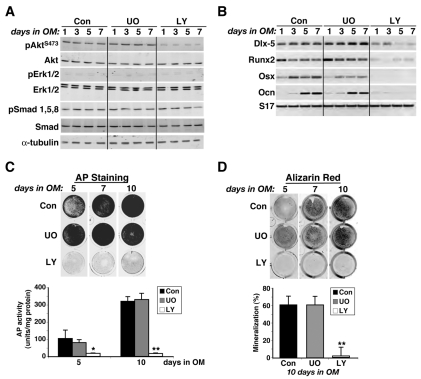
To block potential IGF-regulated signaling cascades, we treated confluent C3H10T1/2 cells with either the Mek inhibitor UO126 or the PI3-kinase inhibitor LY294002, in the presence of BMP2 and osteogenic medium, because both the Grb-Sos-Mek-Erk and PI3-kinase–Akt pathways have been shown to be activated by the IGF-I receptor via the adaptor molecules IRS1 and IRS2 in bone cells (Akune et al., 2002; Kadowaki et al., 1996; Ogata et al., 2000). Addition of UO126 had no effect on BMP2-mediated signaling, or on the rate or extent of osteoblast gene expression or differentiation (Fig. 2A-D), although at the concentration used (10 μM), it completely inhibited IGF-I-stimulated Erk phosphorylation in C3H10T1/2 cells (supplementary material Fig. S1). By contrast, LY294002 (20 μM), which blocked IGF-induced Akt phosphorylation (supplementary material Fig. S1), impaired expression of osteoblast differentiation genes (Fig. 2B) and completely prevented induction of alkaline phosphatase activity and mineralization (Fig. 2C,D), although like UO126, it also did not inhibit BMP2-activated Smad phosphorylation (Fig. 2A).
Fig. 2.
Inhibition of PI3-kinase activity blocks BMP2-induced osteoblast differentiation. Results are shown of experiments in which C3H10T1/2 cells were incubated in osteogenic media (OM) containing BMP2 for up to 10 days without (Con, control) or with the MEK inhibitor UO126 (UO) (10 μM) or the PI3-kinase inhibitor, LY294002 (LY) (20 μM), as described in the Materials and Methods. (A) Immunoblots of whole-cell protein lysates for Akt phosphorylated at Ser473 (pAktS473), total Akt, tyrosine and serine phosphorylated Erk1 and Erk2 (pErk1/2), total Erks, serine phosphorylated Smad1, Smad5 and Smad8 (pSmad1,5,8), total Smads and α-tubulin. (B) Results of RT-PCR assays showing expression of osteoblast-specific genes encoding Dlx-5, Runx2, osterix (Osx) and osteocalcin (Ocn), and the control gene S17 after incubation for up to 7 days in osteogenic medium with or without UO126 or LY294002. (C) Representative images of qualitative alkaline phosphatase (AP) staining in cells after incubation in osteogenic medium with or without UO126 or LY294002 for 5, 7 or 10 days. The graph depicts measurement of alkaline phosphatase activity in lysates of cells incubated for 5 or 10 days in osteogenic medium with or without UO126 or LY294002 (mean ± s.d., n=3; *P<0.01, **P<0.001 vs cells incubated without LY294002). (D) Measurement of osteoblast-mediated mineralization assessed by Alizarin red staining at days 5, 7 and 10 after incubation in osteogenic medium with or without UO126 or LY294002. The graph shows calculation of mineralized area at day 10 (mean ± s.d., n=5; **P<0.01 vs cells incubated without LY294002).
We next considered whether BMP2 could activate the PI3-kinase–Akt pathway, and whether LY294002 could impair BMP2-mediated signaling. To address the first question, we incubated C3H10T1/2 cells with BMP2 in serum-free medium for up to 60 minutes, and measured Akt phosphorylation on Ser473 as an indicator of PI3-kinase activation. Addition of BMP2 stimulated serine phosphorylation of Smad1, Smad5 and Smad8 within 15 minutes, but had no effect on Akt. Conversely, IGF-I induced phosphorylation of Akt but not that of Smad proteins (Fig. 3A). To examine effects of PI3-kinase inhibition on the acute actions of BMP2, we measured expression of Smad target genes Sox9 and JunB (Chalaux et al., 1998; Zehentner et al., 1999) after addition of BMP2 with or without LY294002 to confluent C3H10T1/2 cells in osteogenic medium. Under these conditions, BMP2 stimulated the progressive accumulation of both mRNAs starting at 12 hours, as well as inducing transcripts for Dlx5 and Runx2 (Fig. 3B). Addition of LY294002 had no effect on the kinetics of Sox9 or JunB gene expression, or mRNA abundance, but completely prevented accumulation of Dlx5 or Runx2 mRNA. Thus, LY294002 interferes selectively with BMP2-induced Smad-regulated gene activation.
Fig. 3.
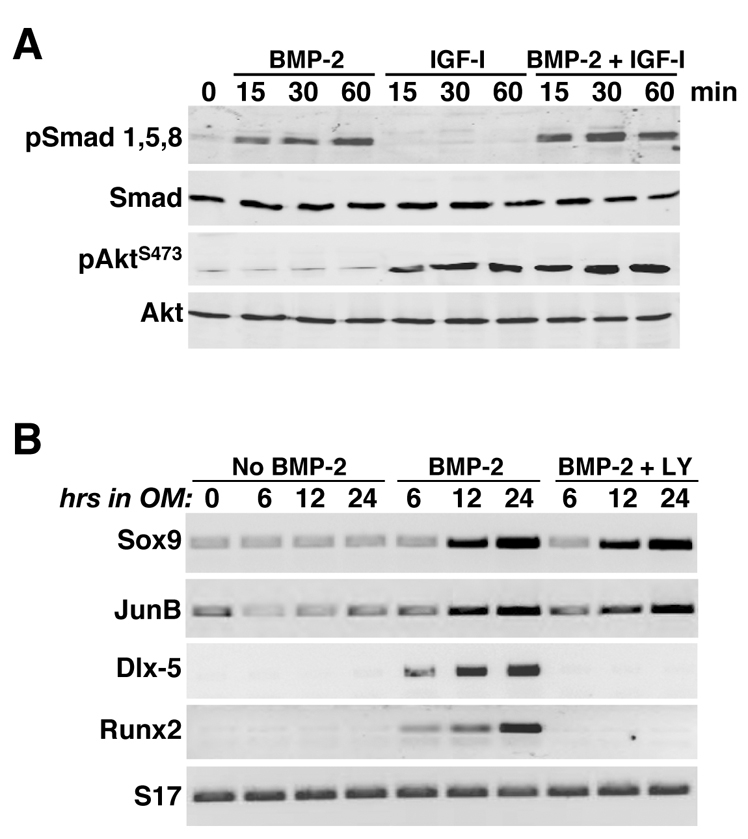
Acute effects of BMP2 on signaling and gene expression. (A) BMP2 activates Smads but not Akt. Immunoblots of whole-cell protein lysates for serine phosphorylated Smad1, Smad5 and Smad8 (pSmad1,5,8), total Smads, Akt phosphorylated at Ser473 (pAktS473), and total Akt after incubation of C3H10T1/2 cells in serum-free medium with BMP2 (200 ng/ml), 10 nM IGF-I or both growth factors for 0, 15, 30 or 60 minutes. (B) Results of RT-PCR experiments for mRNA encoding Sox9, JunB, Dlx-5, Runx2 and S17 after incubation for 0, 6, 12, or 24 hours in osteogenic medium without BMP2, with BMP2 or with BMP2 plus 20 μM LY294002.
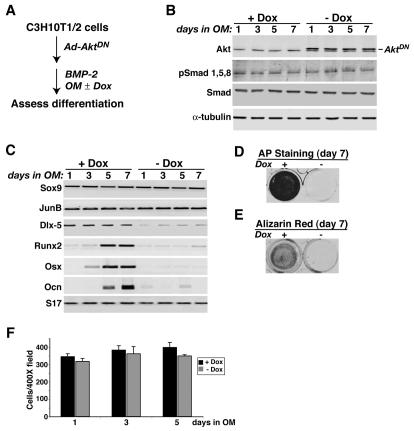
To expand these observations, we next used adenovirus-mediated gene transfer to deliver a regulated dominant-negative version of the serine-threonine protein kinase Akt (AktDN) to C3H10T1/2 cells, to assess whether inhibition of Akt activity also could block BMP2-mediated osteoblast differentiation (see Fig. 4A). Synthesis of AktDN in our adenoviral delivery system was prevented by the antibiotic doxycycline (Dox). In the presence of Dox, no AktDN was produced (Fig. 4B) and osteoblast-specific genes were induced, alkaline phosphatase activity accumulated and bone matrix mineralization proceeded normally (Fig. 4C-E). However, when Dox was omitted from the culture medium, AktDN accumulated in the cells (Fig. 4B), and as a result, the expression of osteoblast genes was impaired, alkaline phosphatase activity was eliminated and mineralization prevented (Fig. 4C-E), even though BMP2-activated Smad phosphorylation and Sox9 and JunB gene expression appeared to be normal (Fig. 4B,C). In other mesenchymal cell derivatives, including skeletal muscle, inhibition of signaling through Akt can block differentiation by promoting cell death (Fujio et al., 2001; Lawlor and Rotwein, 2000), yet under the conditions of these experiments, cell numbers remained constant whether or not Akt signaling was impaired by expression of AktDN (Fig. 4F). Thus, taken together, the results in Figs 2, 3, 4 show that inhibition of either PI3-kinase or Akt activity blocked all aspects of BMP2-mediated osteogenic differentiation of cultured mesenchymal stem cells, apparently without interfering with Smad function or impairing cell viability.
Fig. 4.
Dominant-negative Akt (AktDN) blocks BMP2-stimulated osteoblast differentiation. C3H10T1/2 cells were infected with Ad-AktDN and Ad-tTA and incubated in osteogenic medium with BMP2 with or without doxycycline (Dox) for up to 7 days. (A) Experimental scheme. (B) Immunoblots of whole-cell protein lysates for Akt, AktDN, pSmad1,5,8, total Smads and α-tubulin. (C) Results of RT-PCR experiments for mRNA encoding Sox9, JunB, Dlx-5, Runx2, Osx, Ocn and S17. (D) Results of alkaline phosphatase staining on day 7. (E) Measurement of mineralization by Alizarin red staining on day 7. (F) Measurement of cell numbers after 1, 3, or 5 days in osteogenic medium.
An inducible Akt promotes osteoblast differentiation in the presence of IGFBP5 or the PI3-kinase inhibitor LY294002
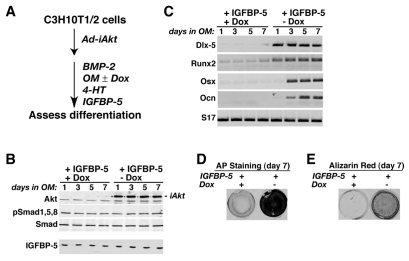
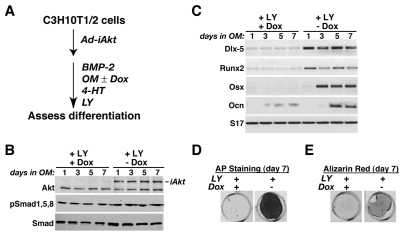
To test the hypothesis that the PI3-kinase–Akt pathway has an essential role in IGF-regulated osteogenic differentiation, we next asked whether an inducible-activated Akt (iAkt) could reverse the inhibition of differentiation seen with either IGFBP5 or LY294002 (see Fig. 5A and Fig. 6A). Synthesis of iAkt by the adenoviral gene delivery vehicle was prevented by Dox, and stimulated in its absence (Fig. 5B and Fig. 6B), and full Akt enzymatic activity of the fusion protein was induced by the selective estrogen receptor modulator 4-hydoxytamoxifen (4-HT), thus bypassing normal regulatory mechanisms (Tureckova et al., 2001). As shown in Fig. 5C, BMP2-mediated osteoblast-specific gene expression was blocked by IGFBP5 but was restored by iAkt, as was accumulation of alkaline phosphatase and mineralization (Fig. 5C-E, lanes `+IGFBP5, –Dox'). Similar results were observed in cells incubated with LY294002 (Fig. 6); once iAkt was produced and activated, osteoblast differentiation proceeded normally, even in the presence of the PI3-kinase inhibitor (Fig. 6C-E, lanes `+LY294002, –Dox'). These latter data additionally show that the dose of LY294002 used was not toxic, because its effects could be reversed. Based on the results depicted in Figs 2, 4, 5 and 6, we conclude that the IGF-stimulated PI3-kinase–Akt pathway is required for BMP2-mediated osteogenic differentiation of cultured mesenchymal stem cells. Since both Smad phosphorylation and activation of Smad-dependent genes appeared to be normal when IGF-stimulated PI3-kinase or Akt were blocked, it seems likely that the point of interaction between the two growth-factor-initiated signaling cascades is downstream of Smad action and upstream of induction of osteoblast-specific gene expression.
Fig. 5.
Active Akt reverses the inhibitory effects of IGFBP5 on BMP2-mediated osteoblast differentiation. C3H10T1/2 cells were infected with Ad-iAkt and Ad-tTA, and incubated in osteogenic medium with BMP2, 4-hydroxytamoxifen (4-HT), purified mouse IGFBP-5 and with or without Dox for up to 7 days. (A) Experimental scheme. (B) Immunoblots of whole-cell protein lysates for Akt, iAkt, pSmad1,5,8 and total Smads, and immunoblot of conditioned medium for IGFBP5. (C) RT-PCR experiments for mRNA encoding Dlx-5, Runx2, Osx, Ocn and S17. (D) Alkaline phosphatase activity on day 7. (E) Assessment of mineralization by Alizarin red staining on day 7.
Fig. 6.
Active Akt promotes osteoblast differentiation in the presence of a PI3-kinase inhibitor. C3H10T1/2 cells were infected with Ad-iAkt and Ad-tTA, and incubated in osteogenic medium with BMP2, LY294002, 4-hydroxytamoxifen (4-HT) with or without Dox for up to 7 days. (A) Experimental scheme. (B) Immunoblots of whole-cell protein lysates for Akt, iAkt, pSmad1,5,8 and total Smads. (C) Results of RT-PCR experiments for mRNA encoding Dlx-5, Runx2, Osx, Ocn and S17. (D) Alkaline phosphatase staining on day 7. (E) Measurement of mineralization by Alizarin red staining on day 7.
Akt activity is required during all phases of osteoblast differentiation and function
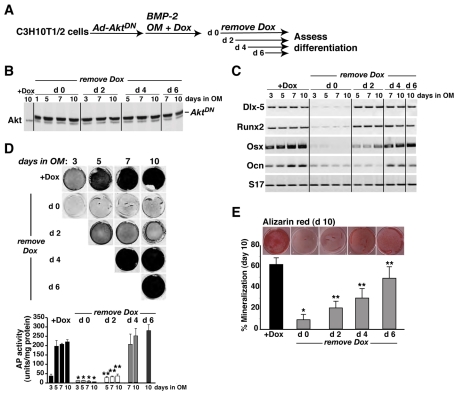
Osteogenic differentiation is a multi-step process, which begins with expression of osteoblast-specific transcription factors (Lian et al., 2006), and proceeds with production of bone-specific proteins, deposition of extracellular matrix and its subsequent mineralization (Balcerzak et al., 2003; Hoshi et al., 2000; Lian et al., 2006). As depicted in Figs 2, 4, 5 and 6, IGF-mediated Akt activity appears to be a necessary collaborator with BMP2-stimulated signaling pathways for initiating osteoblast differentiation. To begin to address whether Akt actions also are needed for later events in bone cell maturation and function, we devised a way to activate AktDN by removal of Dox from the medium at different times during the course of BMP2-mediated osteogenesis of mesenchymal stem cells (see Fig. 7A). As shown in Fig. 7B, in the presence of Dox, no AktDN was synthesized, whereas its sustained production was seen beginning 1 day after Dox removal from culture medium. Analysis of osteoblast gene expression revealed complete inhibition when AktDN was present at the onset of differentiation (Fig. 7C, Dox removal on day 0; also see Fig. 4C). By contrast, Dlx5 and Runx2 mRNA was fully induced even when AktDN was present from day 3 onwards (Fig. 7C, Dox removal on day 2), although expression of genes encoding Osx and Ocn was diminished by ∼50-60%, and only reached maximal values when normal differentiation conditions were sustained for a longer interval (Fig. 7C, Dox removal on day 4). Similarly, alkaline phosphatase enzymatic activity, a measure of differentiated osteoblast function, was inhibited by >85% if AktDN was present by day 3, but was fully induced in cells if the inhibitor did not appear until day 5 (Fig. 7D, compare Dox removal on day 2 and day 4). Taken together, the results in Fig. 7C,D indicate a requirement for continuous IGF-stimulated Akt activity for at least the first few days of BMP2-directed osteoblast differentiation in order for sufficient bone-specific mRNAs and proteins to be produced to sustain differentiated functions. However, matrix mineralization, a later event in the process of osteoblast maturation, appears to have a qualitatively different set of requirements, because the presence of AktDN from day 5 onward prevented 50% of full mineralization by day 10, and expression of AktDN from day 7 onwards still blocked ∼25% of the normal accumulation of mineralized matrix at day 10 (Fig. 7E, compare Dox removal on day 4 and day 6 with +Dox), even though at these time points AktDN had no inhibitory effects on expression of bone genes or on alkaline phosphatase activity. Our provisional interpretation of the results in Fig. 7 is that there are several temporally distinct Akt targets in differentiating osteoblasts that govern different aspects of bone cell development and function.
Fig. 7.
Continual Akt activity is necessary for osteoblast differentiation, maturation and function. C3H10T1/2 cells were infected with Ad-AktDN and Ad-tTA and incubated in osteogenic medium with BMP2 and Dox. Dox was removed sequentially at days 0, 2, 4 or 6 to induce expression of AktDN. (A) Experimental scheme. (B) Immunoblots of whole-cell protein lysates for Akt and AktDN. (C) Results of RT-PCR assays for mRNA encoding Dlx-5, Runx2, Osx, Ocn and S17 at day 3, 5, 7 and 10. (D) Results of alkaline phosphatase activity measured at day 3, 5, 7 and 10 by staining and by in vitro enzymatic assay (graph) after removal of Dox on different days (mean ± s.d., n=3 experiments; *P<0.001, **P<0.01 vs +Dox). (E) Mineralized area assessed by Alizarin red staining at day 10 after removal of Dox on different days (mean ± s.d., n=5 experiments; *P<0.001, **P<0.05 vs +Dox at day 10). Representative images are depicted above the graph.
Signaling through the PI3-kinase–Akt pathway is required for growth of isolated mouse metatarsal bones
Short-term culture of neonatal mouse metatarsal bones has been used previously to study bone growth and endochondral ossification (Krishnan et al., 2003; Mukherjee et al., 2005). As we have shown recently, these bones increased in length by >35% in serum-free medium over a 10-day culture period, but growth was blocked by IGFBP5 (Mukherjee and Rotwein, 2008). As also depicted in Fig. 8, a single addition of 20 μM LY294002 at 1 day after plating reduced growth to <5% over the same time course. We observed similarly dramatic inhibitory effects on longitudinal growth in metatarsals infected with Ad-AktDN at the beginning of ex vivo culture, whereas, by contrast, infection with Ad-EGFP or Ad-AktDN in the presence of Dox, was completely ineffective (Fig. 9). Thus, in this model system, the PI3-kinase–Akt pathway appears to be needed for normal bone growth.
Fig. 8.
A PI3-kinase inhibitor prevents growth of cultured neonatal mouse metatarsal bones. Neonatal mouse metatarsals were incubated in DMEM containing 0.5% BSA with or without 20 μM LY294002 for up to 10 days. (A) Representative images of metatarsal bones after incubation with or without LY294002 for 10 days. (B) Relative change in metatarsal bone length after incubation with or without LY294002 for 4, 7 or 10 days compared with day 0 (mean ± s.d., n=3 experiments; *P<0.001 vs +LY294002).
Fig. 9.
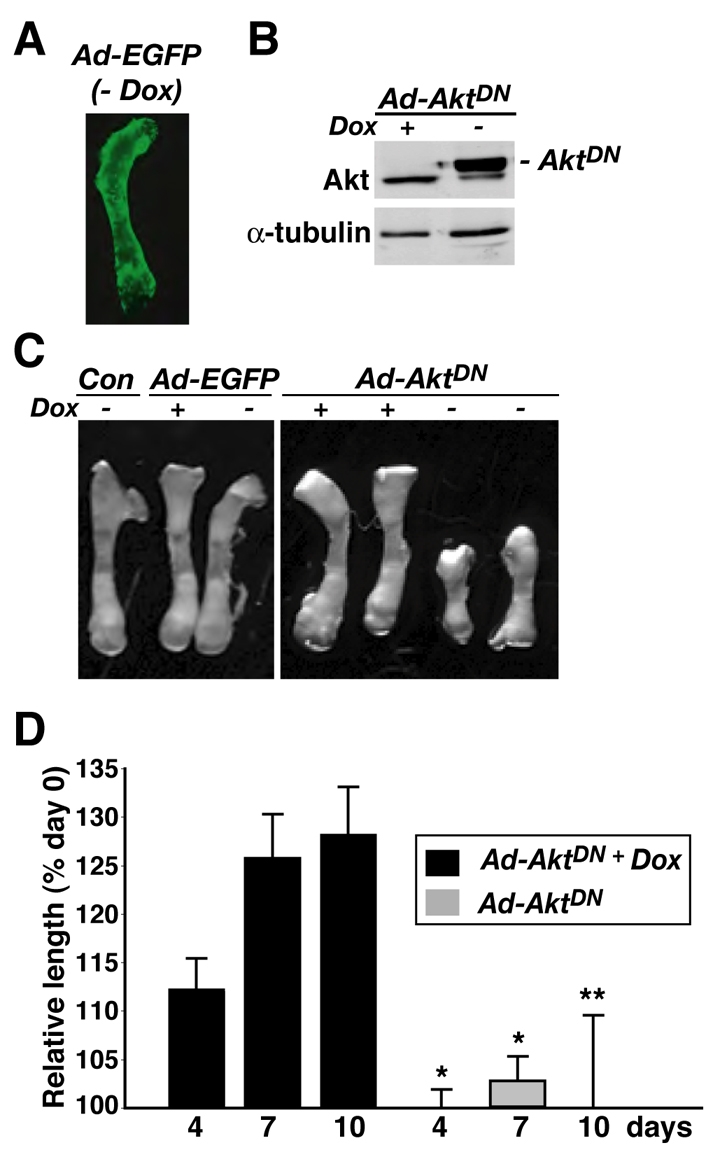
AktDN inhibits growth of cultured neonatal mouse metatarsal bones. Neonatal mouse metatarsals were infected with Ad-EGFP and Ad-tTa, or Ad-AktDN and Ad-tTa, and were incubated in DMEM containing 0.5% BSA with or without Dox for up to 10 days. (A) Representative image of metatarsal bone for EGFP expression at 10 days after infection with Ad-EGFP and Ad-tTa without addition of Dox. In the presence of Dox no EGFP was detected. (B) Immunoblot showing induction of AktDN in the absence of Dox, and expression of endogenous Akt (lower band) and α-tubulin in tissue lysates from metatarsals after 10 days of culture. (C) Representative images of metatarsal bones after incubation with Ad-EGFP or Ad-AktDN with or without Dox, or no adenovirus (Con) for 10 days. (D) Relative change in metatarsal bone length after infection with Ad-AktDN for 4, 7, or 10 days with or without Dox treatment compared with day 0 (mean ± s.d., n=3 experiments; *P<0.001, **P<0.01 vs +Dox).
Inhibition of Akt activity impairs cartilage growth and osteoblast development and function in isolated mouse metatarsal bones
We analyzed histological sections of mouse metatarsal bones incubated ex vivo for different intervals to address potential mechanisms by which Akt signaling was required for bone growth. At the start of the 10-day culture period, proliferating cartilage made up nearly half of the bone length and, together with hypertrophic cartilage, comprised 85% of the total, with the mid-diaphyseal mineralizing zone comprising the remaining 15% (Fig. 10, top panel). By day 10, this central mineralizing zone had increased to almost 30% of the now longer bone, with proliferating cartilage remaining proportionately constant (54%), and the hypertrophic zone decreasing from 39% to 18% (Fig. 10, second panel). We observed nearly identical results on day 10 of culture with bones infected with Ad-AktDN and incubated with Dox (Fig. 10, third panel), thus illustrating the lack of effect of adenoviral infection on the proportion of different cell types in the developing and growing bone. By contrast, expression of AktDN not only prevented longitudinal metatarsal growth, but also completely inhibited lengthening of the zone of proliferating cartilage and expansion of the mineralized zone (Fig. 10, bottom panel). As a consequence, the histological profile after 10 days of AktDN expression resembled that of control metatarsals at the onset of ex vivo incubation (Fig. 10, compare top and bottom panels). Thus, Akt signaling appears to be required for both cartilage and bone growth in isolated metatarsal bones.
Fig. 10.
AktDN inhibits chondrocyte maturation and osteoblast development in cultured neonatal mouse metatarsal bones. Neonatal mouse metatarsals were infected with Ad-AktDN and Ad-tTa, and incubated in DMEM with 0.5% BSA with or without Dox for 10 days followed by histological analysis. Pictured on the left are hematoxylin and eosin stained sections of representative metatarsals at day 0 and day 10 of culture after control incubations (top two panels) or after infection with Ad-AktDN and Ad-tTa (bottom two panels); ×40 magnification. Zones of proliferating (PC) or hypertrophic chondrocytes (HC) are indicated, as is the central mineralized zone (MZ). The charts in the center represent graphical analysis of each component as a percentage of the total length of each bone. Images on the right show the boxed regions on the left panels at ×100 magnification.
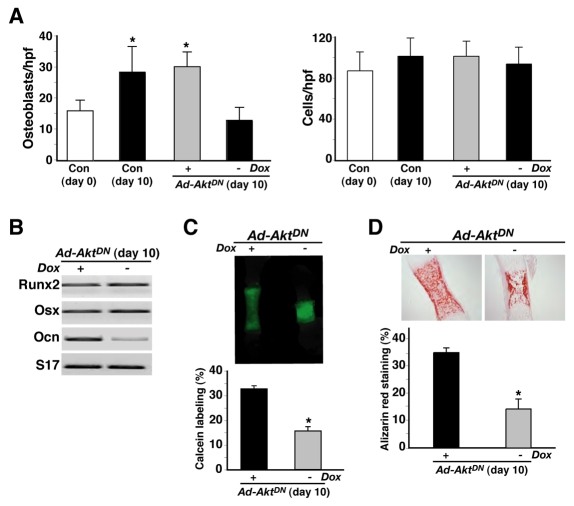
We further examined the effects of inhibiting Akt signaling on osteoblasts within the mid-diaphyseal mineralizing zone. Cell-counting experiments demonstrated that the number of morphologically recognizable osteoblasts per ×400 microscopic field nearly doubled (from 16±3 to 28±7) during 10 days of organ culture, whereas total cell density did not change (Fig. 11A). As the mineralizing zone also expanded twofold in absolute length (Fig. 10), it appears that the total number of osteoblasts increased by a factor of four during ex vivo bone development. We recorded nearly identical results in bones infected with Ad-AktDN and incubated with Dox. By contrast, when AktDN was expressed in the absence of Dox, both osteoblast numbers and total cell density were unchanged at day 10 compared with control metatarsals at day 0 (Fig. 11A). Our interpretation of these results is that Akt appears to be required for osteoblast recruitment and/or differentiation within bone during the early postnatal period in mice, although alternatively, Akt signaling might be necessary for osteoblast viability.
Fig. 11.
AktDN inhibits osteoblast development and function in cultured neonatal mouse metatarsal bones. Neonatal mouse metatarsals were uninfected (Con) or were infected with Ad-AktDN and Ad-tTa, and incubated in DMEM plus 0.5% BSA with or without Dox for 10 days. (A) Cell counts of osteoblasts per field (h.p.f.) in histological sections of the mineralized zone at ×400 magnification [mean ± s.d., n=4; *P<0.001, vs Con (day 0)]. (B) Results of RT-PCR experiments at day 10 for mRNA encoding Runx2, Osx, Ocn, and S17. (C) Representative fluorescent images of calcein-labeled mineralizing zone after incubation for 10 days; graph shows the relative difference in length of the calcein-labeled mineralizing zone at day 10 in metatarsals incubated with or without Dox (mean ± s.d., n=4; *P<0.001, vs +Dox). (D) Representative images showing Alizarin-red-stained histological sections after a incubation for 10 days; graph shows the difference in the Alizarin-red-stained area at day 10 in metatarsals incubated with or without Dox (mean ± s.d., n=4; *P<0.01).
We next assessed osteoblast maturation and function in metatarsal cultures by examining bone-cell-specific gene expression and mineralization. AktDN reduced induction of mRNA encoding Ocn by 70%, but had no effect on transcripts encoding Runx2 or Osx (Fig. 11B). Since Runx2 and Osx are produced by both chondrocytes and osteoblasts, whereas Ocn is synthesized exclusively by differentiated osteoblasts (Karsenty, 1998), we interpret these data to indicate that AktDN interfered with osteoblast development within bone. Similarly, as measured by both calcein labeling of living bones (Fig. 11C) and Alizarin red staining of histological sections (Fig. 11D), AktDN prevented the normal accumulation of mineralized matrix. Thus, based on these results, we conclude that continuous Akt signaling is necessary for full osteoblast maturation and function in vivo, as well as in vitro.
Discussion
BMPs have a central role in bone development and osteoblast differentiation (Li and Cao, 2006), but require interactions with other growth-factor-activated signals. Here, we demonstrate essential crosstalk between BMP2 and the IGF-activated PI3-kinase–Akt pathway to initiate osteogenic differentiation in uncommitted mesenchymal precursor cells, and to promote maturation of committed osteoblasts. We also show that IGF-stimulated and Akt-mediated signaling is crucial for longitudinal bone growth by exerting facilitating effects on both chondrocyte and osteoblast development and function. Based on these results, we conclude that the PI3-kinase–Akt pathway is a crucial component of an interactive osteogenic signaling network.
An IGF-activated PI3-kinase–Akt pathway regulates BMP2-mediated osteoblast differentiation
We previously found that IGFBP5 could block BMP2-regulated osteogenic differentiation of mesenchymal stem cells by sequestering IGF-I and IGF-II from the IGF-I receptor (Mukherjee and Rotwein, 2008), and postulated that through this mechanism it also prevented osteoblast maturation and blocked longitudinal growth of mouse metatarsal bones. We now show that both a chemical PI3-kinase inhibitor and a dominant-negative version of Akt (AktDN), also can inhibit BMP2-initiated osteogenesis (Figs 2 and 4). By contrast, blocking the Mek-Erk signaling pathway had no effect on the onset or progression of BMP2-activated osteoblast differentiation (Fig. 2). As the inhibitory actions of IGFBP5 or LY294002 on BMP2-stimulated osteogenesis could be reversed by a recombinant adenovirus encoding an activated version of Akt (Figs 5 and 6), our results document that Akt-regulated signaling is the key pathway of IGF action in promoting osteoblast differentiation in collaboration with BMPs.
Several previous studies have supported facilitating roles for IGF-mediated signaling in osteogenesis in cell culture models, but with disparate results regarding the intracellular pathways implicated (Merriman et al., 1990; Niu and Rosen, 2005; Strong et al., 1991; Strong et al., 1994). Fujita and colleagues found that either PI3-kinase or Mek inhibitors could reduce the amount of bone-specific alkaline phosphatase produced by cells overexpressing Runx2 (Fujita et al., 2004). Raucci and co-workers also showed that these chemical inhibitors could decrease alkaline phosphatase activity in two additional osteogenic cell lines, and attributed the negative effects of the PI3-kinase inhibitor to enhanced cell death (Raucci et al., 2008). These authors also found that a constitutively active Akt led to increased accumulation of several bone-specific mRNAs, including those encoding Runx2 and Osx (Raucci et al., 2008). Several other groups also showed that chemical inhibition of PI3-kinase or Mek could reduce markers of osteoblast differentiation (Ghosh-Choudhury et al., 2002; Ghosh-Choudhury et al., 2007; Hanai et al., 2006; Noda et al., 2005; Osyczka and Leboy, 2005). Our observations thus appear to be one of the few studies to clearly discriminate between the PI3-kinase–Akt and Mek-Erk pathways in terms of osteogenic outcomes.
What mechanisms might mediate Akt-regulated osteoblast differentiation? A dominant-negative Akt decreased the transcriptional actions of Runx2 in a model system in which Runx2 was overexpressed (Fujita et al., 2004). These authors additionally found that overexpression of Runx2 led to an increase in abundance of Akt and of both regulatory and catalytic PI3-kinase subunits, and postulated the existence of a positive-feedback loop in which Runx2 upregulated components of the PI3-kinase–Akt pathway, which then enhanced the functions of Runx2 (Fujita et al., 2004). Others have found that a dominant-negative Akt could reduce the activity of a BMP2-dependent promoter-reporter gene, possibly by inhibiting the nuclear accumulation of activated Smad1 and Smad5 (Ghosh-Choudhury et al., 2002), but these results have not been replicated, and we see no inhibitory effects of dominant-negative Akt on Smad-mediated Sox9 and JunB gene expression (Fig. 4B). In another mesenchymal derivative, skeletal muscle, Akt has been shown to collaborate with myogenic transcription factors to enhance the abundance of transcriptional co-activators on muscle gene promoters (Wilson and Rotwein, 2007), and to reduce co-repressors (Serra et al., 2007). It thus might be reasonable to postulate an analogous role for Akt signaling in osteoblast differentiation, although to date there is little experimental evidence for or against this idea.
Akt signaling is required in all phases of osteoblast differentiation and maturation
Osteoblast differentiation can be divided into several phases, including lineage commitment, characterized in part by expression of Runx2 and Osx (Komori, 2008), early differentiation, in which other bone-cell-specific mRNAs and proteins are produced (Deng et al., 2008), and maturation, marked by accumulation of bone-specific alkaline phosphatase, extracellular matrix deposition and mineralization (Balcerzak et al., 2003; Hoshi et al., 2000; Zaidi, 2007). We now find that Akt activity appears to be required for each of these stages of differentiation, because AktDN can block progression from one step to the next (Fig. 7). Most remarkable in this regard is the inhibitory action of AktDN on mineralization, which was seen even when AktDN was added relatively late in the differentiation process, at a time when alkaline phosphatase activity was already maximal (compare Fig. 7D with 7E). These latter results, which complement our previous data using IGFBP5 to block osteogenesis (Mukherjee and Rotwein, 2008), are supported by the inhibitory effects of AktDN on osteoblast maturation and function in cultured metatarsal bones (Fig. 11), and also are consistent with the defective mineralization phenotype seen in mice lacking the IGF-I receptor in mature osteoblasts (Zhang et al., 2002). Mineralization represents the outcome of a complex series of steps that include active transport of calcium and inorganic phosphate into osteoblasts, release of matrix vesicles into the extracellular space, and nucleation and deposition of hydroxyapatite granules in the osteoid (Balcerzak et al., 2003; Hoshi et al., 2000). Among factors that control mineralization is the sodium-dependent phosphate transporter Pit-1, which appears to be regulated by IGF-I in osteoblasts (Kavanaugh and Kabat, 1996; Palmer et al., 1997; Selz et al., 1989). As mineralization is an important step in fracture healing (Schindeler et al., 2008), an understanding of the regulatory mechanisms has the potential to lead to better treatment options.
Akt signaling in bone growth and endochondral ossification
We showed previously that incubation with IGFBP5 prevented both longitudinal growth and mineralization of cultured neonatal mouse metatarsal bones, and found that the inhibitory effects of IGFBP5 depended on its ability to bind IGFs with high affinity (Mukherjee and Rotwein, 2008). We now show that a chemical PI3-kinase inhibitor and adenoviral-delivered AktDN also block metatarsal growth (Figs 8 and 9). In these experiments, the metatarsals were incubated in serum-free medium, and because IGFBP5, LY294002 and AktDN all exerted similar inhibitory effects, we conclude that locally produced IGFs are responsible for activating the PI3-kinase–Akt signaling pathway that is essential for longitudinal bone growth. The defects seen in our metatarsal model resemble the bone phenotype in mice lacking the IGF-I receptor (Zhang et al., 2002) or both Akt1 and Akt2 (Peng et al., 2003), in which ossification was delayed and osteopenia resulted. Conversely, the opposite phenotype was observed in transgenic mice lacking Pten in osteoblasts, in which progressive increases in bone volume and density were seen throughout life (Liu et al., 2007). As Pten dephosphorylates and inactivates phosphatidylinositol (3,4,5)-trisphosphate (PIP3), the product of PI3-kinase, and because PIP3 is essential for membrane targeting and activation of Akt (Franke, 2008; Hanada et al., 2004), these results predict enhanced Akt activity in Pten-deficient osteoblasts. In fact, cultured calvarial osteoblasts engineered to lack Pten did show increased Akt phosphorylation and phosphorylation of several Akt substrates, and the cells differentiated more extensively than controls (Liu et al., 2007). No results have been reported yet on mice in which a constitutively active Akt has been targeted to osteoblasts, but a similarly high bone density phenotype might be anticipated.
Akt is essential for optimal chondrogenesis and osteogenesis
Histological analysis of growing mouse metatarsal bones revealed a growth-associated proportional increase in the zone of proliferating chondrocytes, a decline in the extent of terminally differentiated hypertrophic chondrocytes and a more-than-proportional rise in the length of the central mineralized zone, which contained osteoblasts as well as other cell types (Fig. 10). These growth-related changes in the profile of cell types within the cultured metatarsals were completely inhibited by AktDN (Fig. 10), thus demonstrating negative effects on both chondrocyte and osteoblast development. In their analysis of isolated tibias from E15.5 mouse embryos, Ulici and colleagues also found that LY294002 could impair chondrocyte differentiation and inhibit longitudinal bone growth (Ulici et al., 2008). In addition, AktDN blocked the normal twofold increase in osteoblast number seen in the central mineralizing zone, and also impaired both osteoblast maturation and function, as measured by diminished osteocalcin gene expression and reduced mineralization (Fig. 11). Thus, sustained Akt activity appears to be required for the normal cartilage and bone cell development that leads to longitudinal bone growth during the early postnatal period, at least in metatarsals. These observations are additionally supported by previous studies, which have suggested that Akt signaling is required for proteoglycan and collagen production in chondrocytes mediated by Runx2 (Fujita et al., 2004), and that Akt1 is important for normal rates of bone formation and for preventing osteoblast apoptosis (Kawamura et al., 2007).
In summary, we have shown that the IGF-activated PI3-kinase–Akt signaling pathway is a potent facilitator of osteoblast differentiation, bone growth, and mineralization. Our results point to a key role for IGF-mediated signaling in all phases of osteogenesis, and provide an impetus to define the mechanisms of interaction with BMPs and other regulators of cartilage and bone development and function.
Materials and Methods
Reagents
Fetal calf serum, horse serum, Dulbecco's modified Eagle's medium (DMEM), and phosphate-buffered saline (PBS) were purchased from Mediatech-Cellgrow (Herndon, VA). Okadaic acid was from Alexis Biochemicals (San Diego, CA), and NBT/BCIP tablets and protease inhibitor tablets were from Roche Applied Sciences (Indianapolis, IN). Calcein, sodium orthovanadate, alizarin red, ascorbic acid and β-glycerol phosphate were purchased from Sigma (St Louis, MO). Trypsin-EDTA solution and Superscript III first-strand synthesis kit were from Invitrogen (Carlsbad, CA). The BCA protein assay kit was from Pierce Biotechnologies (Rockford, IL) and Immobilon-FL was from Millipore Corporation (Billerico, MA). AquaBlock EIA/WIB solution was from East Coast Biologicals (North Berwick, ME). IGF-I (Gropep) was stored at –80°C at a concentration of 10 mM in 0.01 M HCl until use. Doxycycline (Dox, Clontech, Palo Alto, CA) was used at a final concentration of 1 mg/ml. LY294002 (Biomol Research Laboratories, Plymouth Meeting, PA) was stored in dimethyl sulfoxide at –20°C at a concentration of 20 mM until use; 4-hydroxytamoxifen (HT) was from Sigma, and was stored in ethanol at –20°C at a concentration of 50 mM until use. UO126 (Promega, Madison, WI) was stored in ethanol at –80°C at a concentration of 20 mM until use. Other chemicals were purchased from commercial vendors.
Antibodies
The following polyclonal antibodies were purchased from commercial suppliers: anti-Smad1, anti-Akt, anti-phospho-Akt (Ser473), anti-Erk1 and 2, anti-phospho-Erk1 and -Erk2, Cell Signaling Technology (Beverly, MA); anti-α-tubulin, Sigma; anti-IGFBP-5, anti-phospho-Smad, Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal anti-T7 antibody was from Novagen (San Diego, CA) and anti-HA was from Roche Applied Sciences (Indianapolis, IN). Goat anti-rabbit IgG-IR800 and goat anti-mouse IgG-IR680 were from Rockland Immunochemical (Gilbertsville, PA).
Recombinant adenoviruses
The following adenoviruses (Ad) have been described: Ad-EGFP (Tureckova et al., 2001), Ad-IGFBP-5 (Mukherjee et al., 2008), Ad-BMP2 (Mukherjee and Rotwein, 2008), Ad-tTA (tetracycline transactivator protein), Ad-iAkt (inducible Akt) (Tureckova et al., 2001) and Ad-AktDN [dominant negative Akt (Wilson et al., 2003)]. All viruses were purified by centrifugation through CsCl density gradients and titered as described (Wilson et al., 2003).
Production of BMP2 and IGFBP5
C3H10T1/2 mouse embryonic fibroblasts (ATCC: CCL226) were incubated at 37°C in humidified air with 5% CO2 in DMEM with 10% fetal calf serum. Cells were infected at ∼50% of confluent density with Ad-BMP2 [multiplicity of infection (MOI) of 500]. The following day, medium was replaced with DMEM plus 2% horse serum; 2 days later, conditioned medium was collected, clarified, and stored in aliquots at –80°C until use. The concentration of BMP2 was determined by immunoblotting with purified standards purchased from R&D systems (Minneapolis, MN) (see supplementary material Fig. S2). Mouse IGFBP-5 was produced in C3H10T1/2 cells following infection with Ad-IGFBP-5, and was purified by heparin affinity chromatography (Mukherjee et al., 2008).
Osteogenic differentiation
Confluent C3H10T1/2 cells were incubated in osteogenic medium (DMEM, 10% fetal calf serum, 50 μg/ml ascorbic acid, 10 mM β-glycerol phosphate and 200 ng/ml BMP2) in the absence or presence of LY294002 (20 μM), UO126 (10 μM), or IGFBP-5 (150 nM). osteogenic medium was replaced every 48 hours for up to 10 days. Cell counting was performed as described (Wilson and Rotwein, 2007). Alternatively, C3H10T1/2 cells were infected at ∼50% of confluent density with Ad-tTA at an MOI of 125, and either Ad-EGFP, Ad-iAkt, or Ad-AktDN at MOIs of 500. One day later, cells were washed and osteogenic medium was added, along with other chemicals as described in individual figure legends.
Mouse metatarsal bone culture
Metatarsal bones were isolated from newborn C57BL6 mice (days 0-3 after birth), as described (Krishnan et al., 2001; Mukherjee and Rotwein, 2008) and were incubated in DMEM containing 0.5% bovine serum albumin, 50 μg/ml ascorbic acid, 1 mM β-glycerol phosphate and 100 μg/ml penicillin-streptomycin solution at 37°C in humidified air with 5% CO2 for up to 10 days. In some experiments LY294002 (20 μM) was added the next day. In others, bones were infected the next day with Ad-tTA (1×107 PFU/ml) plus either Ad-EGFP or Ad-AktDN (6×107 PFU/ml). Images were captured at days 1, 4, 7 and 10 with a Nikon DXL1200 camera attached to a Lieca MZ FLIII microscope. Mineralization was assessed by addition of medium containing calcein (500 ng/ml) for 2 hours. After rinsing three times with PBS, fluorescent images were captured with a Roper Scientific Cool Snap FX CCD camera attached to a Nikon Eclipse T300 microscope using an Apple PowerPC computer running IP Labs Scientific Image Processing software v3.9.4r2 (Scanalytics, Rockville, MD).
Bone histology
Metatarsals were fixed in 4% paraformaldehyde for 18 hours at 4°C and stored in 70% ethanol. Bones were embedded in paraffin blocks and sectioned. Staining with hematoxylin and eosin or with alizarin red was performed after hydrating slides in graded concentrations of ethanol and water, as described (Mukherjee et al., 2005; Serra et al., 1999). After staining, sections were dehydrated, coverslips added and images were captured using a MicroPublisher cooled CCD camera (QImaging, Surrey, British Columbia) attached to a Nikon Eclipse E800 compound microscope. For cell counting, osteoblasts were identified in the central mineralized zone by the cuboidal morphology of their nuclei, and were counted at ×400 magnification in histological sections stained with hematoxylin and eosin.
Alkaline phosphatase staining
Cells were washed with PBS, fixed with 70% ethanol for 10 minutes, and incubated with 500 ml NBT/BCIP solution (1 tablet in 10 ml distilled water) for 20 minutes at 20°C (Mukherjee and Rotwein, 2008). After three washes with distilled water, images were captured and analyzed with the LiCoR Odyssey Infrared Imaging System, using software version 1.2 (LiCoR, Lincoln, NE). Alkaline phosphatase activity was determined spectrophotometrically at 405 nM after incubating cell lysates (10 μg) in a 96-well format for 20 minutes at 20°C in 50 μl of a 1 mg/ml solution of p-nitrophenyl phosphate (Mukherjee and Rotwein, 2008).
Alizarin red staining
Cells were fixed in 70% ethanol for 10 minutes, and stained with 2% alizarin red solution (pH 4.1-4.5) for 1 minute at 20°C (Mukherjee and Rotwein, 2008). Images were obtained by scanning plates on a Canon flat-bed scanner or with the LiCoR Odyssey and results were quantified as described (Mukherjee and Rotwein, 2008).
Analysis of gene expression
Whole-cell RNA (2 μg), isolated as described (Mukherjee and Rotwein, 2008), was reverse-transcribed with the Superscript III first-strand synthesis kit, using oligo (dT) primers in a final volume of 20 μl. PCR reactions were performed with 1 μl of cDNA per reaction and previously published primer pairs for mouse Dlx5, Runx2, osterix, osteocalcin and S17 (Mukherjee and Rotwein, 2008). Other oligonucleotide primers are as follows: mouse Sox9: top strand, 5′-AGGAAGCTGGCAGACCAGTA-3′; bottom strand, 5′-CGTTCTTCACCGACTTCCTC-3′; mouse JunB: top strand, 5′-ACGGAGGGAGAGAAAAGCTC-3′; bottom strand, 5′-AAGGCTGTTCCATTTTCGTG-3′. Cycle numbers ranged from 20-30 and results were visualized after agarose gel electrophoresis.
Protein extraction and immunoblotting
Whole-cell protein lysates and conditioned cultured medium were prepared from C3H10T1/2 cells as described (Mukherjee et al., 2008; Mukherjee and Rotwein, 2008), and aliquots were stored at –80°C until use. Metatarsals were homogenized in cell lysis buffer with protease inhibitors using a hand-held Teflon homogenizer. After centrifugation at 14,000 r.p.m. for 10 minutes at 4°C in a microcentrifuge, supernatants were collected and stored at –80°C until use. Protein samples (30 μg/lane) or medium (25 μl/lane) were resolved by SDS-PAGE and transferred to Immobilon-FL membranes. After blocking with 25% AquaBlock solution for 1 hour at 20°C, membranes were incubated sequentially with primary and secondary antibodies (Mukherjee and Rotwein, 2008). The following primary antibodies were used at a dilution of 1:1000: anti-Akt, anti-phospho-Akt (Ser473), anti-IGFBP-5, anti-phospho-Smad, anti-Smad1, anti-HA, anti-Erk1/2 and anti-phospho-Erk1/2. Anti-T7 was used at 1:5000 and anti-α-tubulin at 1:15,000. Secondary antibodies were used at 1:5000. Results were visualized and images captured using the LiCoR Odyssey and version 1.2 analysis software.
Supplementary Material
We thank Svetlana Lutsenko for reagents and Ronen Schweitzer for use of his microscope and imaging system, and appreciate the assistance of the histology core of the Department of Pathology at OHSU. The studies presented in this manuscript were supported by National Institutes of Health R01 grants DK42748 and DK63073 (to P. R). Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/5/716/DC1
References
- Akune, T., Ogata, N., Hoshi, K., Kubota, N., Terauchi, Y., Tobe, K., Takagi, H., Azuma, Y., Kadowaki, T., Nakamura, K. et al. (2002). Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J. Cell Biol. 159, 147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, L. A., Headey, S. J. and Norton, R. S. (2005). IGF-binding proteins-the pieces are falling into place. Trends Endocrinol. Metab. 16, 228-234. [DOI] [PubMed] [Google Scholar]
- Balcerzak, M., Hamade, E., Zhang, L., Pikula, S., Azzar, G., Radisson, J., Bandorowicz-Pikula, J. and Buchet, R. (2003). The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim. Pol. 50, 1019-1038. [PubMed] [Google Scholar]
- Chalaux, E., Lopez-Rovira, T., Rosa, J. L., Bartrons, R. and Ventura, F. (1998). JunB is involved in the inhibition of myogenic differentiation by bone morphogenetic protein-2. J. Biol. Chem. 273, 537-543. [DOI] [PubMed] [Google Scholar]
- Deng, Z. L., Sharff, K. A., Tang, N., Song, W. X., Luo, J., Luo, X., Chen, J., Bennett, E., Reid, R., Manning, D. et al. (2008). Regulation of osteogenic differentiation during skeletal development. Front. Biosci. 13, 2001-2021. [DOI] [PubMed] [Google Scholar]
- Duan, C. and Xu, Q. (2005). Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 142, 44-52. [DOI] [PubMed] [Google Scholar]
- Franke, T. F. (2008). Intracellular signaling by Akt: bound to be specific. Sci. Signal. 1, e29. [DOI] [PubMed] [Google Scholar]
- Fujio, Y., Mitsuuchi, Y., Testa, J. R. and Walsh, K. (2001). Activation of Akt2 Inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 8, 1207-1212. [DOI] [PubMed] [Google Scholar]
- Fujita, T., Azuma, Y., Fukuyama, R., Hattori, Y., Yoshida, C., Koida, M., Ogita, K. and Komori, T. (2004). Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 166, 85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury, N., Abboud, S. L., Nishimura, R., Celeste, A., Mahimainathan, L. and Choudhury, G. G. (2002). Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 277, 33361-33368. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury, N., Mandal, C. C. and Choudhury, G. G. (2007). Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J. Biol. Chem. 282, 4983-4993. [DOI] [PubMed] [Google Scholar]
- Giustina, A., Mazziotti, G. and Canalis, E. (2008). Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 29, 535-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidakis, D. J. and Androulakis, I. I. (2006). Bone remodeling. Ann. NY Acad. Sci. 1092, 385-396. [DOI] [PubMed] [Google Scholar]
- Hanada, M., Feng, J. and Hemmings, B. A. (2004). Structure, regulation and function of PKB/AKT-a major therapeutic target. Biochim. Biophys. Acta 1697, 3-16. [DOI] [PubMed] [Google Scholar]
- Hanai, Y., Tokuda, H., Ishisaki, A., Matsushima-Nishiwaki, R., Nakamura, N., Yoshida, M., Takai, S., Ohta, T. and Kozawa, O. (2006). Involvement of p44/p42 MAP kinase in insulin-like growth factor-I-induced alkaline phosphatase activity in osteoblast-like-MC3T3-E1 cells. Mol. Cell Endocrinol. 251, 42-48. [DOI] [PubMed] [Google Scholar]
- Herpin, A. and Cunningham, C. (2007). Cross-talk between the bone morphogenetic protein pathway and other major signaling pathways results in tightly regulated cell-specific outcomes. FEBS J. 274, 2977-2985. [DOI] [PubMed] [Google Scholar]
- Hoshi, K., Ejiri, S. and Ozawa, H. (2000). Ultrastructural, cytochemical, and biophysical aspects of mechanisms of bone matrix calcification. Kaibogaku Zasshi 75, 457-465. [PubMed] [Google Scholar]
- Jiang, J., Lichtler, A. C., Gronowicz, G. A., Adams, D. J., Clark, S. H., Rosen, C. J. and Kream, B. E. (2006). Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone 39, 494-504. [DOI] [PubMed] [Google Scholar]
- Kadowaki, T., Tobe, K., Honda-Yamamoto, R., Tamemoto, H., Kaburagi, Y., Momomura, K., Ueki, K., Takahashi, Y., Yamauchi, T., Akanuma, Y. et al. (1996). Signal transduction mechanism of insulin and insulin-like growth factor-1. Endocr. J. 43 Suppl., S33-S41. [DOI] [PubMed] [Google Scholar]
- Karsenty, G. (1998). Transcriptional regulation of osteoblast differentiation during development. Front Biosci. 3, d834-d837. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, M. P. and Kabat, D. (1996). Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int. 49, 959-963. [DOI] [PubMed] [Google Scholar]
- Kawamura, N., Kugimiya, F., Oshima, Y., Ohba, S., Ikeda, T., Saito, T., Shinoda, Y., Kawasaki, Y., Ogata, N., Hoshi, K. et al. (2007). Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS. ONE. 2, e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla, S., Westendorf, J. J. and Oursler, M. J. (2008). Building bone to reverse osteoporosis and repair fractures. J. Clin. Invest 118, 421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori, T. (2008). Regulation of bone development and maintenance by Runx2. Front Biosci. 13, 898-903. [DOI] [PubMed] [Google Scholar]
- Krishnan, V., Ma, Y., Moseley, J., Geiser, A., Friant, S. and Frolik, C. (2001). Bone anabolic effects of sonic/indian hedgehog are mediated by bmp-2/4-dependent pathways in the neonatal rat metatarsal model. Endocrinology 142, 940-947. [DOI] [PubMed] [Google Scholar]
- Krishnan, V., Moore, T. L., Ma, Y. L., Helvering, L. M., Frolik, C. A., Valasek, K. M., Ducy, P. and Geiser, A. G. (2003). Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol. Endocrinol. 17, 423-435. [DOI] [PubMed] [Google Scholar]
- Lawlor, M. A. and Rotwein, P. (2000). Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 20, 8983-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. H., Kim, Y. J., Kim, H. J., Park, H. D., Kang, A. R., Kyung, H. M., Sung, J. H., Wozney, J. M., Kim, H. J. and Ryoo, H. M. (2003). BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 278, 34387-34394. [DOI] [PubMed] [Google Scholar]
- Li, X. and Cao, X. (2006). BMP signaling and skeletogenesis. Ann. NY Acad. Sci. 1068, 26-40. [DOI] [PubMed] [Google Scholar]
- Lian, J. B., Stein, G. S., Javed, A., van Wijnen, A. J., Stein, J. L., Montecino, M., Hassan, M. Q., Gaur, T., Lengner, C. J. and Young, D. W. (2006). Networks and hubs for the transcriptional control of osteoblastogenesis. Rev. Endocr. Metab. Disord. 7, 1-16. [DOI] [PubMed] [Google Scholar]
- Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. and Efstratiadis, A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59-72. [PubMed] [Google Scholar]
- Liu, X., Bruxvoort, K. J., Zylstra, C. R., Liu, J., Cichowski, R., Faugere, M. C., Bouxsein, M. L., Wan, C., Williams, B. O. and Clemens, T. L. (2007). Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc. Natl. Acad. Sci. USA 104, 2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman, H. L., La, T. D., Linkhart, T. A., Mohan, S., Baylink, D. J. and Strong, D. D. (1990). Insulin-like growth factor-I and insulin-like growth factor-II induce c-fos in mouse osteoblastic cells. Calcif. Tissue Int. 46, 258-262. [DOI] [PubMed] [Google Scholar]
- Mukherjee, A. and Rotwein, P. (2008). Insulin-like growth factor-binding protein-5 inhibits osteoblast differentiation and skeletal growth by blocking insulin-like growth factor actions. Mol. Endocrinol. 22, 1238-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, A., Dong, S. S., Clemens, T., Alvarez, J. and Serra, R. (2005). Co-ordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mech. Dev. 122, 557-571. [DOI] [PubMed] [Google Scholar]
- Mukherjee, A., Wilson, E. M. and Rotwein, P. (2008). IGF binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions. Mol. Endocrinol. 22, 206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae, J., Kido, Y. and Accili, D. (2001). Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 22, 818-835. [DOI] [PubMed] [Google Scholar]
- Niu, T. and Rosen, C. J. (2005). The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene 361, 38-56. [DOI] [PubMed] [Google Scholar]
- Noda, T., Tokuda, H., Yoshida, M., Yasuda, E., Hanai, Y., Takai, S. and Kozawa, O. (2005). Possible involvement of phosphatidylinositol 3-kinase/Akt pathway in insulin-like growth factor-I-induced alkaline phosphatase activity in osteoblasts. Horm. Metab. Res. 37, 270-274. [DOI] [PubMed] [Google Scholar]
- Ogata, N., Chikazu, D., Kubota, N., Terauchi, Y., Tobe, K., Azuma, Y., Ohta, T., Kadowaki, T., Nakamura, K. and Kawaguchi, H. (2000). Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J. Clin. Invest 105, 935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osyczka, A. M. and Leboy, P. S. (2005). Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 146, 3428-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, G., Bonjour, J. P. and Caverzasio, J. (1997). Expression of a newly identified phosphate transporter/retrovirus receptor in human SaOS-2 osteoblast-like cells and its regulation by insulin-like growth factor I. Endocrinology 138, 5202-5209. [DOI] [PubMed] [Google Scholar]
- Peng, X. D., Xu, P. Z., Chen, M. L., Hahn-Windgassen, A., Skeen, J., Jacobs, J., Sundararajan, D., Chen, W. S., Crawford, S. E., Coleman, K. G. et al. (2003). Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17, 1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phimphilai, M., Zhao, Z., Boules, H., Roca, H. and Franceschi, R. T. (2006). BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 21, 637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, M., Shapiro, P., Kumar, R. and Passaniti, A. (2004). Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J. Biol. Chem. 279, 42709-42718. [DOI] [PubMed] [Google Scholar]
- Raisz, L. G. (2005). Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci, A., Bellosta, P., Grassi, R., Basilico, C. and Mansukhani, A. (2008). Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J. Cell Physiol. 215, 442-451. [DOI] [PubMed] [Google Scholar]
- Schindeler, A., McDonald, M. M., Bokko, P. and Little, D. G. (2008). Bone remodeling during fracture repair: the cellular picture. Semin. Cell Dev. Biol. 19, 459-466. [DOI] [PubMed] [Google Scholar]
- Selz, T., Caverzasio, J. and Bonjour, J. P. (1989). Regulation of Na-dependent Pi transport by parathyroid hormone in osteoblast-like cells. Am. J. Physiol. 256, E93-E100. [DOI] [PubMed] [Google Scholar]
- Serra, C., Palacios, D., Mozzetta, C., Forcales, S. V., Morantte, I., Ripani, M., Jones, D. R., Du, K., Jhala, U. S., Simone, C. et al. (2007). Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell 28, 200-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, R., Karaplis, A. and Sohn, P. (1999). Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J. Cell Biol. 145, 783-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, D. D., Beachler, A. L., Wergedal, J. E. and Linkhart, T. A. (1991). Insulinlike growth factor II and transforming growth factor beta regulate collagen expression in human osteoblastlike cells in vitro. J. Bone Miner. Res. 6, 15-23. [DOI] [PubMed] [Google Scholar]
- Strong, D. D., Merriman, H. L., Landale, E. C., Baylink, D. J. and Mohan, S. (1994). The effects of the insulin-like growth factors and transforming growth factor beta on the Jun proto-oncogene family in MC3T3-E1 cells. Calcif. Tissue Int. 55, 311-315. [DOI] [PubMed] [Google Scholar]
- Tureckova, J., Wilson, E. M., Cappalonga, J. L. and Rotwein, P. (2001). Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J. Biol. Chem. 276, 39264-39270. [DOI] [PubMed] [Google Scholar]
- Ulici, V., Hoenselaar, K. D., Gillespie, J. R. and Beier, F. (2008). The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev. Biol. 8, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, E. M. and Rotwein, P. (2007). Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 282, 5106-5110. [DOI] [PubMed] [Google Scholar]
- Wilson, E. M., Hsieh, M. M. and Rotwein, P. (2003). Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation. J. Biol. Chem. 278, 41109-41113. [DOI] [PubMed] [Google Scholar]
- Wozney, J. M. (1992). The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 32, 160-167. [DOI] [PubMed] [Google Scholar]
- Zaidi, M. (2007). Skeletal remodeling in health and disease. Nat. Med. 13, 791-801. [DOI] [PubMed] [Google Scholar]
- Zehentner, B. K., Dony, C. and Burtscher, H. (1999). The transcription factor Sox9 is involved in BMP-2 signaling. J. Bone Miner. Res. 14, 1734-1741. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Xuan, S., Bouxsein, M. L., von, Stechow, D., Akeno, N., Faugere, M. C., Malluche, H., Zhao, G., Rosen, C. J., Efstratiadis, A. et al. (2002). Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 277, 44005-44012. [DOI] [PubMed] [Google Scholar]
- Zhao, G., Monier-Faugere, M. C., Langub, M. C., Geng, Z., Nakayama, T., Pike, J. W., Chernausek, S. D., Rosen, C. J., Donahue, L. R., Malluche, H. H. et al. (2000). Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141, 2674-2682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.