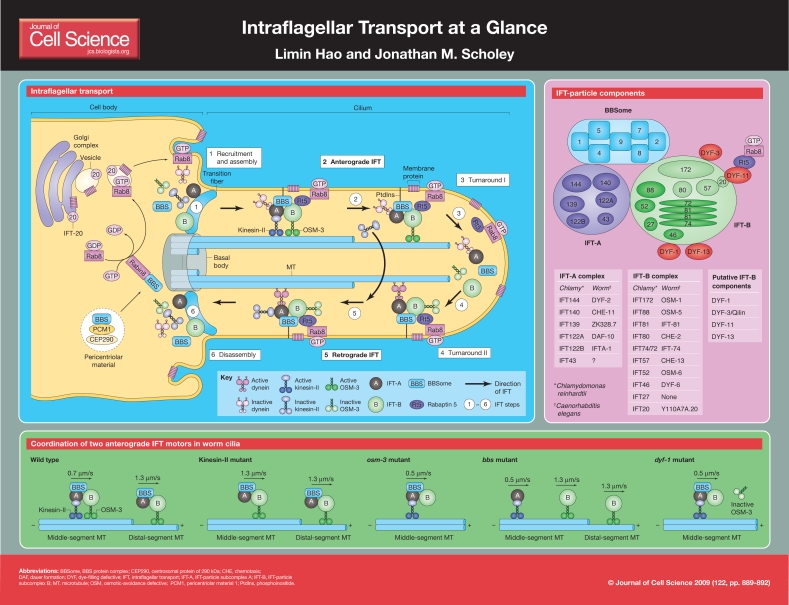
Intraflagellar transport (IFT) is the bidirectional transport of multisubunit protein complexes, called IFT particles, along axonemal microtubules (MTs) beneath the ciliary membrane. IFT plays essential roles in the assembly and function of cilia and flagella by contributing to cell motility, sensory perception and cilium-based signaling (Rosenbaum and Witman, 2002; Scholey, 2003). IFT was first observed as the bidirectional movement of `particles' along Chlamydomonas reinhardtii flagella (Kozminski et al., 1993). Subsequently, time-lapse fluorescence-microscopy assays in transgenic Caenorhabditis elegans allowed observations of the transport of specifically tagged IFT proteins along sensory cilia (Orozco et al., 1999). IFT is now understood to be highly conserved among a broad range of eukaryotes; mutations in IFT proteins in these organisms disrupt cilium biogenesis (Absalon et al., 2008; Avidor-Reiss et al., 2004; Blacque et al., 2008; Follit et al., 2006; Jekely and Arendt, 2006; Nachury et al., 2007; Rosenbaum and Witman, 2002; Scholey, 2003), although, in rare cases, flagella are assembled in the cytoplasm using IFT-independent mechanisms (Briggs et al., 2004; Han et al., 2003; Sarpal et al., 2003). Importantly, defects in IFT and ciliogenesis have been linked to various human diseases such as retinal degeneration, polycystic kidney disease, Bardet-Biedl syndrome (BBS), Jeune asphyxiating thoracic dystrophy, respiratory disease and defective determination of the left-right axis (Blacque and Leroux, 2006).
Figure 1.
Numerous exciting and rapidly expanding topics that are relevant to IFT exist, such as the cellular and developmental functions of cilia and the pathogenesis of ciliary diseases (Adams et al., 2008; Badano et al., 2006; Bisgrove and Yost, 2006; Eggenschwiler and Anderson, 2007; Fliegauf et al., 2007). Our focus here, however, is on summarizing recent work on the composition and mechanism of action of the IFT machinery, including the IFT-particle subcomplexes IFT-A and IFT-B, IFT motors, the BBSome, and other newly identified polypeptides that are associated with IFT-B.
Model for the mechanism of IFT
IFT is proposed to occur in six phases (Pedersen et al., 2008). The first step is to recruit and assemble the IFT machinery and its cargo at the transition fiber, which links the basal body and the membrane around the neck of the cilium (Deane et al., 2001). The second step is anterograde transport of IFT particles; these particles carry cargo and inactive retrograde IFT-dynein motor protein from the basal body to the tip of cilia. The third and fourth steps involve a poorly understood series of events termed `turnaround'. Step 3 includes inactivation of the anterograde motors (kinesin-II or OSM-3), cargo unloading, and dissociation of IFT-A and IFT-B, whereas step 4 involves the activation of the retrograde dynein motor, the assembly of the retrograde IFT machinery and the uploading of retrograde cargo. A structure termed the `flagellar tip complex', which contains the MT-plus-end-binding protein (EB1) is proposed to facilitate turnaround (Sloboda, 2005). The fifth step is the retrograde IFT of ciliary turnover proteins and inactive anterograde motors back from the distal tip to the base of the cilium. Finally, in step 6, the IFT machinery is disassembled for possible re-use.
The molecular composition of the IFT particle
IFT particles were isolated and characterized first from the flagellar matrix of C. reinhardtii, and they can be resolved into two subcomplexes – IFT-A and IFT-B (Cole et al., 1998; Piperno and Mead, 1997). In vivo transport and genetic data from C. elegans also support such a `two subcomplex' model (Ou et al., 2005a). The IFT-A subcomplex in C. reinhardtii comprises six components, namely IFT144, IFT140, IFT139, IFT122A, IFT122B and IFT43. Four of these components (IFT144, IFT140, IFT122A and IFT122B) have been characterized in C. elegans (Blacque et al., 2006; Efimenko et al., 2006; Qin et al., 2001), Trypanosoma brucei (Absalon et al., 2008) and other organisms (Adams et al., 2008; Tsao and Gorovsky, 2008b). It is possible that IFT-A plays a role in returning IFT proteins from the ciliary tip to the cell body, as the knockout or knockdown of IFT-A components results in the accumulation of IFT particles at the ciliary tip (Absalon et al., 2008; Blacque et al., 2006; Efimenko et al., 2006; Iomini et al., 2001; Piperno et al., 1998; Tsao and Gorovsky, 2008b).
An isolated IFT-B subcomplex dissociates under high-salt conditions to yield the IFT-B core – which contains IFT81, IFT74 and IFT72 (IFT74/72), IFT88, IFT52, IFT46 and IFT27 – and four dissociated components – IFT172, IFT80, IFT57 and IFT20. Within the core, IFT74 and IFT72 are protein variants that are encoded by the same gene and can interact with the IFT81 dimer to form a tetramer (Lucker et al., 2005). C. elegans IFT81 and IFT74 single mutants display very similar phenotypes (Kobayashi et al., 2007). IFT88 and IFT52 have been characterized in a few organisms, establishing their essential roles in ciliogenesis (Absalon et al., 2008; Rosenbaum and Witman, 2002; Scholey, 2003). Interestingly, IFT52 has been found to move separately from all other IFT-B proteins in nephrocystin mutants (Jauregui et al., 2008). IFT46 is responsible for the transport of outer-arm dynein along the axoneme in C. reinhardtii (Hou et al., 2007), whereas IFT27 is a Rab-like small G protein that is required for the assembly of C. reinhardtii flagella. There are no orthologs of IFT27 in C. elegans or Drosophila melanogaster, however, implying that it has roles specifically in motile cilia (Blacque et al., 2008). Mutations in the dissociated component IFT80 lead to defects in cilia formation (Qin et al., 2001). IFT20 is found at the Golgi complex and can interact with IFT57 (Follit et al., 2006). IFT20 might also interact with one of the kinesin-II subunits, but this interaction is less certain in vivo (Baker et al., 2003; Follit et al., 2006). Recently, IFT20 has been found to interact with the zebrafish Rabaptin5- and Rab8-interacting protein Elipsa (known as DYF-11 in C. elegans), thus providing a potential bridging mechanism between IFT transport and membrane-associated protein complexes (Omori et al., 2008). IFT172 interacts with EB1, indicating a possible role in `turnaround' (Sloboda, 2005), a proposal that is supported by the analysis of IFT172 in Tetrahymena thermophila and T. brucei (Absalon et al., 2008; Tsao and Gorovsky, 2008a).
Four additional putative IFT-B proteins in C. elegans (DYF-1, DYF-3, DYF-13 and DYF-11) appear to be associated with the IFT-B subcomplex and undergo IFT (Blacque et al., 2005; Omori et al., 2008; Ou et al., 2005a; Ou et al., 2005b). Depletion of these proteins causes defects in cilium formation. All these genes contain X-box sequences in their putative promoter region that are regulated by the transcription factor daf-19, similar to other IFT components. They are conserved in all ciliated, but not non-ciliated, organisms, supporting the idea that they are IFT-particle components, although biochemical data are needed to confirm this (Omori et al., 2008).
Most protein components of IFT particles contain sequence motifs or repeats (WD40 or TPR, respectively) that are associated with protein-protein interactions (Cole, 2003; Jekely and Arendt, 2006). On the basis of the homology between IFT-complex proteins and components of coat protein I (COPI) and clathrin-coated vesicles, it has been proposed that IFT evolved as a specialized form of coated-vesicle transport from a protocoatomer complex. IFT thus shares common ancestry with all protocoatomer derivatives, including all vesicle coats and the nuclear pore complex (NPC) (Jekely and Arendt, 2006).
The BBSome
The BBS1, 2, 4, 5, 7, 8 and 9 proteins form a complex termed the BBSome and are encoded by genes that are associated with the ciliary disease BBS (Nachury et al., 2007). This complex is thought to be conserved among ciliated organisms, in which it promotes ciliary membrane biogenesis by increasing the level of Rab8-GTP (Nachury et al., 2007). BBS4 is distributed between two pools inside the cell: one at centriolar satellites and the other inside the cilium (Kim et al., 2004; Nachury et al., 2007). BBS5 contains two pleckstrin homology (PH)-like domains and binds to phosphoinositides; inhibition of phosphoinositide production prevents ciliogenesis (Nachury et al., 2007), which provides a possible mechanism for tethering the IFT machinery to the cilium membrane. In C. elegans, the loss of BBSome components, such as BBS-7 or BBS-8, leads to the dissociation of the IFT-A and IFT-B subcomplexes (Ou et al., 2005a).
IFT motors
Anterograde and retrograde IFT are powered by two members of the kinesin-2 family (heterotrimeric kinesin-II and homodimeric OSM-3) and by the IFT-dynein motor, respectively, as discussed in a very recent review (Scholey, 2008). In some cilia, it is thought that the heterotrimeric kinesin-2 motor (kinesin-II or KIF3) acts alone to drive anterograde transport, but in other types of cilia, such as in C. elegans sensory neurons and zebrafish photoreceptors, its activity is augmented by a homodimeric kinesin-2 motor [OSM-3 or KIF17 (Insinna et al., 2008)]. In this case, kinesin-II (or KIF3) builds the core of the axoneme, which consists of nine MT doublets called the `middle segment', and dissociates from the IFT machinery at the tip of this domain, whereas OSM-3 (or KIF17) continues moving tip-wards and specifically assembles nine distal singlets, which are thought to be required for cilium-based signaling (Scholey and Anderson, 2006). The recently identified mitogen-activated protein (MAP) kinase DYF-5 is proposed to modulate the processivity of OSM-3 and to mediate the dissociation of kinesin-II at the middle-segment tips (Burghoorn et al., 2007). The two anterograde motors are proposed to be coordinated by the BBSome (Ou et al., 2005a).
The retrograde motor, IFT-dynein, is formed by two identical cytoplasmic dynein heavy chain 1b (Dhc1b) heavy chains, together with several intermediate, light-intermediate and light chains. How the IFT motors interact with the IFT particles is not known, although in C. elegans bbs mutants, in which IFT-A and IFT-B are dissociated and move separately, kinesin-II moves together with IFT-A and OSM-3 moves with IFT-B. IFT-dynein is proposed to associate with IFT-A (Pedersen et al., 2008).
IFT cargos
Two types of IFT cargo have been identified: axonemal precursor proteins, which are associated with motility, and membrane-associated proteins, which are associated with cilium-based signaling. Axonemal components such as radial spokes, outer-arm dynein and inner-arm dynein, are preassembled prior to being loaded onto the IFT machinery (Qin et al., 2004). Recent data have demonstrated that the loading of specific cargo proteins might require specific IFT-particle subunits. For instance, IFT46 and an associated adaptor protein, ODA16, are essential for transporting outer-arm dynein along C. reinhardtii axonemes (Ahmed et al., 2008; Hou et al., 2007). In the case of membrane-protein cargos, it has been observed that mutations in heterotrimeric kinesin-II lead to defects in the transport of opsin and arrestin from the inner to the outer segment in the mouse photoreceptor (Marszalek et al., 2000). The motility of two transient receptor potential vanilloid (TRPV) channels, OSM-9 and OCR-2, in C. elegans ciliary membranes was directly observed to occur at rates that are comparable to that of the IFT machinery (Qin et al., 2005).
Perspectives
A framework for the entire IFT process has been established, but important questions remain. For example, how do the components assemble to form a functional complex? What is the three-dimensional structure of the IFT machinery? What is the role of each component in the complex? And how do the IFT particles interact with the IFT motors? All the motors that are directly involved in IFT-particle transport appear to have been identified and characterized, but how they are activated and deactivated in the right place and right time is not known. Other questions, such as how the IFT machinery turns around at the tip of cilia, how the components are reorganized at the base and tip of cilia, and how the cargo is uploaded and downloaded, need to be elucidated. To answer these questions, a combination of approaches, including biochemistry, genetics, cell biology, electron microscopy and X-ray diffraction, is necessary.
We apologize to the authors whose original work is not cited owing to space restriction. We are indebted to Joel Rosenbaum, George Witman, Frank McNally, Guangshuo Ou and Seyda Acar for critical review of this manuscript. The work on IFT in the laboratory is supported by NIH grant GM50718. Deposited in PMC for release after 12 months.
References
- Absalon, S., Blisnick, T., Kohl, L., Toutirais, G., Dore, G., Julkowska, D., Tavenet, A. and Bastin, P. (2008). Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol. Biol. Cell 19, 929-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, M., Smith, U. M., Logan, C. V. and Johnson, C. A. (2008). Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J. Med. Genet. 45, 257-267. [DOI] [PubMed] [Google Scholar]
- Ahmed, N. T., Gao, C., Lucker, B. F., Cole, D. G. and Mitchell, D. R. (2008). ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 183, 313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss, T., Maer, A. M., Koundakjian, E., Polyanovsky, A., Keil, T., Subramaniam, S. and Zuker, C. S. (2004). Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117, 527-539. [DOI] [PubMed] [Google Scholar]
- Badano, J. L., Mitsuma, N., Beales, P. L. and Katsanis, N. (2006). The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125-148. [DOI] [PubMed] [Google Scholar]
- Baker, S. A., Freeman, K., Luby-Phelps, K., Pazour, G. J. and Besharse, J. C. (2003). IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J. Biol. Chem. 278, 34211-34218. [DOI] [PubMed] [Google Scholar]
- Bisgrove, B. W. and Yost, H. J. (2006). The roles of cilia in developmental disorders and disease. Development 133, 4131-4143. [DOI] [PubMed] [Google Scholar]
- Blacque, O. E. and Leroux, M. R. (2006). Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol. Life Sci. 63, 2145-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque, O. E., Perens, E. A., Boroevich, K. A., Inglis, P. N., Li, C., Warner, A., Khattra, J., Holt, R. A., Ou, G., Mah, A. K. et al. (2005). Functional genomics of the cilium, a sensory organelle. Curr. Biol. 15, 935-941. [DOI] [PubMed] [Google Scholar]
- Blacque, O. E., Li, C., Inglis, P. N., Esmail, M. A., Ou, G., Mah, A. K., Baillie, D. L., Scholey, J. M. and Leroux, M. R. (2006). The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol. Biol. Cell 17, 5053-5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque, O. E., Cevik, S. and Kaplan, O. I. (2008). Intraflagellar transport: from molecular characterisation to mechanism. Front. Biosci. 13, 2633-2652. [DOI] [PubMed] [Google Scholar]
- Briggs, L. J., Davidge, J. A., Wickstead, B., Ginger, M. L. and Gull, K. (2004). More than one way to build a flagellum: comparative genomics of parasitic protozoa. Curr. Biol. 14, R611-R612. [DOI] [PubMed] [Google Scholar]
- Burghoorn, J., Dekkers, M. P., Rademakers, S., de Jong, T., Willemsen, R. and Jansen, G. (2007). Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 7157-7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, D. G. (2003). The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4, 435-442. [DOI] [PubMed] [Google Scholar]
- Cole, D. G., Diener, D. R., Himelblau, A. L., Beech, P. L., Fuster, J. C. and Rosenbaum, J. L. (1998). Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R. and Rosenbaum, J. L. (2001). Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586-1590. [DOI] [PubMed] [Google Scholar]
- Efimenko, E., Blacque, O. E., Ou, G., Haycraft, C. J., Yoder, B. K., Scholey, J. M., Leroux, M. R. and Swoboda, P. (2006). Caenorhabditis elegans DYF-2, an orthologue of human WDR19, is a component of the intraflagellar transport machinery in sensory cilia. Mol. Biol. Cell 17, 4801-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler, J. T. and Anderson, K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf, M., Benzing, T. and Omran, H. (2007). When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell. Biol. 8, 880-893. [DOI] [PubMed] [Google Scholar]
- Follit, J. A., Tuft, R. A., Fogarty, K. E. and Pazour, G. J. (2006). The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. G., Kwok, B. H. and Kernan, M. J. (2003). Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13, 1679-1686. [DOI] [PubMed] [Google Scholar]
- Hou, Y., Qin, H., Follit, J. A., Pazour, G. J., Rosenbaum, J. L. and Witman, G. B. (2007). Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 176, 653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna, C., Pathak, N., Perkins, B., Drummond, I. and Besharse, J. C. (2008). The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 316, 160-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini, C., Babaev-Khaimov, V., Sassaroli, M. and Piperno, G. (2001). Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153, 13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui, A. R., Nguyen, K. C., Hall, D. H. and Barr, M. M. (2008). The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J. Cell Biol. 180, 973-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely, G. and Arendt, D. (2006). Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays 28, 191-198. [DOI] [PubMed] [Google Scholar]
- Kim, J. C., Badano, J. L., Sibold, S., Esmail, M. A., Hill, J., Hoskins, B. E., Leitch, C. C., Venner, K., Ansley, S. J., Ross, A. J. et al. (2004). The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 36, 462-470. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Gengyo-Ando, K., Ishihara, T., Katsura, I. and Mitani, S. (2007). IFT-81 and IFT-74 are required for intraflagellar transport in C. elegans. Genes Cells 12, 593-602. [DOI] [PubMed] [Google Scholar]
- Kozminski, K. G., Johnson, K. A., Forscher, P. and Rosenbaum, J. L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker, B. F., Behal, R. H., Qin, H., Siron, L. C., Taggart, W. D., Rosenbaum, J. L. and Cole, D. G. (2005). Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem. 280, 27688-27696. [DOI] [PubMed] [Google Scholar]
- Marszalek, J. R., Liu, X., Roberts, E. A., Chui, D., Marth, J. D., Williams, D. S. and Goldstein, L. S. (2000). Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 102, 175-187. [DOI] [PubMed] [Google Scholar]
- Nachury, M. V., Loktev, A. V., Zhang, Q., Westlake, C. J., Peranen, J., Merdes, A., Slusarski, D. C., Scheller, R. H., Bazan, J. F., Sheffield, V. C. et al. (2007). A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201-1213. [DOI] [PubMed] [Google Scholar]
- Omori, Y., Zhao, C., Saras, A., Mukhopadhyay, S., Kim, W., Furukawa, T., Sengupta, P., Veraksa, A. and Malicki, J. (2008). Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10, 437-444. [DOI] [PubMed] [Google Scholar]
- Orozco, J. T., Wedaman, K. P., Signor, D., Brown, H., Rose, L. and Scholey, J. M. (1999). Movement of motor and cargo along cilia. Nature 398, 674. [DOI] [PubMed] [Google Scholar]
- Ou, G., Blacque, O. E., Snow, J. J., Leroux, M. R. and Scholey, J. M. (2005a). Functional coordination of intraflagellar transport motors. Nature 436, 583-587. [DOI] [PubMed] [Google Scholar]
- Ou, G., Qin, H., Rosenbaum, J. L. and Scholey, J. M. (2005b). The PKD protein qilin undergoes intraflagellar transport. Curr. Biol. 15, R410-R411. [DOI] [PubMed] [Google Scholar]
- Pedersen, L. B., Veland, I. R., Schroder, J. M. and Christensen, S. T. (2008). Assembly of primary cilia. Dev. Dyn. 237, 1993-2006. [DOI] [PubMed] [Google Scholar]
- Piperno, G. and Mead, K. (1997). Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA 94, 4457-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., Siuda, E., Henderson, S., Segil, M., Vaananen, H. and Sassaroli, M. (1998). Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 143, 1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, H., Rosenbaum, J. L. and Barr, M. M. (2001). An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr. Biol. 11, 457-461. [DOI] [PubMed] [Google Scholar]
- Qin, H., Diener, D. R., Geimer, S., Cole, D. G. and Rosenbaum, J. L. (2004). Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, H., Burnette, D. T., Bae, Y. K., Forscher, P., Barr, M. M. and Rosenbaum, J. L. (2005). Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695-1699. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, J. L. and Witman, G. B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825. [DOI] [PubMed] [Google Scholar]
- Sarpal, R., Todi, S. V., Sivan-Loukianova, E., Shirolikar, S., Subramanian, N., Raff, E. C., Erickson, J. W., Ray, K. and Eberl, D. F. (2003). Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr. Biol. 13, 1687-1696. [DOI] [PubMed] [Google Scholar]
- Scholey, J. M. (2003). Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423-443. [DOI] [PubMed] [Google Scholar]
- Scholey, J. M. (2008). Intraflagellar transport motors in cilia: moving along the cell's antenna. J. Cell Biol. 180, 23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey, J. M. and Anderson, K. V. (2006). Intraflagellar transport and cilium-based signaling. Cell 125, 439-442. [DOI] [PubMed] [Google Scholar]
- Sloboda, R. D. (2005). Intraflagellar transport and the flagellar tip complex. J. Cell Biochem. 94, 266-272. [DOI] [PubMed] [Google Scholar]
- Tsao, C. C. and Gorovsky, M. A. (2008a). Different effects of tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Mol. Biol. Cell 19, 1450-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, C. C. and Gorovsky, M. A. (2008b). Tetrahymena IFT122A is not essential for cilia assembly but plays a role in returning IFT proteins from the ciliary tip to the cell body. J. Cell Sci. 121, 428-436. [DOI] [PubMed] [Google Scholar]